Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3138
Peer-review started: May 20, 2019
First decision: July 21, 2019
Revised: August 17, 2019
Accepted: August 27, 2019
Article in press: August 27, 2019
Published online: October 6, 2019
Processing time: 135 Days and 4.8 Hours
Submucosal tumor (SMT)-like early-stage gastric cancer (GC) has rarely been reported. It is difficult to consider the possibility of GC and differentiate it from other submucosal lesions.
We present the case of a 50-year-old male patient with a 1.6 cm SMT-like flat elevated lesion covered by congested mucosa on the gastric angle. Magnifying endoscopy with narrow-band imaging, endoscopic biopsy, endoscopic ultrasound, and computed tomography were performed for diagnosis. Endoscopic submucosal dissection and gastrectomy with lymph node dissection were performed. The post-resection pathological analysis led to a final diagnosis of GC (Bormann type I, T1bN2M0).
GC should be considered when detecting an SMT-like lesion in the stomach.
Core tip: Gastric adenocarcinoma showing features of a submucosal tumor is unusual. Preoperative diagnosis is difficult due to the generally deep location of the tumor and non-specific and overlapping features on imaging studies. In this case, esophagogastroduodenoscopy revealed a 1.6 cm type 0-Is lesion covered by congested mucosa on the gastric angle. Endoscopic ultrasound and computed tomography reveal no swollen lymph nodes and the usual biopsy cannot give a definite diagnosis. After endoscopic submucosal dissection and gastrectomy with lymph node dissection, this patient was diagnosed with GC (Bormann type I, T1bN2M0).
- Citation: Cheng XL, Liu H. Gastric adenocarcinoma mimicking a submucosal tumor: A case report. World J Clin Cases 2019; 7(19): 3138-3144
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3138.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3138
Gastric cancer (GC) remains the second leading cause of cancer-related mortality worldwide and the most prevalent cancer in Eastern Asia (including China)[1] Approximately 90% of GCs are adenocarcinomas. However, an adenocarcinoma showing features of a submucosal tumor (SMT) is unusual, and its prevalence has been reported to be 0.2%-0.62%[2].
Here, we report a rare case of SMT-like GC, which is easily ignored and misdiagnosed.
A 50yearold Chinese male patient presented for evaluation and treatment of an indeterminate lesion on the gastric angle, which was discovered on esophagogastroduodenoscopy (EGD) during a routine health examination 15 d previously. He had no abdominal pain or other discomfort.
He had a history of hypertension for half a month and was regularly taking oral agents including nifedipine controlled-release tablets and valsartan dispersible tablets once a day. The blood pressure can be well controlled.
He had been smoking for 30 years.
On physical examination, he was 71 kg in weight and 178 cm in height. He had a blood pressure of 120/72 mmHg and pulse rate of 68 beats per minute. Other physical examination on admission revealed no abnormalities.
After admission, the patient underwent evaluations including routine blood tests, routine urine tests, routine fecal tests and occult blood test, blood biochemistry, and some serum tumor markers including carcinoembryonic antigen, carbohydrate antigen 199, alpha-fetoprotein, serum pro-gastrin-releasing peptide, neuron specific enolase, squamous cell carcinoma antigen, and carbohydrate antigen 724. No significant abnormal results were recorded.
