Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.2995
Peer-review started: March 28, 2019
First decision: May 31, 2019
Revised: September 5, 2019
Accepted: September 11, 2019
Article in press: September 11, 2019
Published online: October 6, 2019
Processing time: 185 Days and 19.3 Hours
For a long time, laryngopharyngeal reflux disease (LPRD) has been treated by proton pump inhibitors (PPIs) with an uncertain success rate.
To shed light the current therapeutic strategies used for LPRD in order to analysis the rationale in the LPRD treatment.
Three authors conducted a PubMed search to identify papers published between January 1990 and February 2019 about the treatment of LPRD. Clinical prospective or retrospective studies had to explore the impact of medical treatment(s) on the clinical presentation of suspected or confirmed LPRD. The criteria for considering studies for the review were based on the population, intervention, comparison, and outcome framework.
The search identified 1355 relevant papers, of which 76 studies met the inclusion criteria, accounting for 6457 patients. A total of 64 studies consisted of empirical therapeutic trials and 12 were studies where authors formally identified LPRD with pH-monitoring or multichannel intraluminal impedance-pH monitoring (MII-pH). The main therapeutic scheme consisted of once or twice daily PPIs for a duration ranged from 4 to 24 wk. The most used PPIs were omeprazole, esomeprazole, rabeprazole, lansoprazole and pantoprazole with a success rate ranging from 18% to 87%. Other composite treatments have been prescribed including PPIs, alginate, prokinetics, and H2 Receptor antagonists.
Regarding the development of MII-pH and the identification of LPRD subtypes (acid, nonacid, mixed), future studies are needed to improve the LPRD treatment considering all subtypes of reflux.
Core tip: The treatment of laryngopharyngeal reflux disease (LPRD) has not changed since three decades and it is based on proton pump inhibitors (PPIs). However, the superiority of PPIs over placebo is still controversial and there are a significant number of non-responder patients to treatment. The development of multichannel intraluminal pH impedance monitoring led to the identification of subtypes of LPRD including acid, nonacid and mixed LPRD. The treatment of each subtype could be different in order to have better response rate.
- Citation: Lechien JR, Mouawad F, Barillari MR, Nacci A, Khoddami SM, Enver N, Raghunandhan SK, Calvo-Henriquez C, Eun YG, Saussez S. Treatment of laryngopharyngeal reflux disease: A systematic review. World J Clin Cases 2019; 7(19): 2995-3011
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/2995.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.2995
Laryngopharyngeal reflux disease (LPRD) is an inflammatory condition of the upper aerodigestive tract tissues related to direct and indirect effect of gastric or duodenal content reflux, which induces morphological changes in the upper aerodigestive tract[1]. LPR-related symptoms concern approximately 4 to 10% of outpatients visiting Otolaryngology–Head and Neck Surgery Departments[2] and up to 50% of patients in Division of Laryngology[3]. To date, several controversies persist about the diagnostic and the therapeutic management of LPRD. Although proton pump inhibitors (PPIs) are considered as the main treatment of LPRD since many decades[4], the superiority of PPIs over placebo is still controversial[5]. Thus, more than 40% of patients have less or no symptom relief with an empirical therapeutic trial based on PPIs[5,6]. The aim of this systematic review is to shed light the current therapeutic strategies used for the management of LPRD in order to analysis the rationale in the treatment of LPRD.
The criteria for considering studies for the review were based on the population, intervention, comparison, and outcome framework[7].
Clinical prospective or retrospective studies published in peer-reviewed journals were included. Studies had to explore the impact of medical treatment(s) on the clinical presentation of suspected or confirmed LPRD. Only studies published in English literature were included.
Papers were included if they attempted rigorous diagnosis of LPRD through symptoms, exam findings, or objective testing. Patients with positive pH-monitoring or multichannel intraluminal impedance-pH monitoring (MII-pH) were considered as “LPRD patients”; those with a clinical diagnosis based on symptoms ± findings were considered as “suspected LPRD patients”. Studies focusing on patients who did not respond to treatment were not included.
The primary outcome was review of types and effectiveness of treatment administered to LPRD patients. The secondary outcome was based on the above to define a rational approach to the management of LPRD.
Authors had to treat their patients with conventional medical treatment including PPIs, prokinetics, histamine H2-receptor antagonists (H2R), alginate, magaldrate, baclofen and other drugs that have been reported at least once as treatment of LPRD or gastroeosophageal reflux disease (GERD). Diet and behavioral changes have also been considered as treatment. Studies that reported patients treated with surgery were carefully excluded. The studies had to clearly describe the therapeutic scheme, i.e., drug(s), doses and potential association of drug(s) with other therapeutic approaches (speech and swallowing therapies, etc.).
Lechien JR, Barillari MR, and Calvo-Henriquez C conducted a PubMed search to identify papers published between January 1990 and February 2019. Studies were screened if they had database abstracts, available full texts or titles referring to the condition. The following keywords were used: “Laryngopharyngeal reflux”; “reflux laryngitis”; “gastroesophageal reflux”; “treatment”; and “therapeutic”. These investigators provided a critical analysis of the publication’s content and summarized the data of the selected papers in the publication in order to determine final article selection.
