Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3145
Peer-review started: March 15, 2019
First decision: July 30, 2019
Revised: September 3, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 6, 2019
Processing time: 199 Days and 10.3 Hours
Hypereosinophilia (HE) is a heterogeneous disease of unknown etiology in which tissue and organ injury is inflicted by excess numbers of circulating or infiltrating eosinophils. Herein, we describe a patient with rare organ damage due to HE and review the pertinent literature.
A 43 year-old Chinese man with a 13-year history of eosinophilia and shortness of breath for 7 d presented to our hospital. During the course of his illness, the patient variably presented with gastrointestinal symptoms, eczema, vitiligo, mastitis, joint symptoms, nephrotic syndrome, and interstitial pneumonia. The chronic mastitis proved burdensome, necessitating bilateral mastectomy. HE was diagnosed by repeat bone marrow biopsy, and a kidney biopsy showed focal segmental glomerulosclerosis. Intermittent steroidal therapy is typically initiated to relieve such symptoms, although relapse and organ involvement often ensue once treatment is withdrawn. We administered methylprednisolone sodium succinate (40 mg/d) intravenously for 3 d, followed by oral tablets at the same dose. Subsequent computed tomography (CT) of the chest CT showed relative improvement of the interstitial pneumonia. The patient is currently on a continuous regimen of oral steroid, and his condition is stable.
HE is heterogeneous condition. This is the first reported case of bilateral mastectomy in a male patient with longstanding HE.
Core tip: Hypereosinophilia (HE) is a heterogeneous disease of unknown etiology in which excessive circulating and infiltrating eosinophils cause injury to bodily tissues and organs. We have treated a male patient with HE and unusual organ involvement, namely, mastitis, nephrotic syndrome, and interstitial pneumonia. Intermittent glucocorticoid therapy may relieve symptoms, but relapses and renewed organ damage are common after withdrawal. This particular patient is currently stable on a continuous oral steroid regimen. This is the first reported case of bilateral mastectomy in a male patient with HE.
- Citation: Wu J, Li P, Chen Y, Yang XH, Lei MY, Zhao L. Hypereosinophilia, mastectomy, and nephrotic syndrome in a male patient: A case report. World J Clin Cases 2019; 7(19): 3145-3152
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3145.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3145
Hypereosinophilia (HE) is a disease defined by one or more of the following: (1) Peripheral blood eosinophil counts > 1.5 × 109/L on two occasions at intervals > 1 mo; (2) A ratio of eosinophils to nucleated cells in bone marrow > 20%; (3) Pathologic confirmation of extensive tissue infiltration by eosinophils; and (4) Conditions marked by substantial deposition of eosinophil granuloproteins[1]. Multiorgan involvement and resultant dysfunction are common, given the intensity of eosinophilic infiltration and/or extensive proteinaceous deposits. There may be fibrosis of the lungs, heart, digestive tract, skin, or other organs; thrombosis with or without embolism; cutaneous or mucosal changes [erythema, edema (perhaps vascular in nature), ulceration, pruritus, or eczema]; and peripheral or central nervous system derangements, with or without chronic or recurrent neurologic dysfunction[1]. Herein, we report a male patient with multiorgan involvement, eosinophilic mastitis, and nephrotic syndrome due to longstanding HE.
A 43-year-old Chinese man, who complained of shortness of breath on exertion for 7 d and a 13-year history of peripheral blood eosinophilia, was admitted to our hospital.
