Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3012
Peer-review started: May 21, 2019
First decision: July 30, 2019
Revised: September 2, 2019
Accepted: September 9, 2019
Article in press: September 9, 2019
Published online: October 6, 2019
Processing time: 166 Days and 21.4 Hours
Known ocular manifestations of Alport syndrome include features such as anterior lenticonus and fleck retinopathy. Reports of keratoconus in such patients are limited. We report tomographic findings consistent with keratoconus in a patient with Alport syndrome.
A 52-year-old female was referred to our ophthalmology clinic with decreased vision and increased tearing. She was diagnosed with stage III Alport syndrome two years prior. Upon examination she was found to have average keratometries of 48 D bilaterally with tomographic evidence of keratoconus.
Although a rare presentation, concurrent Alport syndrome and keratoconus should be considered when reviewing the ocular health of Alport syndrome patients and appropriate management steps should be taken upon the diagnosis.
Core Tip: Various ocular manifestations of Alport syndrome have been well described. However, reports of keratoconus in the presence of Alport syndrome is limited in the literature. Keratoconus is a form of corneal ectasia and has shown disorganized and thinning collagen under microscopy. Both Alport syndrome and keratoconus demonstrate collagenous changes which may eventually lead to impaired function of the affected tissue. We present a case of keratoconus in the presence of Alport syndrome.
- Citation: Moshirfar M, Skanchy DF, Gomez AT, Ronquillo YC, Buckner B, Hoopes PC. Keratoconus in a patient with Alport syndrome: A case report. World J Clin Cases 2019; 7(19): 3012-3017
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3012.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3012
Alport syndrome is an inherited disease characterized by progressive renal disease, hearing loss, and ocular abnormalities. It was first described as “familial congenital haemorrhagic nephritis” by Arthur Cecil Alport in 1927[1]. Inherited genetic mutations of COL4A5 (X-linked), COL4A3 and COL4A4 (autosomal recessive) produce defects in the alpha chains that form type IV collagen[2,3]. Type IV collagen is a component of basement membranes throughout the body[4]. Specifically, the heterotrimer α3α4α5 plays a crucial role in the glomerular basement membrane of the kidney, stria vascularis of the cochlea, Descemet’s membrane and Bowman’s layer of the cornea, lens capsule, and both the inner limiting membrane and Bruch’s membrane of the retina[5]. The most common ocular manifestations in Alport patients are corneal opacities, anterior lenticonus, cataract, fleck retinopathies, and temporal retinal thinning[5]. The prevalence of the disease has been estimated around 1:10000 with the X-linked variant accounting for 85% of the disease[6].
Keratoconus is a corneal dystrophy resulting in corneal ectasia due to central or paracentral stromal thinning[7]. The pathophysiology is poorly understood, but breaks in Bowman’s layer, collagen disorganization, and scarring have all been described. Although the etiology remains unknown, both genetic and environmental factors, such as eye rubbing, are believed to contribute to the disease process. Keratoconus has a known association with Down syndrome, Leber’s congenital amaurosis, and connective tissue diseases such as Ehler’s Danlos[8].
Despite the wide variety of ocular manifestations found in patients with Alport syndrome, corneal ectasia appears to be a rare finding. The literature is limited on the presence of keratoconus in these patients[9]. We report an interesting case of tomographic findings consistent with keratoconus in a patient with Alport syndrome.
A 52-year-old female was referred to our ophthalmology clinic with decreased vision and increased tearing.
She was diagnosed with stage III Alport syndrome two years prior which was currently stable per her nephrologist. During these two years had gradual decline in her visual acuity that was not correctable to 20/20.
She had a past medical history of asthma, diabetes mellitus, and hypertension.
Family history was positive for Alport syndrome in her mother and brother but without any known ocular or hearing abnormalities.