The search identified 1355 relevant papers, of which 76 studies met the inclusion criteria, accounting for 6457 patients. A total of 64 studies consisted of empirical therapeutic trials (Table 1)[8-72], and 12 were studies where authors formally identified LPRD with pH-monitoring (n = 10) or MII-pH (n = 2) (Table 2)[40,56,60,73-83].
| Ref. | Design | EL | Characteristics | Outcomes | Treatment |
| Hanson et al[8], 1995 | Pros Uncontr | IIIb | Suspected LPR (n = 141) | Symptom and sign resolution: 51% | 4 wk omeprazole (20 mg, 1/d) and diet |
| Jaspersen et al[9], 1996 | Pros Uncontr | IIIb | Suspected LPR (n = 21) | Laryngeal sign improvement: 100% | 4 wk omeprazole (40 mg, 1/d) |
| Shaw et al[10], 1997 | Pros Uncontr | IIIb | Suspected LPR (n = 96) | Pre to post-score improvement: + | 12 wk omeprazole (20 mg/d) |
| Wo et al[11], 1997 | Pros Uncontr | IIIb | Suspected LPR (n = 21) | Pre to post-score improvement: + | 8 wk omeprazole (40 mg, 1/d) and diet |
| Metz et al[12], 1997 | Pros Uncontr | IIIb | Suspected LPR (n = 10) | Symptom and sign resolution: 60% | 4 wk omeprazole (20 mg/d) |
| Habermann et al[13], 1999 | Pros Uncontr | IIIb | Suspected LPR (n = 29) | Pre to post-score improvement: + | 6 wk pantoprazole (40 mg/d) |
| Havas et al[14], 1999 | Placebo RCT | Ib | Gr1: suspected LPR (n = 7) | Pre to post-score improvement: + | Gr1-2: 12 wk placebo/lanzoprazole (30 mg 2/d) and Diet |
| Gr2: suspected LPR (n = 8) | |||||
| El-Serag et al[15], 2001 | Placebo RCT | Ib | Gr1: suspected LPR (n = 10) | 54% of symptom resolution | Gr1-2: 12 wk placebo/lansoprazole (30 mg 2/d) |
| Gr2: suspected LPR (n = 10) | |||||
| Langevin et al[16], 2001 | Placebo RCT | Ib | Gr1: suspected LPR (n = 14) | Pre to post-score improvement | Gr1-2: 12 wk placebo/omeprazole (40 mg/d) |
| Gr2: suspected LPR (n = 16) | |||||
| Hamdan et al[17], 2001 | Pros Uncontr | IIIb | Suspected LPR (n = 22) | Pre to post-score improvement: + | 4 wk pantoprazole (40 mg, 2/d), cisapride (20 mg, 2/d) and diet |
| Rodríguez-Téllez et al[18], 2002 | Pros Uncontr | IIIb | Suspected LPR (n = 21) | Pre to post-score improvement: + | 12 wk omeprazole (20 mg, 2/d) |
| Habermann et al[19], 2002 | Pros Uncontr | IIIb | Suspected LPR (n = 24) | Pre to post-score improvement: + | 6 wk pantoprazole (40 mg/d) |
| DelGaudio et al[20], 2003 | Pros Uncontr | IIIb | Gr1: LPR responder (n = 19) | 50% symptom improvement: 63% | 8 wk esomeprazole (40 mg 1/d) and diet |
| Bilgen et al[21], 2003 | Pros Contr | IIIb | Gr1: suspected LPR (n = 36) | Improvement of ≥ 1-point RSI and RFS: 68% | 24 wk lansoprozole (30 mg, 2/d) and diet |
| Gr2: CT (n = 23) | |||||
| Garrigues et al[22], 2003 | Pros Uncontr | IIIb | Suspected LPR (n = 91) | Symptom improvement/resolution: 86-41% | 24 w omeprazole (20 mg, 2/d) |
| Laryngoscopic sign resolution: 83% | |||||
| Beaver et al[23], 2003 | Pros Uncontr | IIIb | Suspected LPR (n = 49) | Pre to post-LPR sign score improvement: +1 | 6 wk lansoprazole (30 mg, 2/d) or pantoprazole (40 mg, 2/d) or Omeprazole/Rabeprazole (20 mg, 2/d) |
| Siupsinskiene et al[24], 2003 | Pros Contr | IIb | Gr1: suspected LPR (n = 113) | Symptom improvement of Gr1: 65% | Gr1-2: 5 wk omeprazole (20 mg, 1-2/d) and diet |
| Gr2: healthy (n = 113) | |||||
| Williams et al[25], 2004 | Pros Uncontr | IIIb | Suspected LPR (n = 20) | Improvement of ≥ 1-point level LGS: 63% | 12 wk omeprazole (20 mg, 3/d) and diet |
| Improvement of symptom score: 40%-45% | |||||
| Issing et al[26], 2004 | Pros Uncontr | IIIb | Suspected LPR (n = 22) | Improvement of symptom score: + | 8 wk esomeprazole (20 mg, 2/d) |
| Sereg-Bahar et al[27], 2005 | Pros Uncontr | IIIb | Suspected LPR (n = 43) | Pre to post-RFS improvement: +1 | 8 wk esomeprazole (40 mg/d) and diet |