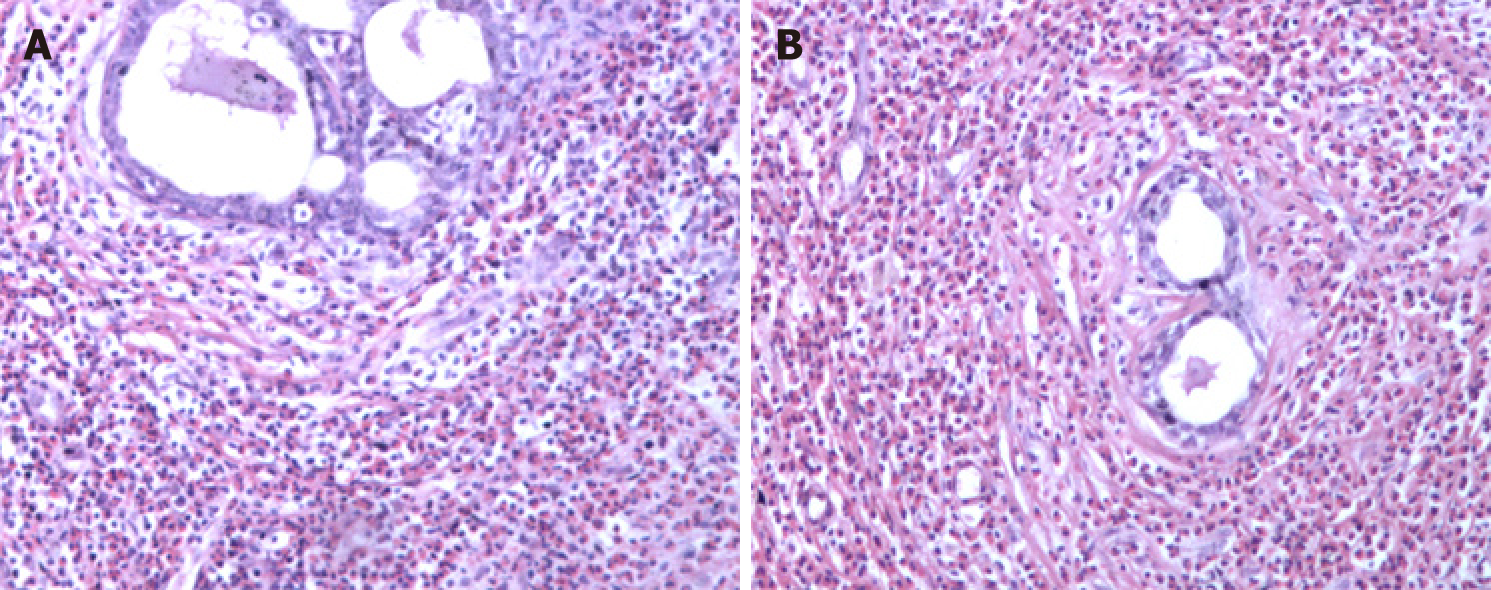
The patient was treated at a local hospital in August 2005 because routine blood examination showed a white blood cell count of 8.5 × 109/L, with 24.2% of eosinophils (2 × 109/L). In a bone marrow biopsy, eosinophils accounted for 13.5%, most with normally segmented rod nuclei. Once oral triamcinolone was initiated (4 mg, tid), the eosinophil counts rapidly normalized. After gradual dosage reduction in a span of 6 mo and eventual discontinuation, hyperplastic and nodular changes developed in both breasts, accompanied by abnormal lactation and bilateral axillary node enlargement. This condition was sufficiently burdensome to require surgery. Bilateral mastectomy was subsequently performed (January 2006) for chronic mastitis. Postoperative pathology examination disclosed male breast development and severe chronic inflammation (i.e., dilatation of mammary ducts with interstitial influx of eosinophils) (Figure 1).
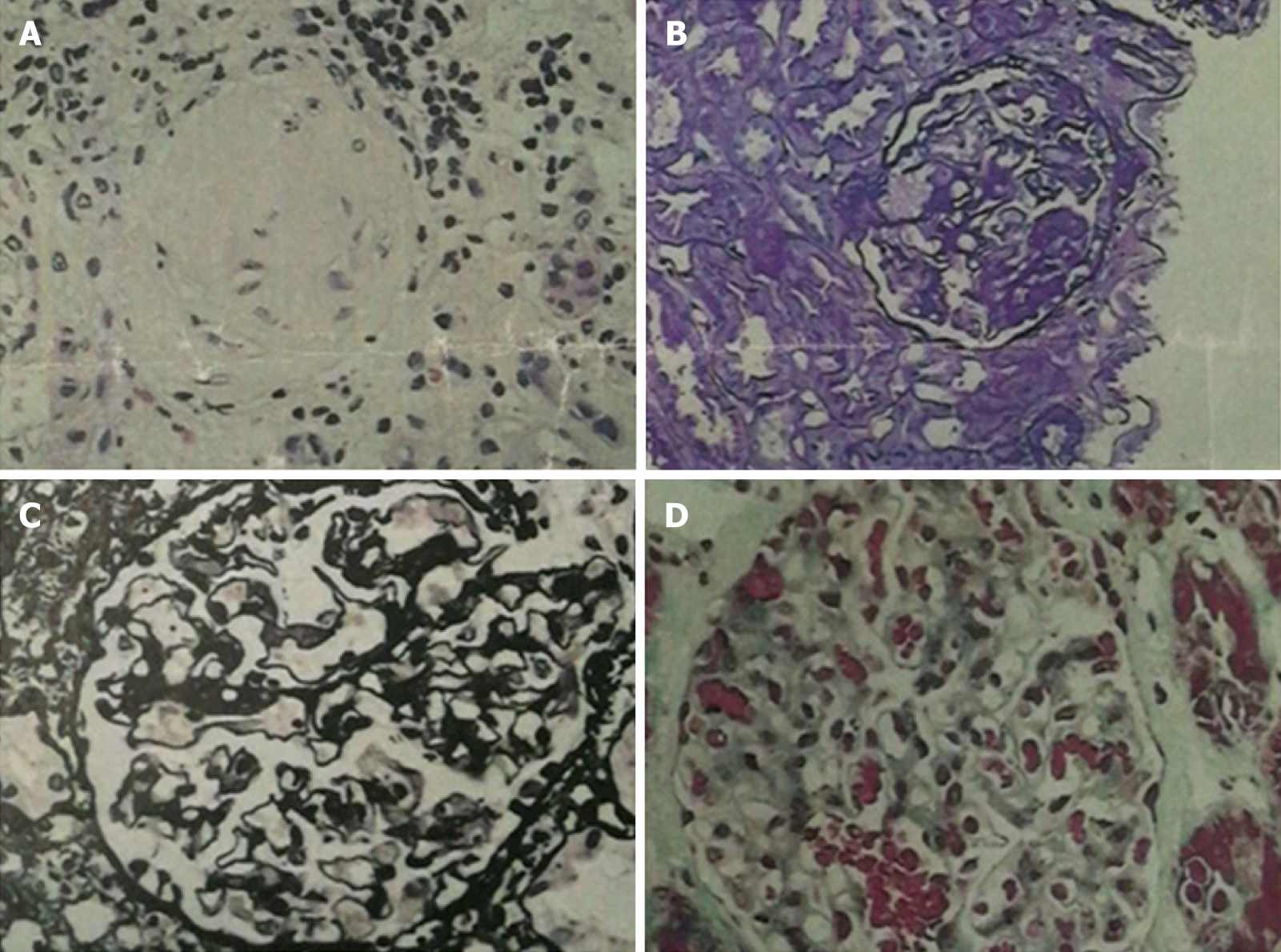
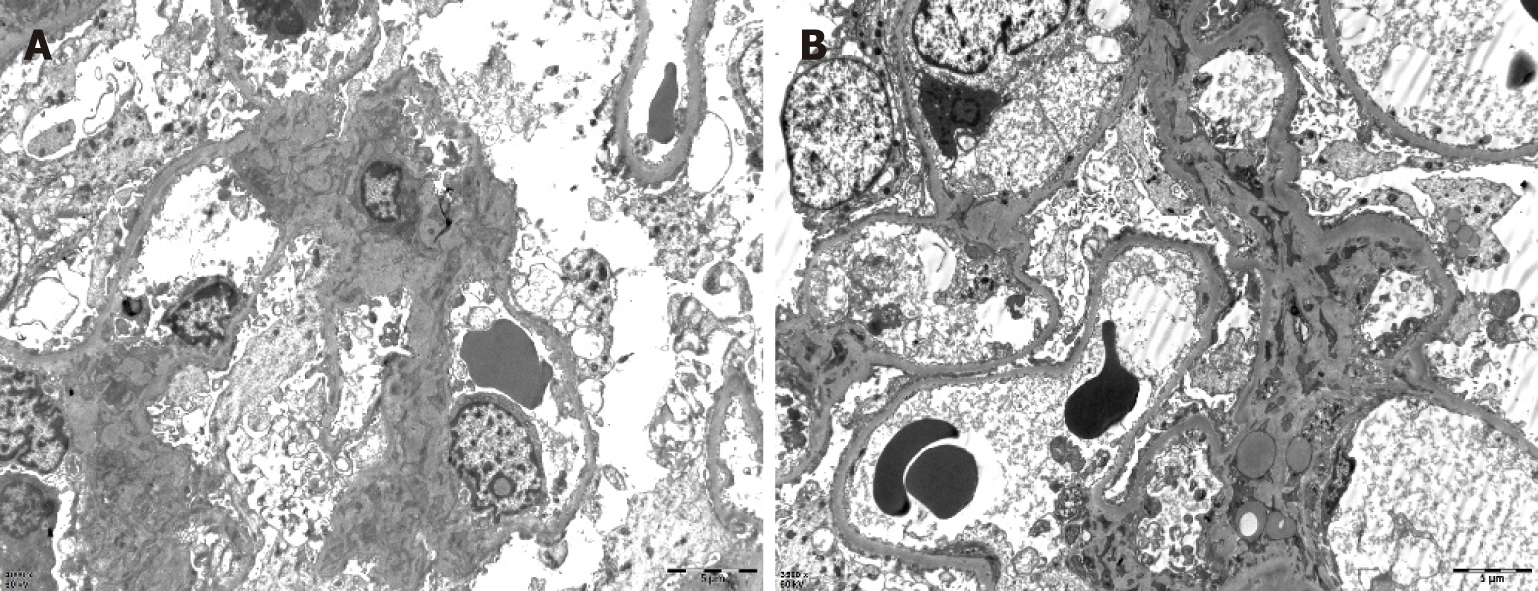