On initial examination (Table 1), uncorrected distance visual acuity was 20/60 on the right and 20/30 on the left, with a corrected visual acuity of 20/30 bilaterally.
| Encounter | Initial encounter | 2 mo follow up | Initial encounter | 2 mo follow up |
| Eye | OD | OD | OS | OS |
| Vision Acuity | ||||
| UDVA | 20/60- | 20/60 | 20/30- | 20/40 |
| BCVA | 20/30 | 20/25-2 | 20/30 | 20/25+ |
| Man. Refraction | -1.50-1.75 x 031 | -0.75-2.25 x 037 | -1.00-2.75 x 149 | -0.75-3.75 x 147 |
| Tomography | ||||
| Front | ||||
| K1 (flat) | 47.0 D | 46.7 D | 47.1 D | 47.1 D |
| K2 (steep) | 48.9 D | 49.5 D | 49.6 D | 50.1 D |
| Km | 47.9 D | 48.1 D | 48.3 D | 48.5 D |
| Axis | 31.0° | 36.1° | 146.4° | 148.7° |
| Back | ||||
| K1 | -6.5 | -6.5 | -6.7 | -6.7 |
| K2 | -6.9 | -6.9 | -6.9 | -7.0 |
| Km | -6.7 | -6.7 | -6.8 | -6.8 |
| Axis | 40.3° | 53.6° | 123.9° | 134.2° |
| Pachymetry | ||||
| Pupil center | 506 μm | 502 μm | 474 μm | 472 μm |
| Pachy apex | 505 μm | 499 μm | 472 μm | 463 μm |
| Thinnest | 496 μm | 489 μm | 454 μm | 448 μm |
Slit lamp examination showed bilateral eyelid laxity, papillary conjunctival changes, prominent nerves, superficial punctate keratitis, unilateral (OD) anterior basement membrane changes, with no guttata, apical scarring, or corneal striae. Dilated fundus exam revealed bilateral floaters in the vitreous humor and normal retinal vasculature.
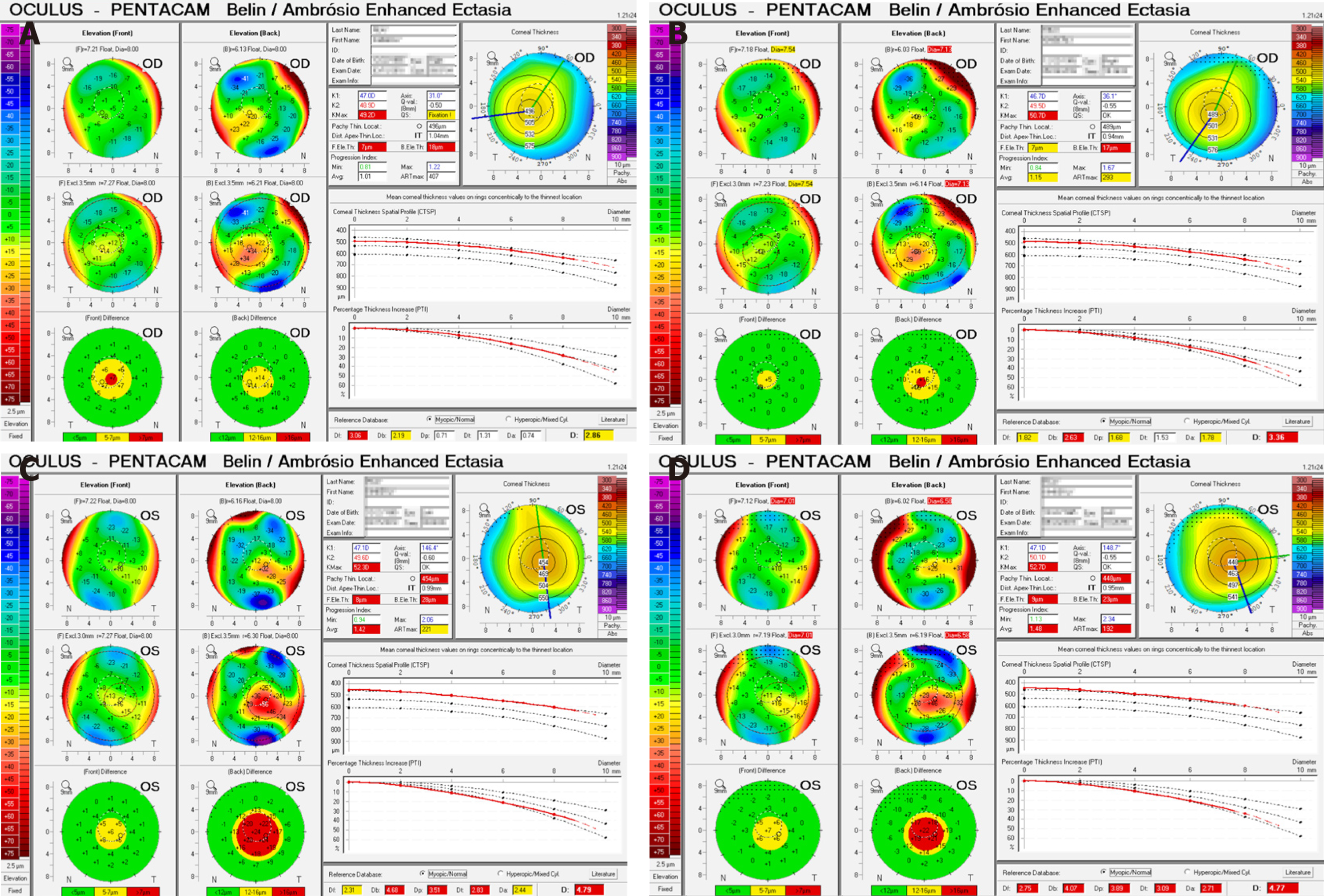
Initial Pentacam (Oculus, Wetzlar, Germany) tomography revealed mean keratometry of 47.9 D in the right and 48.3 D in the left, with a 2 mo follow-up scan revealing a mean keratometry of 48.1 D and 48.5 D respectively (Table 1). During this 2 mo period progressive corneal thinning occurred bilaterally. There was also bilateral anterior curvature steepening with no appreciable changes in the posterior curvature.
