Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3734
Peer-review started: September 3, 2019
First decision: September 23, 2019
Revised: October 18, 2019
Accepted: October 30, 2019
Article in press: October 29, 2019
Published online: November 26, 2019
Processing time: 83 Days and 23.8 Hours
Hepatocellular carcinoma (HCC) is the world’s sixth most common malignant tumor and the third cause of cancer death. Although great progress has been made in hepatectomy, it is still associated with a certain degree of risk of post-hepatectomy liver failure (PHLF), which extends the length of hospital stay and remains the leading cause of postoperative death. Studies have shown that assessment of hepatic functional reserve before hepatectomy is beneficial for reducing the incidence of PHLF.
To assess the value of model for end-stage liver disease (MELD) score combined with standardized future liver remnant (sFLR) volume in predicting PHLF in patients undergoing hepatectomy for HCC.
This study was attended by 238 patients with HCC who underwent hepatectomy between January 2015 and January 2018. Discrimination of sFLR volume, MELD score, and sFLR/MELD ratio to predict PHLF was evaluated according to the area under the receiver operating characteristic curve.
The patients were divided into two groups according to whether PHLF occurred after hepatectomy. The incidence of PHLF was 8.4% in our research. The incidence of PHLF increased with the decrease in sFLR volume and the increase in MELD score. Both sFLR volume and MELD score were considered independent predictive factors for PHLF. Moreover, the cut-off value of the sFLR/MELD score to predict PHLF was 0.078 (P < 0.001). This suggests that an sFLR/MELD ≥ 0.078 indicates a higher incidence of PHLF than an sFLR/MELD < 0.078.
MELD combined with sFLR is a reliable and effective PHLF predictor, which is superior to MELD score or sFLR volume alone.
Core tip: Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the second leading cause of death from cancer worldwide. At present, Post-hepatectomy liver failure (PHLF) is still one of the main causes of death for HCC patients undergoing hepatectomy. Although standardized future liver remnant (sFLR) or model for end-stage liver disease (MELD) can predict the occurrence of PHLF to a certain extent, their sensitivity and specificity do not sufficiently meet clinical needs. The combination of sFLR volume with MELD score is a reliable predictor of PHLF. This measurement can effectively guide the early management after hepatectomy, thereby improving the prognosis and reducing the mortality. Also, the model can provide a new strategy for the preoperative evaluation of hepatectomy.
- Citation: Kong FH, Miao XY, Zou H, Xiong L, Wen Y, Chen B, Liu X, Zhou JJ. End-stage liver disease score and future liver remnant volume predict post-hepatectomy liver failure in hepatocellular carcinoma. World J Clin Cases 2019; 7(22): 3734-3741
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3734.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3734
Hepatocellular carcinoma (HCC) is the world’s sixth highest incidence and death rate third malignant tumor. In the past 10 years, there has been great progress in the treatment of HCC. There are many ways to treat HCC, such as hepatectomy, image-guided transcatheter tumor therapy, liver transplantation, and systemic therapy (drugs such as sorafenib were indicated to improve survival rates in patients with advanced liver cancer)[1-3]. However, hepatectomy is still the first-line treatment for primary and secondary liver cancer and non-cirrhotic hepatocellular carcinoma, and the best method for long-term survival[4,5]. The widespread use of large-area hepatectomy enhances the danger of post-hepatectomy liver failure (PHLF), which is related to the incidence of complications, mortality, and length of prolonged hospital stay[6]. Despite improvements in surgical and postoperative management, the parameters determining the degree of hepatectomy remain largely uncertain. Many preoperative factors, intraoperative factors, and postoperative factors are the causes of PHLF[6]. Preoperative evaluation, including the assessment of liver volume and residual liver function, is a prerequisite for major hepatectomy. At present, PHLF remains one of the worst complications in hepatectomy, and it is one of the main causes of death after hepatectomy[7,8]. Therefore, accurate preoperative prediction of PHLF risk in patients with liver cancer is key for surgeons to evaluate the feasibility and safety of hepatectomy.