One month later, the patient presented with joint pain of the trunk and limbs, movement dysfunction, and an exacerbated skin rash. Dexamethasone and prednisone were prescribed. An herbal extract (Tripterygium wilfordii) was also used shortly and then abandoned due to severe treatment-related alopecia. Thereafter, the joint symptoms gradually relented, allowing the patient to resume work. In April 2017, however, bilateral lower extremity edema developed spontaneously (blood pressure, 150/100 mmHg; plasma albumin, 15.6 g/L; low-density lipoproteins, 6.87 mmol/L; and 24-h urinary protein, 14.78 g/d), prompting a clinical diagnosis of nephrotic syndrome. A kidney biopsy was performed, establishing a pathologic diagnosis of focal segmental glomerulosclerosis, non-specific type (Figures 2 and 3). A second bone marrow puncture again was indicative of eosinophilia, but qualitative polymerase chain reaction failed to detect the FIP1L1-PDGFRA fusion gene. The patient was given prednisone orally at an initial dose of 60 mg (12 tablets) per day. This was gradually reduced and discontinued in March 2018, at which time all parameters of nephrotic syndrome were stable.
One month after medication withdrawal, hospital readmission was brought on by shortness of breath (April 2018).
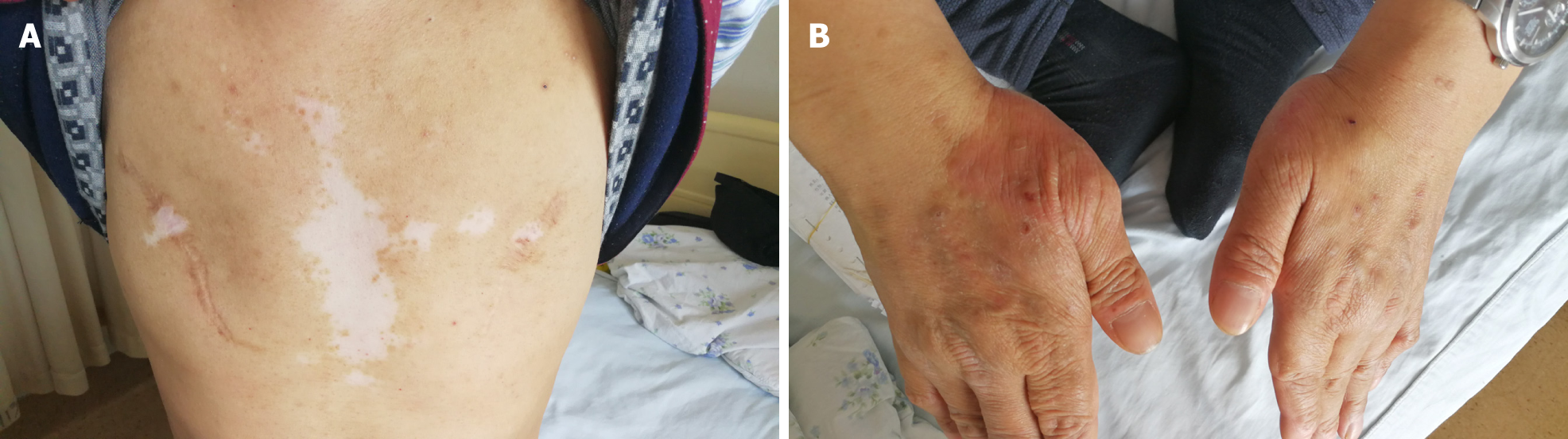
He initially presented in December 2004 with constipation, abdominal distension, and persistent vomiting. Gastroscopic inspection revealed superficial gastritis, duodenal bulbar inflammation, and gastric retention. Enteroscopy was then performed, showing rectosigmoid mucositis. Interstitial inflammatory infiltrates were prominent in gastric and colonic mucosal biopsies. After symptomatic treatment, the digestive symptoms gradually abated and disappeared. Several months later, the patient developed an erythematous papular skin rash and vitiligo. The rash coalesced in places but was largely confined to the dorsa of hands and limbs in a symmetric distribution, without itching. Vitiligo chiefly affected the face and trunk.