| Park et al[28], 2005 | Pros Contr | IIb | Gr1: suspected LPR (n = 30) | Symptom improvement (Gr1-2):68%-46% | Gr1: 16 wk lansoprazole (30 mg, 2/d) and diet |
| Gr2: suspected (n = 30) | Sign improvement (Gr1-2): 50%-18% | Gr2: Omeprazole (20 mg, 2/d) and ranitidine (300 mg/d) and diet | |||
| Gr3: suspected (n = 25) | Gr3: esomeprazole (40 mg, 1/d) and diet | ||||
| Vaezi et al[29], 2006 | Placebo RCT | Ib | Gr1: suspected LPR (n = 95) | Symptom resolution: 15% | Gr1-2: 16 wk placebo/esomeprazole (40 mg, 2/d) |
| Gr2: suspected LPR (n = 50) | |||||
| Dore et al[30], 2007 | Pros Uncontr | IIIb | Suspected LPR (n = 266) | Symptom improvement/resolution: 68%-12% | 12 wk rabeprazole/pantoprazole (20 mg, 2/d), and diet or esomeprazole (20 mg, 2/d) or lanzoprazole (30 mg, 2/d), |
| Qua et al[31], 2007 | Pros Contr | IIIb | Suspected LPR (n = 32) | Gr1-2: Symptom improvement: 67%-18% | 8 wk lanzoprazole (30 mg, 2/d) |
| Gr1: GERD (n = 21) | Gr1-2: LGS improvement: 86%-36% | ||||
| Gr2: non-GERD (n = 11) | |||||
| Oridate et al[32], 2008 | Pros Uncontr | IIIb | Suspected LPR (n = 52) | > 50% improvement of RSI and GERD: 50%-78% | 9 wk rabeprazole (20 mg/d) |
| Pre to post-improvement of DLS: + | |||||
| Reichel et al[33], 2008 | Placebo RCT | Ib | Gr1: suspected LPR (n = 30) | RSI improvement: 78% | Gr1-2: 12 wk placebo/esomeprazole (20 mg, 2/d) |
| Gr2: suspected LPR (n = 28) | |||||
| McGlashan et al[34], 2009 | Placebo RCT | Ib | Gr1: suspected LPR (n = 24) | Pre to post-RSI improvement | Gr1-2: 24 wk placebo/gaviscon (4/d) and diet |
| Gr2: suspected LPR (n = 25) | |||||
| Vashani et al[35], 2010 | Placebo RCT | Ib | Gr1: suspected LPR (n = 16) | Pre to post-RSI improvement: + | Gr1: 6 wk voice therapy + Omeprazole (20 mg, 2/d) |
| Gr2: suspected LPR (n = 16) | Gr 2: Placebo (2/d) | ||||
| Fass et al[36], 2010 | Placebo RCT | Ib | Gr1: suspected LPR (n = 24) | Pre to post-symptom improvement: + | Gr1-2: 12 wk placebo/esomeprazole (20 mg, 2/d) and diet |
| Gr1: suspected LPR (n = 17) | Pre to post-RFS improvement: - | ||||
| Lam et al[37], 2010 | Placebo RCT | Ib | Gr1: suspected LPR (n = 42) | Pre to post-RSI and RFS improvement: + | Gr1-2: 18 wk placebo/rabeprazole (20 mg, 2/d) and diet |
| Gr2: suspected LPR (n = 40) | |||||
| Ezzat et al[38], 2011 | Placebo RCT | Ib | Gr1: suspected LPR (n = 42) | RFS improvement (Gr1-2): 48%-20% | Gr1-2: 8 wk pantoprazole (40 mg/d) and itopride (50 mg, 3/d) |
| Gr2: suspected LPR (n = 45) | Pre to post-symptom improvement: + | /Pantoprazole and placebo and diet | |||
| Chiba et al[39], 2011 | Pros Uncontr | IIIb | Suspected LPR (n = 27) | Pre to post-GERD Score improvement: + | 8 wk lanzoprazole (30 mg/d) or rabeprazole (10 mg/d) |
| Friedman et al[40], 2011 | Retrospective | IV | Gr1: LPR (n = 73) | Improvement of main complaint Gr1-2: 49%-41% | 24 wk PPI (20 or 40 mg, 2/d) |
| Gr2: suspected LPR (n = 70) | Resolution of main complaint Gr 1-2: 14%-3% | ||||
| Lee et al[41], 2011 | Pros Uncontr | IIIb | Suspected LPR (n = 455) | Reduction of > 50% of RSI: 75% | 12 wk rabeprazole (10/20 mg/d) and diet |
| Masaany et al[42], 2011 | Pros Uncontr | IIIb | Suspected LPR (n = 47) | Reduction of ≥ 10-point of RSI: 79% | 16 wk pantoprazole (40 mg, 2/d) |
| Naiboglu et al[43], 2011 | Pros Uncontr | IIIb | Suspected LPR (n = 50) | Pre to post-RSI and RFS improvement: + | 12 wk lansoprazole (30 mg/d) and diet |
| Patigaroo et al[44], 2011 | Pros Uncontr | IIIb | Suspected LPR (n = 50) | Pre to post-RSI and RFS improvement: + | 16 wk esomeprazole (20 mg, 2/d)/pantoprazole (40 mg/d) |
| Lansoprazole (30 mg, 2/d) | |||||
| Habermann et al[45], 2012 | Pros Uncontr | IIIb | Suspected LPR (n = 1044) | Pre to post-RSI and RFS improvement: + | 12 wk pantoprazole (20 or 40 mg, 2/d) |