The quality and quantity of hepatocytes determine the liver function reserve. In 2000, Malinchoc et al[9] used model for end-stage liver disease (MELD) score to predict the mortality of patients with end-stage liver disease after tranjugular intrahepatic portosystemic shunt, and the authors confirmed that MELD score could predict mortality and survival time in end-stage liver disease. MELD scores are used to prioritize patients most in need of organ transplantation according to objective criteria (creatinine level, international standardized ratio (INR), and bilirubin level)[10,11]. However, MELD score can also predict the survival rate of patients with liver cirrhosis caused by infection, variceal bleeding, fulminant liver failure, and alcoholic hepatitis. Moreover, MELD score can also be used to select surgical patients other than liver transplantation patients and to determine the best treatment for HCC patients[12-14]. It is reported that MELD score can predict the occurrence and death of PHLF after hepatectomy[13,15].
Standardized future liver remnant (sFLR) volume has been considered to be an important factor affecting the outcome of major hepatectomy[16]. In recent years, liver computed tomography (CT) volumetry has been used to evaluate liver function reserve, especially in the selection of patients with HCC for major hepatectomy. Before the operation, the residual volume of the liver is measured by a 3D CT reconstruction method, which can accurately reflect the size of the residual liver. Patients with smaller FLR volumes have a higher risk of PHLF[17]. Although MELD score and sFLR volume are two popular markers for evaluating liver function reserve in the clinic, no studies have been performed on the effect of the MELD score and sFLR volume to predict the incidence of PHLF after hepatectomy.
In the past, sFLR volume was used to measure the size of the residual liver[17]. Although both measurements can predict the occurrence of PHLF after hepatectomy to a certain extent, the sensitivity and specificity do not sufficiently meet clinical needs; therefore, an urgent issue is the need for a new method to predict the risk of PHLF after hepatectomy in order to better reduce the incidence of PHLF. In view of this, we compared the roles of MELD score, sFLR volume, and sFLR/MELD ratio in predicting PHLF after hepatectomy.
Patients who accepted 3D CT reconstruction prior to hepatectomy for HCC from January 2015 to January 2018 at the Second Xiangya Hospital of Central South University were considered for this retrospective study. The inclusion criteria were: (1) HCC was not treated before operation, without cardiopulmonary dysfunction, renal insufficiency, or severe encephalopathy before hepatectomy; (2) Open hepatectomy with curative intent performed by a single team of surgeons; and (3) None of these patients had biliary obstruction prior to surgery or evidence of hepatitis C virus-specific antibodies or alcoholic cirrhosis.
Informed consent was waived for this retrospective research. This research was approved by the Central South University Agency Review Committee.
To overcome the limitation of MELD score in predicting postoperative prognosis, in recent years, the liver CT volume method has been used to estimate the liver function reserve, especially in selecting patients with HCC for major hepatectomy[18]. The FLR volume of the liver was measured by 3D CT reconstruction, and the residual liver size could be truly reflected[19]. sFLR volume, calculated as FLR/estimated total liver volume, was used to reflect the percentage of residual liver after resection[20]. MELD score is calculated on the basis of INR, serum creatinine (Cre), and the total bilirubin (TBil): MELD = 9.57 × ln (Cre, mg/dL) + 3.78 × ln (TBil, mg/dL) + 11.2 × ln (INR) + 6.43 × (etiology: 0 if cholestatic or alcoholic, 1 otherwise)[21]. A diagnosis of HCC was made according to the postoperative pathological examination. PHLF was accounted as the total bilirubin value was more than 50 μmol/L and the prothrombin time index was less than 50% (INR > 1.7) on or after the 5th d after operation[22,23].
Continuous variables are represented as intermediate values (ranges) and compared by the Mann-Whitney U test. Univariate analysis and multivariate Logistic regression analysis were used to determine the risk factors related to PHLF. Determination of the cut-off value of PHLF was performed by receiver operating characteristic (ROC) curve analysis. Comparison of discrete variables was performed by the χ2 test. Statistical analyses were performed using SPSS 24.0 (IBM, United States). P values < 0.05 were considered statistically significant.