Historically, the patient’s birth was premature (8 mo of gestation). There was one episode of pertussis during childhood and many instances of pneumonia. The patient’s father had succumbed to gastric cancer at the age of 50.
Physical examination revealed alopecia, loss of facial and trunk pigmentation, and an erythematous rash on the dorsa of both hands and limbs (Figure 4). No dry or wet rales were detected in either lung.
Pertinent laboratory results were as follows: Blood eosinophil count, 0.41 × 109/L; total IgE, 1491 IU/ml; PaO2, 53.6 mmHg; and SaO2, 91.2%. In bronchoscopic alveolar lavage fluid, eosinophils represented 30.0% of cells (CD4+/CD8+ = 0.15).
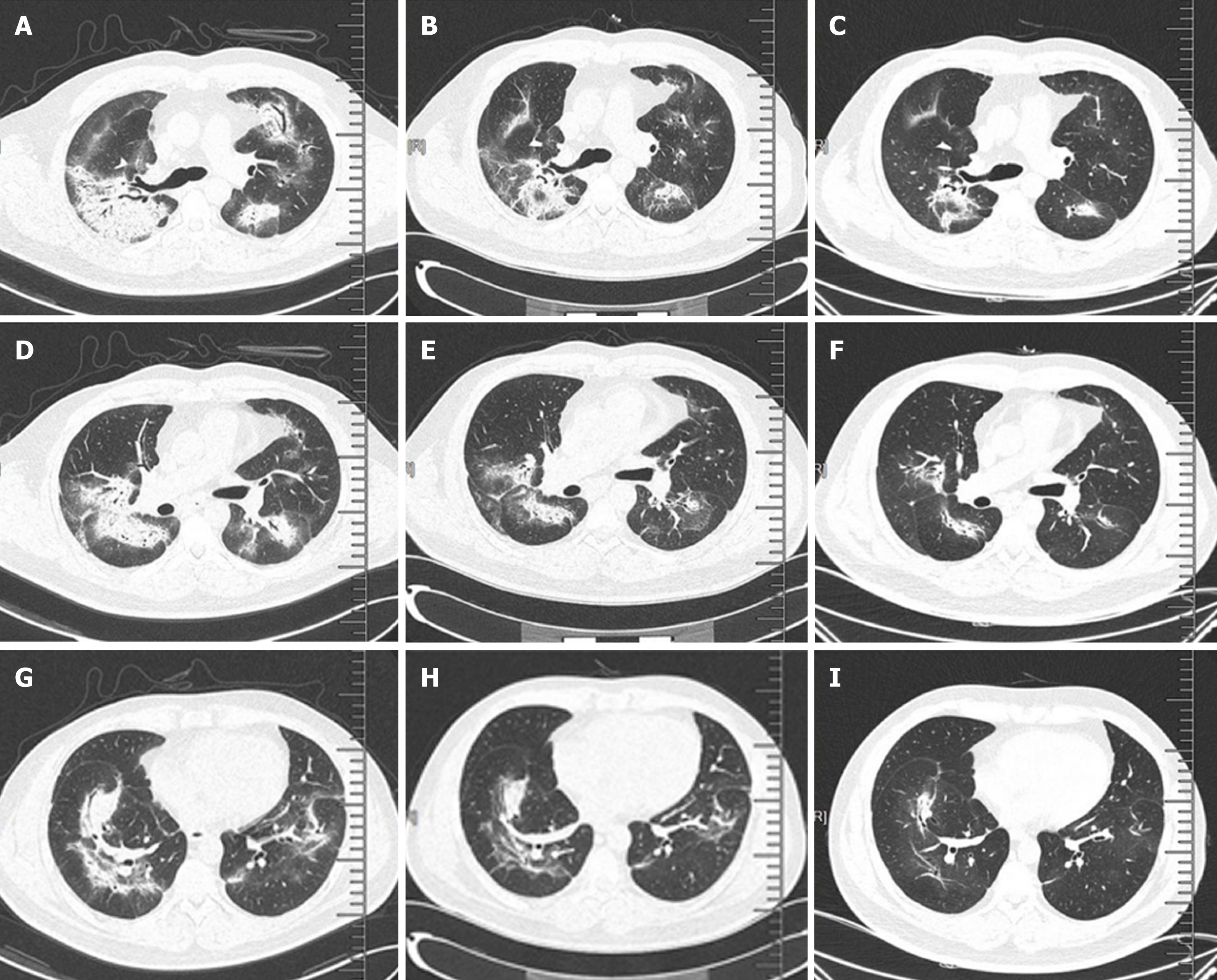
Chest computed tomography (CT) showed interstitial pneumonia (Figure 5A).