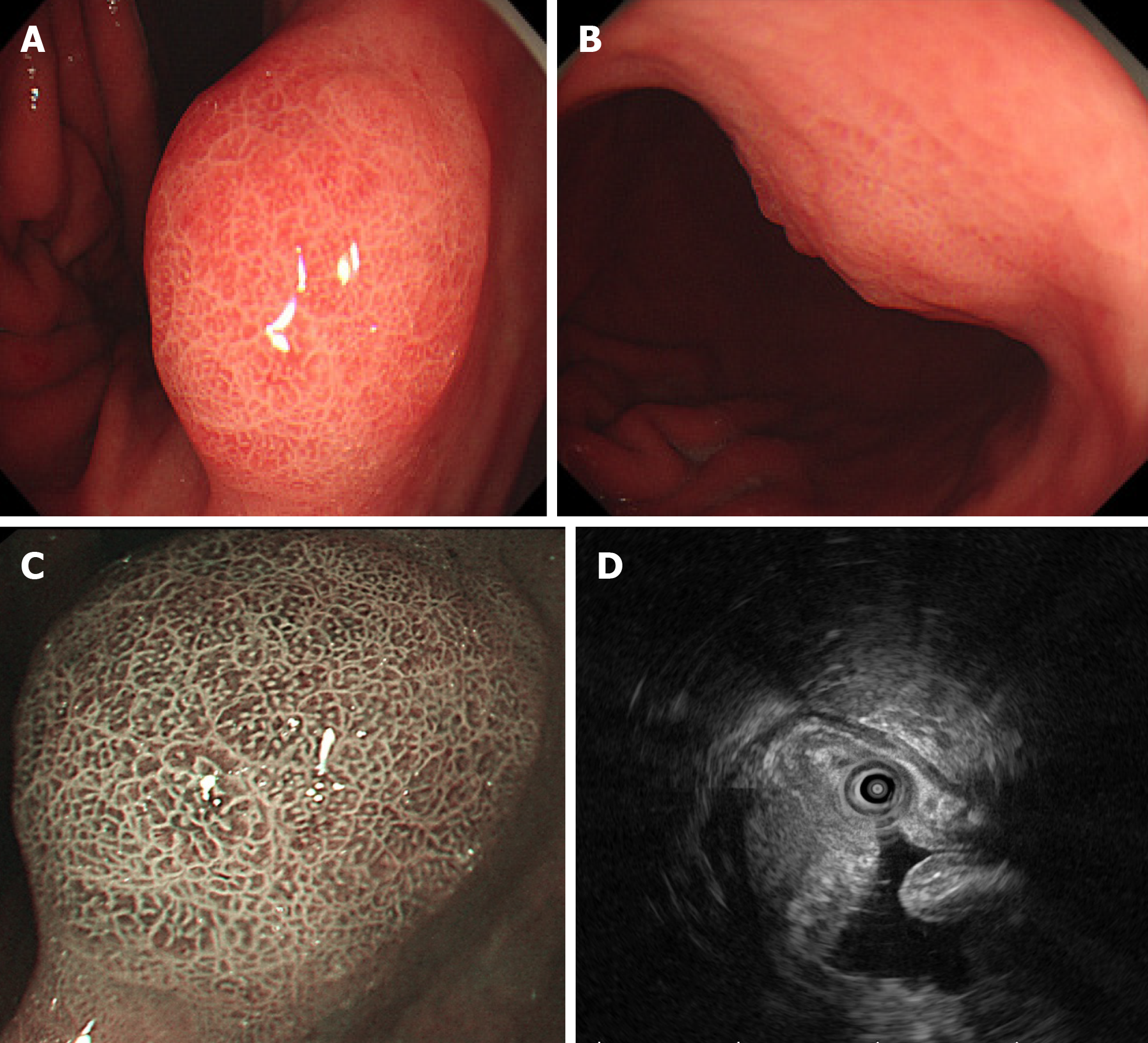
EGD revealed a 1.6 cm type 0-Is lesion covered by congested mucosa on the gastric angle (Figure 1A and B). Magnifying endoscopy with narrow-band imaging revealed a regular microvascular pattern, presence of a demarcation line, irregular and smaller crypt opening, and widened intervening part (Figure 1C).
Endoscopic ultrasound (EUS; GF-UM2000, Olympus, Tokyo, Japan) revealed a 15.3 mm × 9 mm hypoechoic mass within the submucosa, thickened mucosal and submucosal layers (Figure 1D), and no swollen lymph nodes.
Endoscopic biopsy specimens revealed moderately chronic super gastritis with little intestinal metaplasia.