In this study, 238 patients were divided into two groups according to whether PHLF occurred after hepatectomy. The median age of PHLF (+) patients was 57 years (range, 26-66 years), and that of PHLF (-) patients was 51 years (range, 18-74 years). Comparisons showed that there was no difference in prothrombin time, age, sex ratio, INR, alanine aminotransferase, HBsAg positivity, maximum tumor size, or major hepatectomy (P > 0.05; Table 1) between the two groups. However, platelet count (P < 0.05), total bilirubin level (P < 0.01), albumin level (P < 0.05), sFLR volume (P < 0.001), and MELD score (P < 0.01) were significantly different (Table 1). In China, many patients already have liver cirrhosis and poor liver function during outpatient visits. Of the patients included in the research, the percentage of patients with HBsAg positivity was as high as 90%, and many of the patients with HCC also had liver cirrhosis, which led to the high incidence of PHLF.
| Variable | PHLF (+) (n = 20) | PHLF (-) (n = 218) | P value |
| Age, years | 57 (26-66) | 51 (18-74) | 0.077 |
| Gender ratio, M/F | 16/4 | 189/29 | 0.407 |
| Platelet count, ×109/L | 123 (28-240) | 158 (30-535) | 0.016 |
| Prothrombin time, s | 13.9 (10.8-16.4) | 13.1 (9.9-17.1) | 0.289 |
| International normalized ratio | 1.06 (1.00-1.26) | 1.06 (0.88-1.44) | 0.971 |
| Total bilirubin, μmol/L | 20.1 (6.2-43.1) | 12.9 (3.9-37.1) | 0.001 |
| Albumin, g/L | 37.3 (26.1-43.3) | 38.8 (25.6-48.9) | 0.029 |
| Alanine aminotransferase, U/L | 46.2 (16.9-94.7) | 34.1 (8.5-302.6) | 0.127 |
| HBsAg positivity | 90.0% | 92.7% | 0.667 |
| Maximum tumor size, cm | 6.5 (2.5-13.3) | 5.5 (1.5-18.0) | 0.270 |
| sFLR | 0.503 (0.378-0.780) | 0.666 (0.361-0.995) | < 0.001 |
| Major hepatectomy | 40.0% | 25.2% | 0.152 |
| MELD score | 9 (6-12) | 7 (6-12) | 0.001 |