Our final diagnosis was HE with pulmonary involvement.
Methylprednisolone sodium succinate (40 mg/d) was administered intravenously for 3 d, followed by the oral formulation at the same dose.
After one week of steroid treatment, repeat chest CT suggested some resolution of the interstitial pneumonia (Figure 5B). Four months after resuming steroid therapy, a number of scattered high-density patches in both lungs were further reduced in CT images (Figure 5C). The patient is currently continuing an oral steroid regimen.
Multiorgan organ involvement is a precarious aspect of HE. When analyzing the clinical data of 188 patients with HE, Ogbogu et al[2] documented cutaneous manifestations in 37% of cases, respiratory symptoms in 25%, and gastrointestinal complaints in 14%. Heart involvement in early-stage disease was evident in < 5%, and there were no clinical symptoms attributable to eosinophilia in 6%. Over the course of > 10 years, this patient has been plagued by gastrointestinal, skin, breast, joint, kidney, lung, and other organ involvement. Some of the developments, such as gastroente-ritis, mastitis, and arthritis, were transient, whereas others (i.e., eczema and vitiligo) had persisted. Although HE may incite mastitis in male patients, bilateral mastectomy has not been reported in the literature to date, and HE with nephrotic syndrome is exceedingly rare.
In past reports of HE, we have discovered only one case of breast involvement[3]. The patient was a 51-year-old woman who complained of bilateral mammary erythema, pain, and swelling for 1 wk. Histologically, tissue from both breasts displayed ductal eosinophilic infiltration, moderate to severe ductal dilatation, ductal epithelial necrosis, and substantial infiltration of surrounding matrix by eosinophils. However, the patient’s prognosis after steroid therapy was good. These symptoms and pathologic features resemble those of our patient, confirming that the breast is one of the rare target organs of HE. Because our patient was male, gender proclivity seems unlikely, but no prior reports of breast involvement in men with HE has surfaced. Bilateral mastectomy was performed in this particular patient due to the morbidity imposed by symmetric enlargement, nodularity, abnormal lactation, swelling, pain, and enlarged axillary nodes. Had steroid therapy been otherwise considered, surgery may have been avoidable.
Although kidney involvement is rare in the setting of HE, it may well be life-threatening. There are two main causes: (1) Direct kidney damage by eosinophilic granules; and (2) Iatrogenic insults during the treatment of HE (i.e., cholesterol embolism, adverse drug reactions, immunologic reactions, eosinophilic helminth infection, and ongoing hemodialysis)[4]. In relevant clinical case reports with glomerulonephritis as the chief clinical manifestation[5-8], only one instance of nephrotic syndrome is reported in a 7-year-old boy[9]. The pathologic spectrum of renal damage is rather broad in this regard, including capillary proliferative glomerulonephritis[5], eosinophilic interstitial nephritis[6], crescent glomerulone-phritis[7], and membranous nephropathy[8]. It is likely that immune reactivity leads to deposition of complexes within glomeruli. The release of damaging cytokines and other mediators, especially leukotrienes[4], by multinucleated eosinophils may be implicated as well. A non-specific type of focal segmental glomerulosclerosis was identified in our patient. Unfortunately, pathologic classification of renal injury due to HE was specified in one case report only[10] and in the absence of nephrotic syndrome. The authors refer to “focal segmental sclerotic-like” in their description. The patient in question showed various manifestations of nephrotic syndrome (hypertension, massive proteinuria, hyperlipidemia, and hypoproteinemia). Thus, although the pathologic underpinnings may be shared, there is an inherent heterogeneity that remains unexplained.