| Park et al[46], 2012 | Pros Contro | IIIb | Gr1: suspected LPR (n = 50) | Reduction of ≥ 5-point of RSI Gr1-2:46-68% | Gr1: 12 wk omeprazole (20 mg, 2/d) |
| Gr2: suspected LPR (n = 50) | Reduction of ≥ 3-point of RFS Gr1-2:18-50% | Gr2: Omeprazole + voice therapy | |||
| Becker et al[47], 2012 | Pros Uncontr | IIIb | Suspected LPR (n = 30) | Reduction of RSI: 20% | 12 wk pantoprazole (40 mg, 2/d) |
| Hunchaisri et al[48], 2012 | Pros Contro | IIb | Gr1: suspected LPR (n = 32) | RSI reduction: 73% | Gr1: 12 wk domperidone (10mg, 3/d) and omeprazole (20 mg, 2/d) and diet |
| Gr2: suspected LPR (n = 33) | > 50% of RSI reduction: 67% | Gr2: Omeprazole (20 mg, 2/d) and diet | |||
| Chung et al[49], 2012 | Pros Contro | IIb | Gr1: suspected LPR (n = 22) | Pre to post-RSI and RFS improvement: + | Gr1: 8 wk Lanzoprazole (30 mg/d) |
| Gr2: suspected LPR (n = 20) | Gr2: Lanzoprazole + SGB | ||||
| Oridate et al[50], 2012 | Pros Contro | IIb | Gr1: suspected LPR (n = 60) | Pre to post-RFS improvement: - | Gr 1: 4 wk rabeprazole (10 mg/d) |
| Gr2: suspected LPR (N=13) | Gr 2: No treatment | ||||
| Chun et al[51], 2013 | Pros Contro | IIb | Gr1: suspected LPR (n = 32) | Pre to post-RSI and RFS improvement: + | Gr1: 12 wk lanzoprazole (30 mg/d) |
| Gr2: suspected LPR (n = 29) | Gr2: Lanzoprazole and itopride (50 mg 3/d) | ||||
| Beech et al[52], 2013 | Pros Uncontr | IIIb | Suspected LPR (n = 74) | Reduction of ≥ 1-point of RSI: 71% | 24 wk lansoprazole (30 mg 2/d) and diet |
| Improvement of pre to post-VSS: + | |||||
| Vailati et al[53], 2013 | Pros Uncontr | IIIb | Suspected LPR (n = 22) | Reduction of ≥1-point of RSI: 59% | 12 wk pantoprazole (40 mg, 2/d) |
| Lee et al[54], 2014 | Pros Uncontr | IIIb | Suspected LPR (n = 180) | Pre to post-RSI and RFS improvement: + | 12 wk lansoprazole (15 mg, 2/d) and diet |
| Chappity et al[55], 2014 | RCT | IIb | Gr1: suspected LPR (n = 117) | Pre to post-score improvement: + | Gr1: 12 wk omeprazole (20 mg, 2/d) and diet |
| Gr2: suspected LPR (n = 117) | Gr2: Diet | ||||
| Wan et al[56], 2014 | Pros Contro | IIb | Gr1: suspected LPR (n = 35) | Pre to post-RSI and RFS improvement: + | 4 wk esomeprazole (20 mg, 2/d) and diet |
| Gr2: LPR (n = 23) | |||||
| Semmanaselvan et al[57], 2015 | Pros Uncontr | IIIb | Suspected LPR (n = 50) | Reduction of ≥ 1-point of RSI/RFS: 87%-98% | 12 wk rabeprazole (20 mg/d) and domperidone (30 mg/d) |
| Ozturan et al[58], 2016 | Pros Contro | IIb | Gr1: suspected LPR (n = 65) | Pre to post-RSI and RFS improvement: + | 8 wk esomeprazole, (20 mg, 2/d) and diet |
| Gr2: Control (n = 35) | |||||
| Gupta et al[59], 2016 | Retrospective | IV | Suspected LPR (n = 188) | Pre to post-RSI and RFS improvement: + | 10 wk PPIs (2/d) |
| Nennstiel et al[60], 2016 | Retrospective | IV | Gr1: LPR (n = 21) | Symptom VAS improvement: 60% | 12 wk pantoprazole (40 mg, 2/d) and diet |
| Cross-sectional | Gr2: suspected LPR (n = 24) | ||||
| Batıoğlu-Karaaltın et al[61], 2016 | Pros Uncontr | IIIb | Suspected LPR (n = 84) | Reduction of ≥ 1-point of RSI/RFS: 21%-56% | 12 wk lansoprazole (30 mg, 2/d) |
| Dulery et al[62], 2016 | Pros Uncontr | IIIb | Suspected LPR (n = 24) | Symptom resolution: 10% | 8 wk esomeprazole (40 mg, 2/d) |
| Joshi et al[63], 2017 | Pros Uncontr | IIIb | Suspected LPR (n = 100) | Pre to post-RSI and RFS improvement: + | 24 wk omeprazole (20 mg, 2/d) and diet |
| Pullarat et al[64], 2017 | Pros Uncontr | IIIb | Suspected LPR (n = 30) | Pre to post-RSI and RFS improvement: + | 8 wk pantoprazole (40 mg/d) |
| Zalvan et al[65], 2017 | Retrospective | IV | Gr1: suspected LPR (n = 85) | Reduction of ≥ 6-points of RSI Gr1-2: 54-63% | Gr1: 6 wk PPI (1 or 2/d) and diet |