Average keratometries above 47 to 48 D have been shown to correlate with keratoconus[10,11]. Based on the Amsler-Krumeich Classification system our patient was diagnosed with stage 2 keratoconus, with signs of progression over two months, including further corneal thinning and increased steepening. the disease[12,13]. This diagnosis was reinforced with the Pentacam Belin/Ambrosio Enhanced Ectasia Display which highlights tomographic abnormalities consistent with keratoconus (Figure 1).
Although no curative treatment is available for patients with Alport syndrome, Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone inhibitors reduce stress on the kidneys and may slow the progression of renal failure[14,15]. Yearly hearing and vision tests are recommended for patients diagnosed with Alport syndrome from the time the patient is 7 or 8 years old[14].
Ophthalmologic manifestations of Alport syndrome are treated symptomatically, including lens removal and intraocular lens placement for lenticonus and cataract. Our patient was advised to continue to use glasses and avoid eye rubbing.
While her keratoconus was mild, continued signs of disease progression would warrant discussion of corneal collagen crosslinking while reserving corneal transplantation for advanced disease.
The concurrent presentation of Alport syndrome and keratoconus, to our knowledge, has only been described once in the literature. Chugh et al[9] reported that 3 of 45 (6.7%) patients diagnosed with Alport syndrome were found to have associated keratoconus. This is the first report in the literature of tomographic findings consistent with keratoconus in a patient with Alport syndrome. While the inheritance patterns for Alport syndrome are well defined, the inheritance of keratoconus is under much investigation. Variants of the COL4A3 and COL4A4 genes are two of many possible genetic causes of keratoconus[16-18]. One study found that seven polymorphisms of COL4A3 and COL4A4 were associated with keratoconus, although no mutations were directly linked with the disease[17]. A meta-analysis of genetic associations for keratoconus concluded that COL4A4 had differential effects on keratoconus between ethnic groups[18]. Specific mutations of the COL4A3 and COL4A4 genes are known to cause of Alport syndrome.
A range of kidney-related diseases including chronic renal failure, kidney transplant, hypertension, diabetes mellitus, and various medications have been associated with keratoconus[19]. One theory is that both renal and ocular tissues express the PAX6 gene. PAX6 is linked to corneal gelatinase B, which is a metalloprotease responsible for corneal structure regulation. Thus, a mutated PAX6 gene may contribute to keratoconus and renal pathology[19]. Aldave et al[20] reported cases of polymorphous corneal dystrophy (PPCD) in Alport patients[5]. However, the process of corneal steepening found in PPCD is likely independent from that found in keratoconus, as the steepening often presents with the absence of other clinical features of keratoconus.
In Alport syndrome, the normal collagen IV α3α4α5 network is lost and the immature α1α1α2 network persists. This α1α1α2 network has fewer crosslinks and more proteolytic cleavage sites. This produces basement membranes that are more susceptible to biomechanical strain. It also induces an increase in matrix metalloprotease activity[5]. Increased activity of protease enzymes and decreased protease inhibitors have been found in keratoconus[11]. Disorganization of collagen fibrils and a diminished number of crosslinks within and between such fibrils contributes to the weakened biomechanical state of the cornea in keratoconus[21]. Increased protease activity and decreased crosslinking may be a possible connection between the two diseases.