Univariate and multivariate analyses were used to determine the risk factors related to PHLF. The correlation between PHLF and the sFLR volume combined with MELD score was analyzed. Univariate logistic regression analysis showed that platelet count, albumin level, MELD score, and sFLR volume were risk factors for PHLF (P < 0.05; Table 2). Multivariate logistic regression analysis revealed that platelet count, albumin level, MELD score, and sFLR volume were independent risk factors for PHLF (P < 0.05; Table 2).
| Variable | Univariate analysis | Multivariate analysis | ||
| Odds ratio | P value | Odds ratio | P value | |
| Age, years | 1.60 (0.58-4.40) | 0.360 | 3.02 (0.77-11.78) | 0.112 |
| Male sex | 0.61 (0.19-1.96) | 0.411 | 0.68 (0.15-3.07) | 0.616 |
| Platelet count, ×109/L | 2.97 (1.14-7.73) | 0.026 | 5.17 (1.31-20.35) | 0.019 |
| Blood loss, mL | 2.27 (0.87-5.92) | 0.092 | 1.14 (0.33-3.91) | 0.839 |
| Tumor number (≥ 3) | 0.58 (0.13-2.62) | 0.479 | 0.31 (0.05-2.14) | 0.235 |
| Albumin, g/L | 3.67 (1.29-10.46) | 0.015 | 3.90 (1.14-13.35) | 0.030 |
| Prothrombin time, s | 1.45 (0.57-3.68) | 0.439 | 1.39 (0.40-4.82) | 0.605 |
| sFLR | 10.08 (3.49-29.10) | < 0.001 | 29.92 (6.51-137.46) | < 0.001 |
| MELD | 5.98 (2.31-15.45) | < 0.001 | 7.89 (2.23-28.01) | 0.001 |
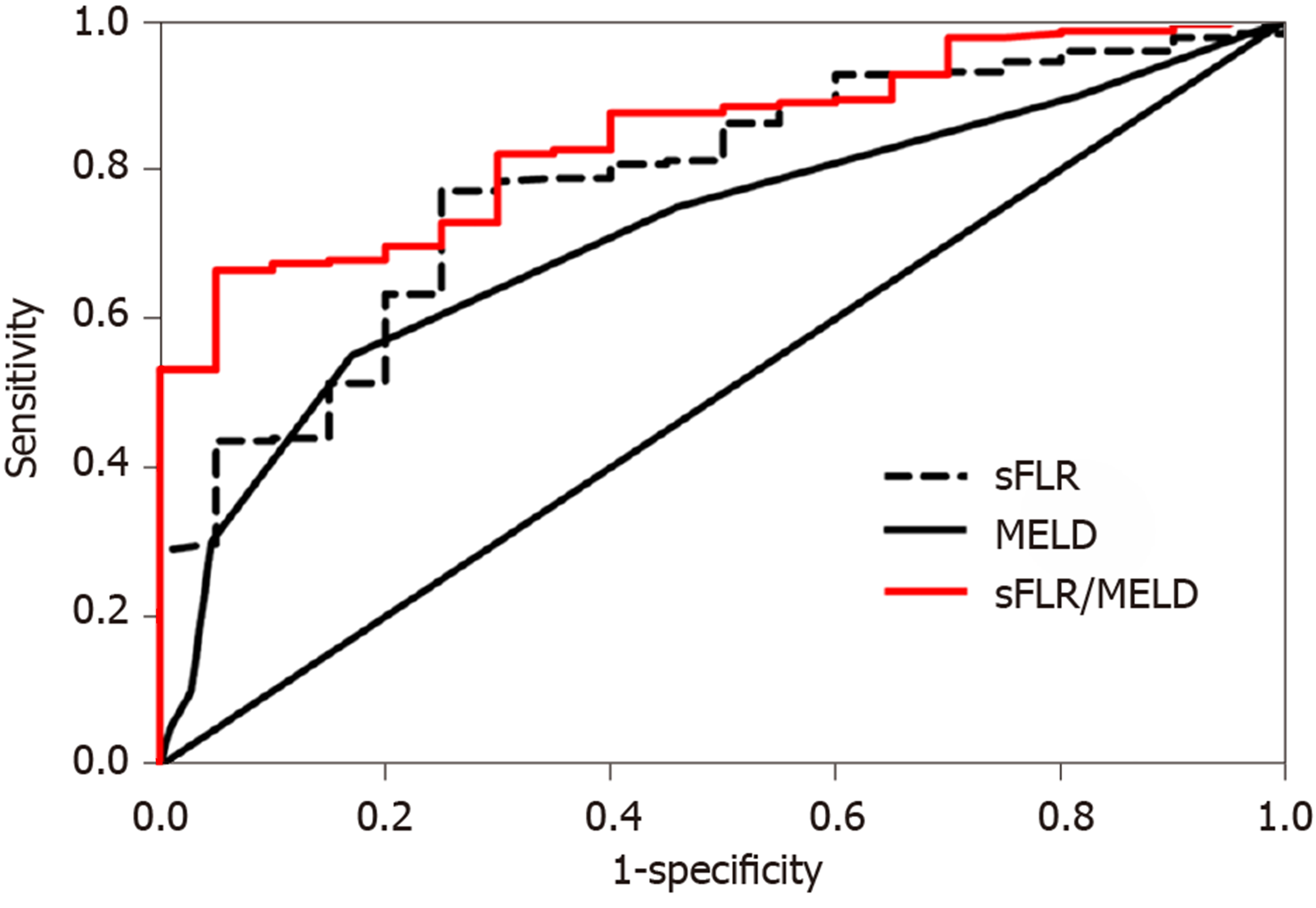
The ROC curve analysis revealed that the area under curve (AUC) of MELD score to predict PHLF was 0.715 (Figure 1), with a 55.0% sensitivity and 83.0% specificity, and the best MELD score cut-off value for the prediction of PHLF was 8.5 (P < 0.01, Table 3). Similarly, the AUC of sFLR volume for predicting PHLF was 0.782 (Figure 1), and the cut-off value was 0.544, with a 77.1% sensitivity and 75.0% specificity (P < 0.001, Table 3). Moreover, the AUC of the sFLR/MELD score for the prediction of PHLF was 0.845 (Figure 1), and the cut-off value was 0.078, with a 66.5% sensitivity and 95.0% specificity (P < 0.001, Table 3).