| Gr2: suspected LPR (n = 99) | Gr2: Diet | ||||
| Carroll et al[66], 2017 | Retrospective | IV | Suspected LPR (n = 97) | RSI < 13: 49% | 12 wk omeprazole (40 mg/d) and ranitidine (300 mg/d) |
| Lechien et al[67], 2018 | Pros Uncontr | IIIb | Suspected LPR (n = 80) | Post-therapy RSI < 13 and RFS < 7: 74% | 12 wk pantoprazole (20 mg, 2/d) and diet |
| Mozzanica et al[68], 2018 | Pros Uncontr | IIIb | Suspected LPR (n = 34) | Pre to post-RSI, RFS, VoiSS improvement: + | 8 wk omeprazole (20 mg, 2/d) and diet |
| Wilkie et al[69], 2018 | Pros Contro | IIb | Gr1: suspected LPR (n = 39) | Reduction of RSI: 94% | Gr1: 12 wk gaviscon advance (4/d) and diet |
| Gr2: suspected LPR (n = 33) | Pre to post-RSI improvement: - | Gr2: Gaviscon (4/d) and PPI (NA) and diet | |||
| Yang et al[70], 2018 | Retrospective | IV | Suspected LPR (n = 105) | Reduction of ≥ 1-point of RSI: 91% | 8 wk PPI (40 mg/d) ± H2 blocker (300 mg/d) and diet |
| Kirti et al[71], 2018 | Pros Uncontr | IIIb | Suspected LPR (n = 80) | Unblinded RFS < 7: 95% | 8 wk PPI (2/d) and diet |
| Suzuki et al[72], 2019 | Pros Contro | IIb | Gr1: suspected LPR (n = 20) | Pre to post-RSI, RFS improvement: + | Gr1: 8 wk esomeprazole (20 mg/d) |
| Gr2: suspected LPR (n = 20) | Gr2: 8 wk famotidine (20 mg/d) |
| Ref. | Design | EL | Characteristics | Outcomes | Treatment |
| Noordzij et al[73], 2001 | Placebo RCT | Ib | Gr1: LPR (n = 15); Gr2: LPR (n = 15) | Pre to post-symptom improvement: +; Pre to post-sign improvement: - | Gr1-2: 8 wk placebo/omeprazole (40 mg, 2/d) |
| Belafsky et al[74], 2001 | Pros Uncontr | IIIb | LPR (n = 39) | Pre to post-RSI and RFS improvement: + | 24 wk omeprazole/rabeprazole (20 mg, 2/d) or lansoprazole (30 mg, 2/d) and diet |
| Belafsky et al[75], 2002 | Pros Uncontr | IIIb | LPR (n = 25) | Pre to post-RSI improvement: + | 26 wk PPIs (2/d) and diet |
| Eherer et al[76], 2003 | Placebo RCT | Ib | Gr1: LPR (n = 7); Gr2: LPR (n = 7) | Symptom/sign improvement: 80%-100% | Gr1-2: 12 wk placebo/pantoprazole (40 mg, 2/d) |
| Steward et al[77], 2004 | Placebo RCT | Ib | Gr1: LPR (n = 21); Gr2: LPR (n = 21) | Symptom improvement: 53% | Gr1-2: 8 wk placebo/rabeprazole (20 mg 2/d) and diet |
| Wo et al[78], 2006 | Placebo RCT | Ib | Gr1: LPR (n = 19); Gr2: LPR (n = 20) | Symptom improvement: 40% | Gr1-2: 12 wk placebo/pantoprazole (40 mg/d) |
| Jin et al[79], 2008 | Pros Uncontr | IIIb | LPR (n = 40) | Pre to post-RSI and RFS improvement: + | 20 wk lansoprazole (30 mg/d) and mosapride (5 mg, 3/d) or levosulpride (25 mg, 3/d) |
| Friedman et al[40], 2011 | Retrospective | IV | Gr1: LPR (n = 73); Gr2: suspected LPR (n = 70) | Improvement of main complaint Gr1-2: 49%-41%; Resolution of main complaint Gr 1-2: 14%-3% | 24 wk PPI (20 or 40 mg, 2/d) and diet |
| Lien et al[80], 2013 | Pros Contr | IIIb | Gr1: GERD and LPR (n = 65); Gr2: LPR (n = 42) | Reduction of > 50% of RSI (Gr1-2): 63%-17% | 12 wk esomeprazole (40 mg, 2/d) and diet |
| Wan et al[56], 2014 | Pros Contr | IIb | Gr1: suspected LPR (n = 35); Gr2: LPR (n = 23) | Pre to post-RSI and RFS improvement: + | 4 wk esomeprazole (20 mg, 2/d) and diet |
| Waxman et al[81], 2014 | Retrospective | IV | LPR (n = 43) | Reduction of ≥ 1-point of RSI: 67% | 4 wk omeprazole (40 mg, 2/d) |
| Nennstiel et al[60], 2016 | Retrospective | IV | Gr1: LPR (n = 21); Gr2: suspected LPR (n = 24) | Symptom VAS improvement: 60% | 12 wk pantoprazole (40mg, 2/d) and diet |
| Cross-sectional | |||||
| Tseng et al[82], 2018 | Placebo RCT | Ib | Gr1: LPR (n = 39); Gr2: LPR (n = 40) | Pre to post-RSI and RFS improvement: + | Gr1-2: 8 wk alginate/placebo and diet |
| Agrawal et al[83], 2018 | Pros Uncontr | IIIb | LPR (n = 33) | Reduction of > 50% of RSI: 45% | 8-12 wk omeprazole and diet |