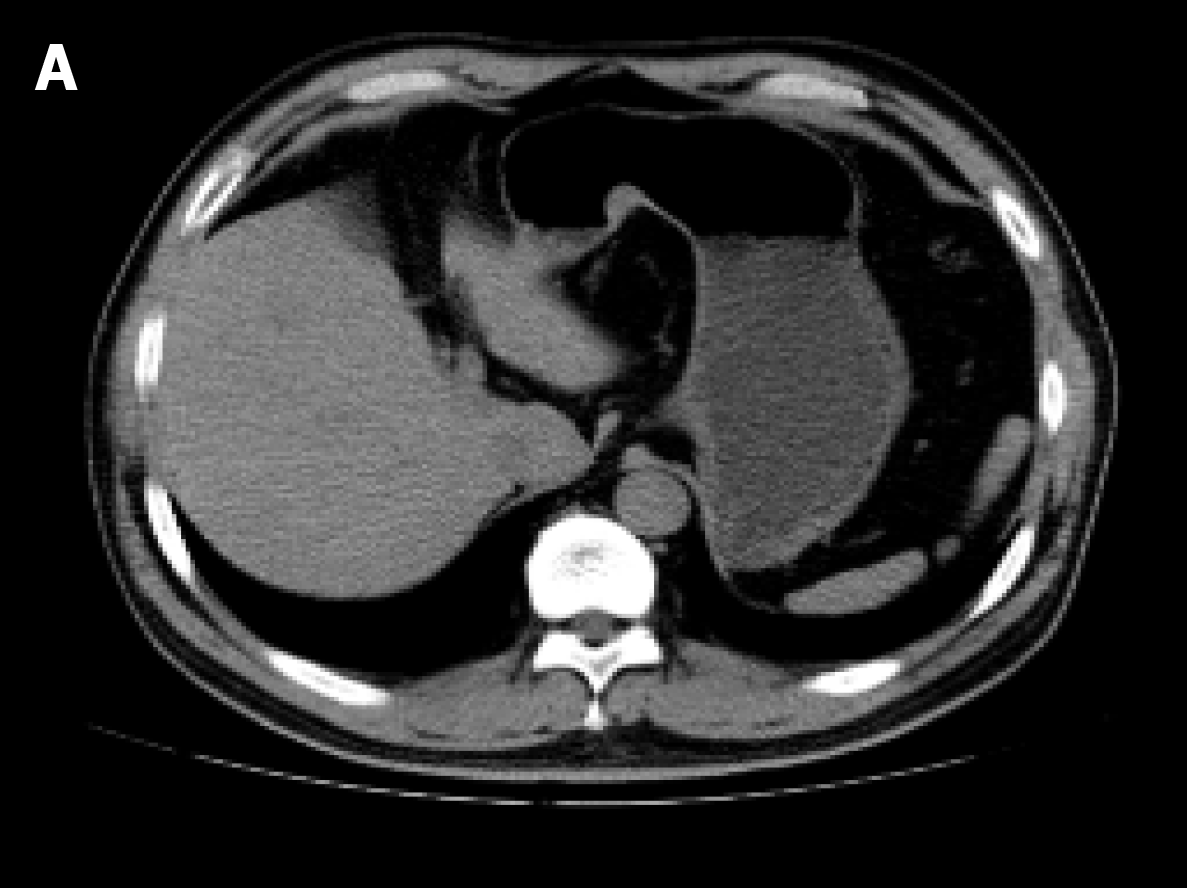
Computed tomography of the upper abdomen revealed a 15 mm × 12 mm nodule protruding on the lesser curvature of the stomach and no evidence of swollen lymph nodes or distant metastasis (Figure 2).
According to the imaging findings and the histopathologic examination, this patient was diagnosed with GC (Bormann type I, T1bN2M0).