| Index | AUC | 95%CI | Cut-off value | P value | Sensitivity (%) | Specificity (%) |
| MELD | 0.715 | 0.581-0.849 | 8.5 | 0.001 | 55.0 | 83.0 |
| sFLR | 0.782 | 0.687-0.877 | 0.544 | < 0.001 | 77.1 | 75.0 |
| sFLR/MELD | 0.845 | 0.778-0.912 | 0.078 | < 0.001 | 66.5 | 95.0 |
To validate the sFLR/MELD score, we used the sFLR/MELD ratio as the basis for dividing all patients into two groups. The incidence of PHLF was 13.01% (19/146) in patients with an sFLR/MELD score ≥ 0.078 and 1.09% (1/92) in patients with an sFLR/MELD score < 0.078 (χ2 = 9.065, P = 0.001. Table 4). When sFLR/MELD ≥ 0.078, the incidence of PHLF was much higher than that in patients with an sFLR/MELD < 0.078. The regression coefficients of the sFLR/MELD score were statistically significant. Therefore, 0.078 can be used as the favorable cut-off value to predict PHLF based on sFLR/MELD. Finally, our data showed that sFLR/MELD score, as a good indicator of PHLF, can predict PHLF better than MELD score or sFLR volume alone.
| Group | n | PHLF (+) | PHLF (-) |
| sFLR/MELD ≥ 0.078 | 146 | 19 | 127 |
| sFLR/MELD < 0.078 | 92 | 1 | 91 |
In this research, we evaluated the value of MELD score, sFLR volume, and their combination to predict the occurrence of PHLF after hepatectomy. Although both MELD score and sFLR volume can predict the occurrence of PHLF to some extent, we found that their combination could improve the accuracy, sensitivity, and specificity for predicting PHLF. Thus, this combination score has good guiding significance in the clinic.
For years, hepatectomy has remained one of the most complex surgical procedures. The mortality rate after hepatectomy ranges from 0 to 5%[24], while PHLF remains the main cause of high mortality after hepatectomy[25]. PHLF refers to the failure of one or more synthetic and excretory functions, including hyperbilirubinemia, prolonged prothrombin time, hypoalbuminemia, elevated serum lactate, and different grades of hepatic encephalopathy[25-27]. Although the incidences of both PHLF and mortality have improved considerably over the past 10 years as a result of improvements in surgical techniques and critical care, the incidence of PHLF is still 8% to 10%[25,28]. However, the incidence of PHLF can be as low as 1% to 2% in some countries[29]. This outcome may be associated with a low incidence of HBV infection and liver cirrhosis[29]. Therefore, it is of great clinical significance to evaluate the risk for PHLF before surgery to reduce the incidence of PHLF after hepatectomy.
MELD score is commonly used as an objective criterion for evaluating the severity of end-stage liver disease and has also been applied in the treatment of patients with chronic liver disease without transplantation[30]. Additionally, MELD score has also been indicated to have the ability to predict PHLF and mortality following hepatic resection in HCC[13,15]. According to multivariate analysis in this study, MELD score is an independent predictor of PHLF after hepatectomy. However, in some other studies, MELD score was not a significant predictor of PHLF[10,31]. To the best of our knowledge, MELD score is frequently used in patients with advanced cirrhosis[10] who are often not eligible for hepatic resection because of poor liver function reserve. It seems that MELD score exhibits low value in predicting postoperative liver function in HCC patients with no chronic liver diseases or cirrhosis[32]. In China, most HCC patients have a background of HBV infection with liver function impairment[33]. It was found in our study that 92.4% of the patients had HBV infection. This might be one of the reasons that we obtained a relatively high rate of PHLF (8.4%). In addition, the AUC of the MELD score for prediction of PHLF was 0.715 (P < 0.01), showing a relatively good predictive performance. This finding may indicate that MELD score has good predictive value in HCC patients with chronic liver diseases.