The main therapeutic scheme consisted of once or twice daily PPIs (n = 63) for a duration ranged from 4 to 24 wk. The most used PPIs were omeprazole, esomeprazole, rabeprazole, lansoprazole and pantoprazole (Table 3). The efficacy of these treatments was reported in the majority of studies using different outcomes, yielding the comparison between studies difficult (Tables 1 and 2). Overall, authors reported a success rate with PPI therapy ranging from 18% to 87%. Other composite treatments have been prescribed including PPIs, alginate, prokinetics, and H2R antagonists (Table 4).
| Drugs and duration | Study numbers |
| 4-5 wk | |
| Omeprazole 40mg 1/d | 1 |
| Omeprazole 20mg 1/d | 3 |
| Omeprazole 40mg 2/d | 1 |
| Esomeprazole 20mg 2/d | 2 |
| Rabeprazole 10mg 1/d | 1 |
| 6-7 wk | |
| Pantoprazole 40mg 2/d | 1 |
| Pantoprazole 40mg 1/d | 2 |
| Lansoprazole 30mg 2/d | 1 |
| Omeprazole 20mg 2/d | 2 |
| 8-9 wk | |
| Omeprazole 40mg 1/d | 1 |
| Omeprazole 20mg 2/d | 1 |
| Omeprazole 40mg 2/d | 1 |
| Esomeprazole 40mg 2/d | 1 |
| Esomeprazole 40mg 1/d | 2 |
| Esomeprazole 20mg 2/d | 2 |
| Esomeprazole 20mg 1/d | 1 |
| Lansoprazole 30mg 2/d | 1 |
| Lansoprazole 30mg 1/d | 2 |
| Rabeprazole 20mg 1/d | 1 |
| Rabeprazole 20mg 2/d | 1 |
| Rabeprazole 10mg 1/d | 1 |
| Pantoprazole 40mg 1/d | 2 |
| 12 wk | |
| Omeprazole 40mg 1/d | 1 |
| Omeprazole 20mg 3/d | 1 |
| Omeprazole 20mg 2/d | 4 |
| Omeprazole 20mg 1/d | 1 |
| Esomeprazole 20mg 2/d | 3 |
| Esomeprazole 40mg 2/d | 1 |
| Lansoprazole 30mg 2/d | 4 |
| Lansoprazole 15mg 2/d | 1 |
| Rabeprazole 20mg 2/d | 1 |
| Rabeprazole 10mg 1/d | 1 |
| Pantoprazole 20 mg 2/d | 3 |
| Pantoprazole 40 mg 1/d | 1 |
| Pantoprazole 40 mg 2/d | 6 |
| 16-20 wk | |
| Lansoprazole 30 mg 2/d | 2 |
| Esomeprazole 40 mg 2/d | 1 |
| Esomeprazole 40 mg 1/d | 1 |
| Esomeprazole 20 mg 2/d | 1 |
| Rabeprazole 20 mg 2/d | 1 |
| Pantoprazole 40 mg 2/d | 1 |
| Pantoprazole 40 mg 1/d | 1 |
| 24 wk | |
| Omeprazole 20 mg 2/d | 2 |
| Omeprazole 20 mg 1/d | 1 |
| Lansoprazole 30 mg 2/d | 3 |
| Drugs and duration | Study numbers |
| PPIs and antihistamines | |
| Omeprazole 20 mg 2/d and Ranitidine 300 mg/d (16 wk) | 1 |
| Omeprazole 40 mg/d and Ranitidine 300 mg/d (12 wk) | 1 |
| PPIs 40 mg/d and antihistamine 300 mg/d (8 wk) | 1 |
| PPIs and gastroprokinetic | |
| Pantoprazole 40 mg 2/d and Cisapride 20 mg 2/d (4 wk) | 1 |
| Pantoprazole 40 mg 1/d and Itopride 50 mg 3/d (8 wk) | 1 |
| Omeprazole 20 mg 2/d and Domperidone 10 mg 3/d (12 wk) | 1 |
| Lansoprazole 30 mg 1/d and Itopride 50 mg 3/d (12 wk) | 1 |
| Rabeprazole 20 mg/d and Domperidone 30 mg/d (12 wk) | 1 |
| Lansoprazole 30 mg 1/d and Mosapride 5 mg 3/d (20 wk) | 1 |
| PPIs and alginate | |
| PPIs (NA) and gaviscon 4/d (12 wk) | 1 |
| Aligante 3-4/d (8 wk) | 1 |
| Other | |
| Famotidine 20 mg 1/d (8 wk) | 1 |
| Gaviscon 4/d (24 wk) | 1 |
| Gaviscon 4/d (12 wk) | 1 |
LPRD has been defined as a different entity other than GERD in the end of the nineties[84]. Since then, the number of clinical studies dedicated to the treatment of LPRD have progressively increased[1]. This review has shown that the most preferred treatment for LPRD is still the administration of once or twice daily PPIs. This therapeutic approach is however associated with an uncertain success rate and, depending of the therapeutic outcomes used, a significant number of patients are found to be resistant to treatment. According to a recent systematic review, the non-response rate would be close to 40% of patients[85]. The critical analysis of the different therapeutic schemes and their related success rate has to consider the respective pharmacological properties of the drugs used.