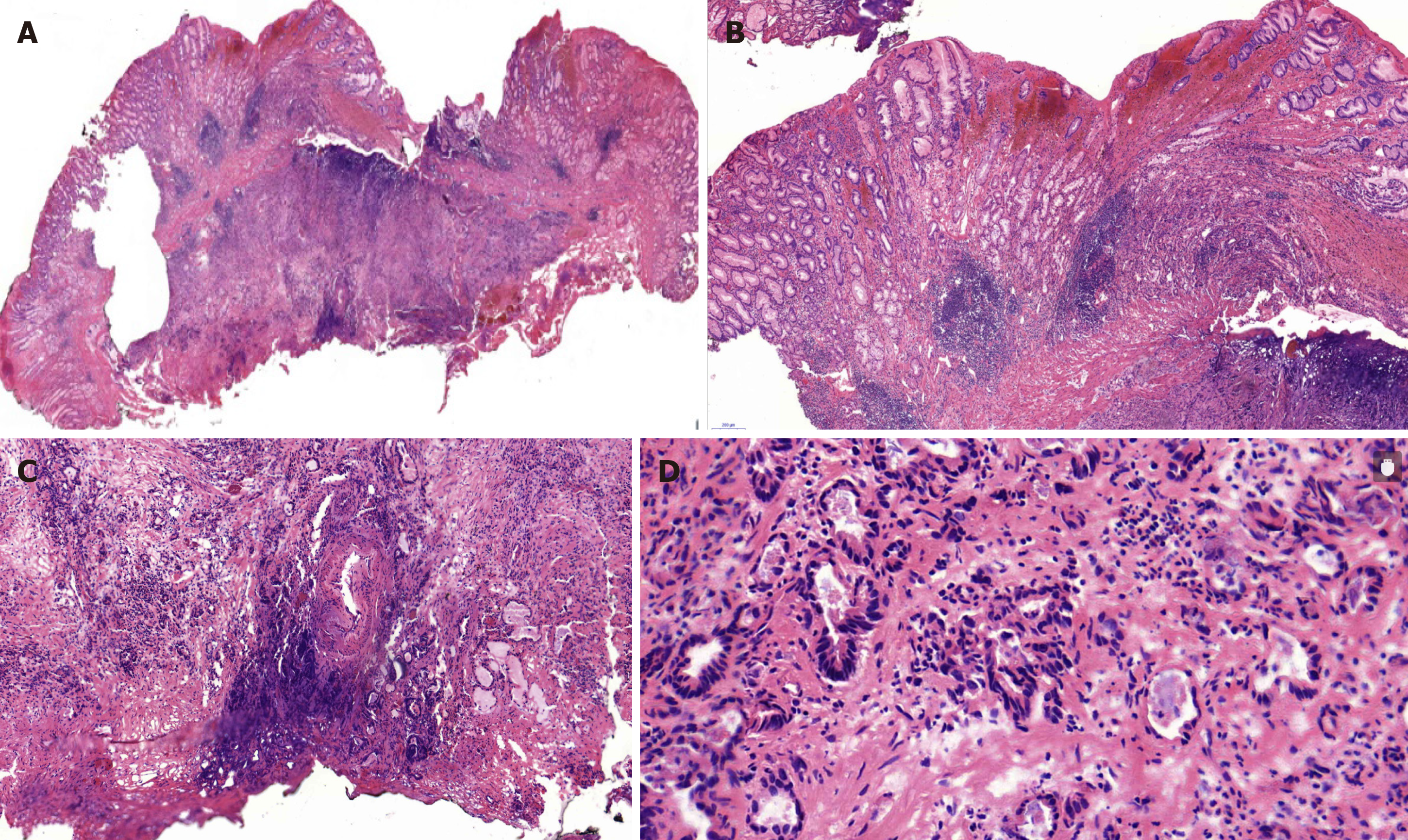
According to the examination results, an initial diagnosis of an SMT-like lesion was made and endoscopic submucosal dissection (ESD) was performed to confirm the diagnosis. Based on the pathology of the resected specimen, we suspected moderately to poorly differentiated adenocarcinoma (Figure 3). Consequently, immunostaining was performed. The lesion was positive for cytokeratin, partly positive for Villin, and weakly positive for CDX-2, but negative for Syn, CgA, CD56, CD3, and CD20. Ki-67 was positive in 30% cells. Accordingly, the final diagnosis was confirmed as poor-differentiated adenocarcinoma. Because the basal cutting edge was involved, subsequent gastrectomy with lymph node dissection was performed. The final histopathologic examination revealed positive metastasis in 5 of 25 lymph nodes of the lesser curvature and negative results for not only other lymph nodes including those in the hepatoduodenal ligament, the periphery of the hepatic artery, the left gastric arteria, and the large curvature but also, the resection margin and omentum tissue. Residual cancer was not observed on parallel mucosal biopsy examination.
After surgery, he was cured. Six months later, the follow-up upper gastrointestinal endoscopy and the biopsies showed no abnormalities.