In addition, we also found that platelet count, albumin level, and sFLR volume were independent risk factors for PHLF. It is interesting that in our previous study, albumin level, bilirubin level, and sFLR volume were also significant predictors of PHLF, and their combination was more effective in predicting PHLF after hepatectomy[2]. Given this finding and to improve the predictive value, we combined MELD score with sFLR volume to develop a new model. ROC curve analysis showed that sFLR/MELD score had a larger AUC in predicting PHLF than sFLR volume or MELD score alone. This result demonstrated that the combination of MELD score and sFLR volume could gain a better predictive performance for PHLF. Patients with an sFLR/MELD score ≥ 0.078 had a significantly higher incidence of PHLF than those with an sFLR/MELD score < 0.078 (13.01% vs 1.09%). Therefore, patients with an sFLR/MELD score ≥ 0.078 are at a high risk of developing PHLF, and prompt clinical intervention is needed for these patients in order to reduce postoperative complications and PHLF.
The current research still has some limitations. First, all patients in the study came from a single research center, and selective bias may be exhibited. In addition, the number of patients in this study was low, so there is a need to recruit more patients from more research centers in future studies. Furthermore, as some patients did not undergo CT scanning at our hospital, three-dimensional CT reconstruction was not available for those patients. Therefore, the patients were not included. Finally, because of the short follow-up time, it was impossible to analyze the relationship between the sFLR/MELD score and patient survival, which is needed in the future.
In conclusion, sFLR volume combined with MELD score is a reliable and effective predictor to predict PHLF after hepatectomy. This measurement can effectively guide the early management after hepatectomy, improve the prognosis, and reduce the mortality. This tool also provides a new strategy for preoperative evaluation of hepatectomy.
Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer. HCC predominantly develops in patients with liver cirrhosis. At present, hepatectomy is still the main treatment for HCC. However, post-hepatectomy liver failure (PHLF) is one of the most serious complications following hepatic resection, despite improvements in surgical and post-operative management. Thus, it is of great clinical significance to evaluate the risk of PHLF before operation to reduce its incidence after hepatectomy.
At present, the models of predicting the occurrence of PHLF after hepatectomy do not meet the clinical needs. We need to have new forecasting indicators to further improve the models for predicting the occurrence of PHLF. The purpose of our study was to evaluate the value of model for end-stage liver disease (MELD) score combined with standardized future liver remnant (sFLR) volume in predicting PHLF in patients undergoing hepatectomy for liver cancer.
To study the value of MELD score combined with sFLR volume in predicting PHLF in patients undergoing hepatectomy for HCC, and explore the application of sFLR/MELD score in the hepatectomy and treatment of HCC, so as to provide reference for clinical treatment of this malignancy.
A total of 238 patients with HCC treated at our hospital from January 2015 to January 2018 were selected as a study group. Discrimination of sFLR volume, MELD score, and sFLR/MELD ratio to predict PHLF was evaluated according to the univariable and multivariable analyses, χ2 test, and receiver operating characteristic curve analysis.
The incidence of PHLF increased with the decrease of sFLR volume and the increase of MELD score. Moreover, both sFLR volume and MELD score were independent risk factors for PHLF. The cut-off value of the sFLR/MELD score to predict PHLF was 0.078, with an AUC of 0.845, which was superior to MELD score or sFLR volume alone.
sFLR volume combined with MELD score can effectively guide early treatment after hepatectomy, so as to improve prognosis and reduce mortality. The model also provides a new strategy for preoperative evaluation of hepatectomy.
Future studies are needed to further confirm the relationship between sFLR/MELD score and patient survival rate so that it can be better used in clinical practice. What’s more, to further consummate the follow-up time of patients and improve the accuracy of sFLR/MELD score is the next step for further analysis.