PPI decreases the H+ gastric secretion by covalent binding with H+/K+ ATPase. The inhibition of proton pump increases the pH of the gaseous refluxate droplets and limits the extracellular activity of pepsin on upper aerodigestive tract tissues[86]. From a pathophysiological standpoint, PPIs have no impact on the intracellular activity of pepsin[87], and a low impact on the activity of trypsin and non-conjugated bile salts, which could injure the laryngopharyngeal mucosa in a nonacid environment[88,89]. Moreover, the PPI intake does not change the total number of daily reflux episodes[90].
This review shows that the doses and administration frequency of PPIs varies from one to another study. PPIs have a short half-life (90 min) and an oral unique dose of 20 mg inhibits 70% of the pump enzymes[91]. In practice, the half-life of the inhibition of gastric acid secretion lasts an estimated 24 h. Approximately 20% of proton pumps are newly synthesized over a 24-h period with greater pump synthesis at night than during the day. With regard to the 90 min blood half-life of PPI, the addition of bedtime administration will not add to inhibition of nocturnal acid breakthrough, because the drug will have disappeared by the time nighttime acid secretion is evident. Assuming that about 70% of pumps are activated by breakfast and that the PPI is given 30 to 60 min beforehand, it can be calculated that steady state inhibition on once-a-day dosing is about 66% of maximal acid output. In other words, and regarding the pharmacological properties of the drug, increasing the dose has virtually no effect once optimal dosage has been reached. However, increasing the dose frequency does have some effect; a morning dose and an evening dose before meals results in about 80% inhibition of maximal acid output[91,92]. Thus, twice daily PPI could be better because a more complete control of both daytime and nocturnal esophageal acid exposure[93]. In LPRD literature, only the study by Park et al[28] compared once vs twice daily PPIs in LPRD. These authors suggested a superiority of twice daily vs once daily PPI(s), which seems to be in accordance with the pharmacological properties of PPIs[28,93]. Pharmacologically, the use of twice daily 20 mg PPIs could be the most effective approach in order to inhibit the acid secretion but, as mentioned above, this approach has low effect on nonacid or weakly acid LPRD variants.
PPIs have been associated with H2R in four studies[28,66,70,72]. In comparison with twice daily PPIs, the use of H2R does not make sense regarding their short duration of action (6 to 12 h)[94,95]. The studies comparing the efficacy of PPIs vs H2R + PPIs did not report a clinical evidence of the use of H2R in LPRD[28,72]. Moreover, the association of once daily PPI with ranitidine at bedtime being more expensive approach than 6-mo twice daily PPIs[66].
The addition of prokinetics to PPIs is still controversial in GERD[96], despite their role in the increase of the esophageal sphincter pressure[94,97,98]. Six studies showed interest in the role of prokinetics for the management of LPRD[17,38,48,51,57,79] and these authors reported mixed evidence about the superiority of PPIs and prokinetics over PPIs alone[99]. Precisely, two RCTs suggested that the addition of prokinetics to PPI(s) would be associated with better symptom improvement[38,51], while the study by Hunchaisri et al[48] did not find similar findings. The controversy about the efficacy of prokinetics in LPRD illustrates the lack of evidence in the occurrence of esophageal dysmotility disorder in this condition[100,101].
The development of MII-pH led to the identification of new subtypes of LPRD, being acid, weakly acid, mixed and nonacid LPRD. In that way, three recent studies found that the majority of patients have in fact nonacid or mixed LPRD[102-104]. The pathophysiological mechanisms of nonacid and mixed LPRD are still unknown but they could involve the activity of trypsin, conjugated and non-conjugated bile salts in the mucosa of the upper aerodigestive tract[1,105]. Precisely, non-conjugated bile salts and trypsin are effective in pH above 6.0 while conjugated bile salts are more effective in acid environment. Consequently, the use of alginate or magaldrate could make sense in the primary management of LPRD.
Alginates form a raft floating over gastric contents that can be maintained within the stomach for up to 4 h. Gaviscon is endowed with bio-adhesive potential, a property due primarily to its polymer chain length and ionizable groups that provides a protective biofilm on the mucosa of esophagus and, potentially, upper aerodigestive tract[106]. Interestingly, these drugs are able to reduce the number of acid reflux events[94,107].