GC usually derives from the lamina propria layer, accounting for 8% of all cancers[3] and adenocarcinomas account for 95% of all GCs[4]. But adenocarcinoma showing features of an SMT is unusual with a prevalence of 0.2%-0.62%[2]. Preoperative diagnosis of SMT-like GC is difficult due to the generally deep location of the tumor and the non-specific and overlapping features on imaging studies.
When an SMT-like lesion is encountered in clinical practice, we should consider the possibility of GC and carefully differentiate it from other submucosal lesions, such as gastric neuroendocrine tumors (GI-NETs)[5], smooth muscle tumor, stromal tumor, and lipoma; heterotopic pancreas; and other unusual cases, such as metastatic carcinoma[6], gastric glomus tumor[7],and gastric hamartomatous inverted polyp[8]. According to available reported cases of GC presenting as SMT, the pathologic diagnosis varies including gastric adenocarcinoma[9], gastric mucinous adenocarcinoma[10,11], and gastric lymphoepithelioma-like carcinoma[12].
The mechanism of SMT-like adenocarcinoma is obscure. Heterotopic gastric glands (HGGs) are gastric glands observed in the submucosa, which have been recognized as aberrant lamina propria components associated with repeated erosion and regeneration, which is inferred to develop to a wide variety of adenocarcinomas[9]. Gastritis cystica profunda (GCP), also related to repeated erosion and regeneration induced by chronic inflammation, causes an aberration of the gastric glands and may be related to carcinogenesis as well[13]. In this case, we did not find any submicosal heterotopic gastric gland or gastritis cystic profunda. Moreover, Epstein-Barr virus-positive GC is associated with Epstein-Barr virus infection and severe lymphocytic infiltration in the submucosa[14]; mucinous gastric carcinoma is due to abundant mucin pools in the submucosa[10,11], and they all show the features of SMT-like cancer. Gastric adenocarcinoma of fundic gland type (GA-FG) has been recognized as a tumor typically arising from the normal gastric mucosa of the fundic gland region without atrophic changes or intestinal metaplasia[9,11]. There is no conclusion if SMT-like GC is the earlier stage of Bormann type IV GC or of linitis plastica.
Upper gastrointestinal endoscopy has become an important tool in the diagnosis of patients with GC. However, up to 6.7% of GCs may be missed when endoscopy shows no initial cancer findings[15], especially when early GC (EGC) mimicks an SMT. In this case, EGD revealed a type 0-Is lesion, covered by nearly normal mucosa on the gastric angle, which is distinguished from that of a past report which appeared as small circumscribed, sometimes ulcerated thickening of the gastric wall[16].
EGD was also performed to obtain a biopsy specimen of suspected gastric lesion. But in previous reports, the cancer cells exposed on the surface accounted for less than 20%-30% of the whole tumor in GC mimicking an SMT, and hence, a usual biopsy cannot confirm the diagnosis. In this case, a usual biopsy was performed twice and two or three pieces of tissue were taken at a time; however, a diagnosis was not confirmed. Multiple biopsies or larger forceps may improve the yield of confirmed diagnosis.
Performance of EUS is important in the initial clinical staging of GC. EGCs are identified on EUS as areas of focal thickening, irregularity, or disruption of layers[17]. However, the diagnostic accuracy of EUS is operator-dependent, ranging from 57%-88% for T staging and 30-90% for N staging[18]. For T1b cancers, the coincidence rate of EUS and pathological diagnosis is only 40%. Ulcers and undifferentiated cancer are two independent factors of EUS mis-staging[19]. In this case, EUS showed that the mucosal and submucosal layers were thickening and the hypoechoic mass was within the submucosal layer, which is consistent with the pathological result. Unfortunately, we did not perform EUS-guided fine needle aspiration (FNA), which is recommended if areas of wall thickening are seen[20], and we were unable to distinguish it from other SMT-like lesions or provide any indications on abnormal lymph nodes. Considering the aforementioned findings, EUS diagnostic performance cannot be considered optimal, either for disease confirmation or for exclusion, especially for the ability of EUS to distinguish T1a (mucosal) vs T1b (submucosal) cancers and positive vs negative lymph node status[21].