Our patient also had vitiligo, which was self-limited and stable in recent years. In our review of the literature, we found no correlation between HE and vitiligo other than two patients with eosinophilic fasciitis (Shulman syndrome) and vitiligo[11,12]. This discovery hints at a suspected link for which further research is needed.
Pulmonary involvement in HE is common, typically marked by symmetric ground-glass opacities of both lungs and consolidation. At the same time, the blood eosinophil count may be within the normal range[13], which is inconsistent with the actual clinical picture.
Glucocorticoids are primarily indicated as a treatment for HE, using hydroxyurea, interferon-α, imatinib, or mepolizumab as alternative agents. The prognosis of patients with HE is generally good[14].
HE is a heterogeneous disease of unknown etiology. Herein is the first reported case of bilateral mastectomy for mastitis in a male patient with HE and nephrotic syndrome. In retrospect, surgery may have been avoided by using a continuous steroidal regimen instead.
| 1. | Leukemia and Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. Chinese expert consensus on the diagnosis and treatment of eosinophilia (2017). Zhonghua Xue Ye Xue Za Zhi. 2017;38:561-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 2. | Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE, Leiferman KM, Nutman TB, Pfab F, Ring J, Rothenberg ME, Roufosse F, Sajous MH, Sheikh J, Simon D, Simon HU, Stein ML, Wardlaw A, Weller PF, Klion AD. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol. 2009;124:1319-25.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 3. | Thompson AB, Barron MM, Lapp NL. The hypereosinophilic syndrome presenting with eosinophilic mastitis. Arch Intern Med. 1985;145:564-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 4. | Giudicelli CP, Didelot F, Duvic C, Desrame J, Herody M, Nedelec G. Eosinophilia and renal pathology. Med Trop (Mars). 1998;58:477-481. [PubMed] |
| 5. | Walker A, Ellis J, Irama M, Senkungu J, Nansera D, Axton J, Coward RJ, Peat DS, Bode HH, Mathieson PW. Eosinophilic glomerulonephritis in children in Southwestern Uganda. Kidney Int. 2007;71:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Bulucu F, Can C, Inal V, Baykal Y, Erikçi S. Renal involvement in a patient with idiopathic hypereosinophilic syndrome. Clin Nephrol. 2002;57:171-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Richardson P, Dickinson G, Nash S, Hoffman L, Steingart R, Germain M. Crescentic glomerulonephritis and eosinophilic interstitial infiltrates in a patient with hypereosinophilic syndrome. Postgrad Med J. 1995;71:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Frigui M, Hmida MB, Jallouli M, Kechaou M, Frikha F, Bahloul Z. Membranous glomerulopathy associated with idiopathic hypereosinophilic syndrome. Saudi J Kidney Dis Transpl. 2010;21:320-322. [PubMed] |
| 9. | Zhong YL, Dang XQ. Hypereosinophilic syndrome involving nephrotic syndrome: a case report. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:850-851. [PubMed] |
| 10. | Grcevska L, Polenaković M, Breskovska G. Focal segmental glomerular-sclerosis-like lesions associated with eosinophilic pneumonia and trimethoprim and penicillin treatment. Nephron. 1993;64:325-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Lakjiri S, Meziane M, Benani A, Harmouch T, Amarti A, Mernissi FZ. Eosinophilic fasciitis, morphea and vitiligo in a single patient. Ann Dermatol Venereol. 2014;141:598-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Stork J, Nĕmcová D, Hoza J, Kodetová D. Eosinophilic fasciitis in an adolescent girl with lymphadenopathy and vitiligo-like and linear scleroderma-like changes. A case report. Clin Exp Rheumatol. 1996;14:337-341. [PubMed] |
| 13. | Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010;126:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Dulohery MM, Patel RR, Schneider F, Ryu JH. Lung involvement in hypereosinophilic syndromes. Respir Med. 2011;105:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yorioka N S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Xing YX