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 2. | Zou H, Wen Y, Yuan K, Miao XY, Xiong L, Liu KJ. Combining albumin-bilirubin score with future liver remnant predicts post-hepatectomy liver failure in HBV-associated HCC patients. Liver Int. 2018;38:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4366] [Article Influence: 545.8] [Reference Citation Analysis (6)] |
| 4. | Cauchy F, Soubrane O, Belghiti J. Liver resection for HCC: patient's selection and controversial scenarios. Best Pract Res Clin Gastroenterol. 2014;28:881-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Fonseca AL, Cha CH. Hepatocellular carcinoma: a comprehensive overview of surgical therapy. J Surg Oncol. 2014;110:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CH, Garden OJ, Farges O, Kokudo N, Vauthey JN, Clavien PA, Demartines N. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations. World J Surg. 2016;40:2425-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 426] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 8. | Hernaez R, Solà E, Moreau R, Ginès P. Acute-on-chronic liver failure: an update. Gut. 2017;66:541-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 456] [Article Influence: 50.7] [Reference Citation Analysis (1)] |
| 9. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2106] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 10. | Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, Wolfe RA, Krom R; United Network for Organ Sharing Liver Disease Severity Score Committee. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 1896] [Article Influence: 82.4] [Reference Citation Analysis (1)] |
| 11. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3778] [Article Influence: 151.1] [Reference Citation Analysis (2)] |
| 12. | Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for end-stage liver disease (MELD). Hepatology. 2007;45:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1255] [Article Influence: 66.1] [Reference Citation Analysis (0)] |
| 13. | Delis SG, Bakoyiannis A, Biliatis I, Athanassiou K, Tassopoulos N, Dervenis C. Model for end-stage liver disease (MELD) score, as a prognostic factor for post-operative morbidity and mortality in cirrhotic patients, undergoing hepatectomy for hepatocellular carcinoma. HPB (Oxford). 2009;11:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, Zanello M, Grazi GL, Pinna AD. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 200] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Hsu KY, Chau GY, Lui WY, Tsay SH, King KL, Wu CW. Predicting morbidity and mortality after hepatic resection in patients with hepatocellular carcinoma: the role of Model for End-Stage Liver Disease score. World J Surg. 2009;33:2412-2419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, Mahvash A, Gupta S, Wallace MJ, Vauthey JN. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 17. | Zou H, Tao Y, Wang ZM. Integration of Child-Pugh score with future liver remnant yields improved prediction of liver dysfunction risk for HBV-related hepatocellular carcinoma following hepatic resection. Oncol Lett. 2017;13:3631-3637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Lim MC, Tan CH, Cai J, Zheng J, Kow AW. CT volumetry of the liver: where does it stand in clinical practice? Clin Radiol. 2014;69:887-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Wigmore SJ, Redhead DN, Yan XJ, Casey J, Madhavan K, Dejong CH, Currie EJ, Garden OJ. Virtual hepatic resection using three-dimensional reconstruction of helical computed tomography angioportograms. Ann Surg. 2001;233:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Cieslak KP, Runge JH, Heger M, Stoker J, Bennink RJ, van Gulik TM. New perspectives in the assessment of future remnant liver. Dig Surg. 2014;31:255-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 611] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The "50-50 criteria" on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828-discussion 829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 839] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 23. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (1)] |
| 24. | Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Kauffmann R, Fong Y. Post-hepatectomy liver failure. Hepatobiliary Surg Nutr. 2014;3:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 67] [Reference Citation Analysis (0)] |
| 26. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 374] [Reference Citation Analysis (0)] |
| 27. | van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 313] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 28. | Schreckenbach T, Liese J, Bechstein WO, Moench C. Posthepatectomy liver failure. Dig Surg. 2012;29:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 29. | Ray S, Mehta NN, Golhar A, Nundy S. Post hepatectomy liver failure - A comprehensive review of current concepts and controversies. Ann Med Surg (Lond). 2018;34:4-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Asrani SK, Kamath PS. Model for end-stage liver disease score and MELD exceptions: 15 years later. Hepatol Int. 2015;9:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Mai RY, Ye JZ, Long ZR, Shi XM, Bai T, Chen J, Li LQ, Wu GB, Wu FX. Preoperative aspartate aminotransferase-to-platelet-ratio index as a predictor of posthepatectomy liver failure for resectable hepatocellular carcinoma. Cancer Manag Res. 2019;11:1401-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Shirata C, Kokudo T, Arita J, Akamatsu N, Kaneko J, Sakamoto Y, Kokudo N, Hasegawa K. Albumin-Indocyanine Green Evaluation (ALICE) grade combined with portal hypertension to predict post-hepatectomy liver failure. Hepatol Res. 2019;49:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol. 2019;71:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Liu JH