Lymph node metastasis is an independent risk factor for the survival of patients with EGC. In the past three reports, the rate of lymph node metastasis for submucosal EGC was 12.5%[22], 11%-20%[23], and 16.7%[24], respectively. One study reported poorly differentiated adenocarcinoma, with a lymph node metastasis rate of 17.0%[25]. In another study, T1b tumors with lymphovascular invasion had a 64.3% rate of positive LN[26]. The probability of lymph node metastasis in EGC is influenced by tumor characteristics and increases with increasing tumor size, submucosal invasion, poorly differentiated tumors, and lymphatic and vascular invasion[24,27]. In our case, LN metastasis in the lesser curvature (5/25) may be related with submucosal invasion and poor differentiation.
In this case, when the SMT-like lesion was found on EGD, we could not distinguish it from other SMTs and we failed to perform EUS-FNA. According to the new consensus guidelines, we directly performed the diagnostic ESD. In contrast to other methods, ESD may increase the economic burden. Subsequently, because of the positive edge, subsequent gastrectomy with lymph node dissection was performed and final histopathologic examination revealed that lymph node metastasis was positive for 5 of 25 lymph nodes in the lesser curvature. Long-term follow-up and monitoring were suggested.
From this case, we infer that some SMT-like lesions pose difficulties in distinguishing between benign tumors and cancers. Thus, during the diagnosis of a sub-mucosal tumor, if usual biopsies cannot confirm the diagnosis, multiple biopsies using larger forceps or even EUS-guided FNA or fine needle biopsy may improve the clinical diagnosis and staging. Sometimes, diagnostic ESD is the preferred method to offer further information to a pathologist such as the degree of differentiation and the depth of infiltration[28]. Moreover, ESD can potentially be used for therapeutic purposes[29]. Additional therapy by gastrectomy with lymphadenectomy should be taken into consideration for lesions that are poorly differentiated, invade into the deep submucosa, and have positive lateral or deep margins with lymph node metastases.
| 1. | Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1235] [Article Influence: 88.2] [Reference Citation Analysis (2)] |
| 2. | Umehara Y, Kimura T, Okubo T, Sano Y, Nakai K, Oi S, Higashi Y, Funai K. Gastric carcinoma resembling submucosal tumor. Gastric Cancer. 1999;2:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25605] [Article Influence: 1707.0] [Reference Citation Analysis (11)] |
| 4. | Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 552] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 5. | Gheorghe AV, Rimbas M, Ginghina O, Spanu A, Voiosu TA. An atypical type I gastric neuroendocrine tumor. Rom J Intern Med. 2017;55:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Inagaki C, Suzuki T, Kitagawa Y, Hara T, Yamaguchi T. A case report of prostate cancer metastasis to the stomach resembling undifferentiated-type early gastric cancer. BMC Gastroenterol. 2017;17:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Handa Y, Kano M, Kaneko M, Hirabayashi N. Gastric Glomus Tumor: A Rare Cause of Upper Gastrointestinal Bleeding. Case Rep Surg. 2015;2015:193684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Aoki M, Yoshida M, Saikawa Y, Otani Y, Kubota T, Kumai K, Wakabayashi G, Omori T, Mukai M, Kitajima M. Diagnosis and treatment of a gastric hamartomatous inverted polyp: report of a case. Surg Today. 2004;34:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Manabe S, Mukaisho KI, Yasuoka T, Usui F, Matsuyama T, Hirata I, Boku Y, Takahashi S. Gastric adenocarcinoma of fundic gland type spreading to heterotopic gastric glands. World J Gastroenterol. 2017;23:7047-7053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Yu BC, Lee WK. Two cases of mucinous adenocarcinoma of the stomach mistaken as submucosal tumor. J Korean Surg Soc. 2013;84:118-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Yoo CH, Park SJ, Park MI, Moon W, Kim HH, Lee JS, Song JY, Jang HK. Submucosal tumor-like early-stage mucinous gastric carcinoma: a case study. Korean J Gastroenterol. 2013;62:122-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 12. | Takahashi T, Otani Y, Yoshida M, Furukawa T, Kameyama K, Akiba Y, Saikawa Y, Kubota T, Kumai K, Kuramochi S, Mukai M, Ishii H, Kitajima M. Gastric cancer mimicking a submucosal tumor diagnosed by laparoscopic excision biopsy. J Laparoendosc Adv Surg Tech A. 2005;15:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Tsuji T, Iwahashi M, Nakamori M, Ueda K, Ishida K, Naka T, Ojima T, Akamatsu H, Yamaue H. Multiple early gastric cancer with gastritis cystica profunda showing various histological types. Hepatogastroenterology. 2008;55:1150-1152. [PubMed] |