Further studies are necessary to elucidate the underlying molecular mechanisms possibly linking keratoconus and Alport syndrome. One of the limitations of this report is an absence of genetic testing. We were unable to obtain sequencing and analysis of the patient’s exome or genome. These tests would have the potential to reveal specific genetic mutations leading to Alport syndrome in this patient and a more definitive link to keratoconus. When obtained, these tests will provide much value.
Although a rare presentation, concurrent Alport syndrome and keratoconus should be considered when reviewing the ocular health of Alport syndrome patients and appropriate management steps should be taken upon the diagnosis.
| 1. | Alport AC. Hereditary familial congenital haemorrhagic nephritis. Br Med J. 1927;1:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 404] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science. 1990;248:1224-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 553] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 3. | Nagel M, Nagorka S, Gross O. Novel COL4A5, COL4A4, and COL4A3 mutations in Alport syndrome. Hum Mutat. 2005;26:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 519] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 5. | Savige J, Sheth S, Leys A, Nicholson A, Mack HG, Colville D. Ocular features in Alport syndrome: pathogenesis and clinical significance. Clin J Am Soc Nephrol. 2015;10:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 164] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Hertz JM, Thomassen M, Storey H, Flinter F. Clinical utility gene card for Alport syndrome. Eur J Hum Genet. 2012;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Rosen ES. Keratoconus. J Cataract Refract Surg. 2012;38:927-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Davidson AE, Hayes S, Hardcastle AJ, Tuft SJ. The pathogenesis of keratoconus. Eye (Lond). 2014;28:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 9. | Chugh KS, Sakhuja V, Agarwal A, Jha V, Joshi K, Datta BN, Gupta A, Gupta KL. Hereditary nephritis (Alport's syndrome)-clinical profile and inheritance in 28 kindreds. Nephrol Dial Transplant. 1993;8:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995;11:371-379. [PubMed] |
| 11. | Mas Tur V, MacGregor C, Jayaswal R, O'Brart D, Maycock N. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv Ophthalmol. 2017;62:770-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 311] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 12. | Amsler M. Le kèratocône fruste au Javal. Ophthalmologica. 1938;96:77-83. |
| 13. | Amsler M. [Not Available]. Ophthalmologica. 1946;111:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 14. | Kashtan CE, Ding J, Gregory M, Gross O, Heidet L, Knebelmann B, Rheault M, Licht C; Alport Syndrome Research Collaborative. Clinical practice recommendations for the treatment of Alport syndrome: a statement of the Alport Syndrome Research Collaborative. Pediatr Nephrol. 2013;28:5-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Stock J, Kuenanz J, Glonke N, Sonntag J, Frese J, Tönshoff B, Höcker B, Hoppe B, Feldkötter M, Pape L, Lerch C, Wygoda S, Weber M, Müller GA, Gross O. Prospective study on the potential of RAAS blockade to halt renal disease in Alport syndrome patients with heterozygous mutations. Pediatr Nephrol. 2017;32:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Saravani R, Hasanian-Langroudi F, Validad MH, Yari D, Bahari G, Faramarzi M, Khateri M, Bahadoram S. Evaluation of possible relationship between COL4A4 gene polymorphisms and risk of keratoconus. Cornea. 2015;34:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 17. | Stabuc-Silih M, Ravnik-Glavac M, Glavac D, Hawlina M, Strazisar M. Polymorphisms in COL4A3 and COL4A4 genes associated with keratoconus. Mol Vis. 2009;15:2848-2860. [PubMed] |
| 18. | Rong SS, Ma STU, Yu XT, Ma L, Chu WK, Chan TCY, Wang YM, Young AL, Pang CP, Jhanji V, Chen LJ. Genetic associations for keratoconus: a systematic review and meta-analysis. Sci Rep. 2017;7:4620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Bahar I, Vinker S, Livny E, Kaiserman I. Possible Association between Keratoconus and Renal Diseases. J Clin Exp Ophthalmol. 2010;01. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Aldave AJ, Ann LB, Frausto RF, Nguyen CK, Yu F, Raber IM. Classification of posterior polymorphous corneal dystrophy as a corneal ectatic disorder following confirmation of associated significant corneal steepening. JAMA Ophthalmol. 2013;131:1583-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Ambekar R, Toussaint KC, Wagoner Johnson A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J Mech Behav Biomed Mater. 2011;4:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang Y S-Editor: Zhang L L-Editor: A E-Editor: Liu MY