| 14. | Kato M, Hayashi Y, Fukumoto K, Nagai K, Tsujii Y, Shinzaki S, Iijima H, Takehara T. Early gastric cancer with lymphoid stroma presenting as a subepithelial lesion diagnosed by endoscopic submucosal dissection. Clin J Gastroenterol. 2018;11:382-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Raftopoulos SC, Segarajasingam DS, Burke V, Ee HC, Yusoff IF. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010;105:1292-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Angelelli G, Ianora AA, Scardapane A, Pedote P, Memeo M, Rotondo A. Role of computerized tomography in the staging of gastrointestinal neoplasms. Semin Surg Oncol. 2001;20:109-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 17. | Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Winawer SJ, Urmacher C, Brennan MF. Preoperative staging of gastric cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 187] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Cardoso R, Coburn N, Seevaratnam R, Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E, Tinmouth J. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer. 2012;15 Suppl 1:S19-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Cheng JY, Wu X, Yang AM, Zou L, Yao F, Guo T, Wu DS, Feng YL, Jiang QW, Zhou WX, Lu XH. Evaluation of preoperative endoscopicultrasonography for diagnostic and therapeutic decision making in superficial gastric cancers. Zhonghua Xiaohuaneijing Zazhi. 2016;33:663-666. [DOI] [Full Text] |
| 20. | Lightdale CJ, Botet JF, Kelsen DP, Turnbull AD, Brennan MF. Diagnosis of recurrent upper gastrointestinal cancer at the surgical anastomosis by endoscopic ultrasound. Gastrointest Endosc. 1989;35:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Mocellin S, Pasquali S. Diagnostic accuracy of endoscopic ultrasonography (EUS) for the preoperative locoregional staging of primary gastric cancer. Cochrane Database Syst Rev. 2015;CD009944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Ludwig K, Klautke G, Bernhard J, Weiner R. Minimally invasive and local treatment for mucosal early gastric cancer. Surg Endosc. 2005;19:1362-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Adachi Y, Shiraishi N, Kitano S. Modern treatment of early gastric cancer: review of the Japanese experience. Dig Surg. 2002;19:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Jin EH, Lee DH, Jung SA, Shim KN, Seo JY, Kim N, Shin CM, Yoon H, Jung HC. Clinicopathologic factors and molecular markers related to lymph node metastasis in early gastric cancer. World J Gastroenterol. 2015;21:571-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Lai JF, Xu WN, Noh SH, Lu WQ. Effect of World Health Organization (WHO) Histological Classification on Predicting Lymph Node Metastasis and Recurrence in Early Gastric Cancer. Med Sci Monit. 2016;22:3147-3153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Ahmad R, Setia N, Schmidt BH, Hong TS, Wo JY, Kwak EL, Rattner DW, Lauwers GY, Mullen JT. Predictors of Lymph Node Metastasis in Western Early Gastric Cancer. J Gastrointest Surg. 2016;20:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Folli S, Morgagni P, Roviello F, De Manzoni G, Marrelli D, Saragoni L, Di Leo A, Gaudio M, Nanni O, Carli A, Cordiano C, Dell'Amore D, Vio A; Italian Research Group for Gastric Cancer (IRGGC). Risk factors for lymph node metastases and their prognostic significance in early gastric cancer (EGC) for the Italian Research Group for Gastric Cancer (IRGGC). Jpn J Clin Oncol. 2001;31:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 109] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1954] [Article Influence: 217.1] [Reference Citation Analysis (1)] |
| 29. | Akiyama M, Ota M, Nakajima H, Yamagata K, Munakata A. Endoscopic mucosal resection of gastric neoplasms using a ligating device. Gastrointest Endosc. 1997;45:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Merrett ND, Park WS, Kwon KA S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wu YXJ