In practice, McGlashan et al[34] have demonstrated the superiority of alginate over placebo in the treatment of LPRD patients. More recently, Wilkie et al[69] found that a treatment based on the single use of alginate is quite competitive with a treatment combining PPIs and alginate. Our recent results also support that the addition of alginate or magaldrate to PPIs seems to significantly improve symptoms in patients with mixed and nonacid LPRD[102].
Diet and behavioral changes remain the first therapeutic step of the LPRD treatment. Additionally, this approach is the best cost-effective empirical treatment for patients with mild LPRD. In practice, patients who respect diet and behavioral changes have better symptom improvement than those who did not respect diet[108]. Furthermore, recent studies suggested that a well-conducted diet could be as efficiently as PPI treatment[65,70]. Alkaline, protein, low-fat and low-acid diet is effective because these types of foods are well digested, also decreasing the number of transient relaxations of esophageal sphincters and thereby the related number of LPRD episodes.
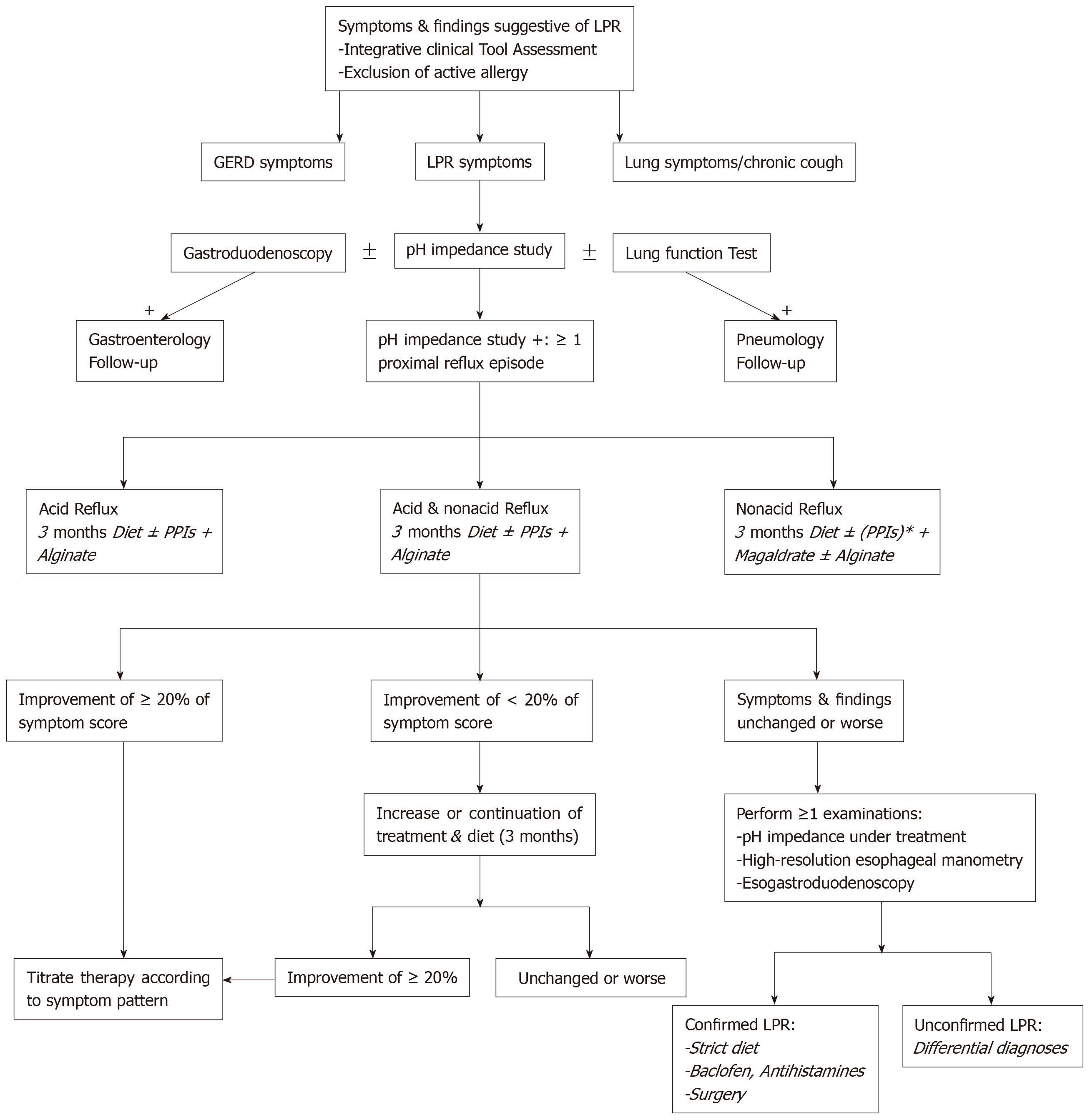
The development of MII-pH as diagnostic tool is an important step in the improvement of daily clinical practices related to LPRD. MII-pH studies showed that there are a large number of patients with nonacid or mixed LPRD, which are both less controlled by conventional PPI therapy. It is highly likely that a significant part of the patients who were called “resistant LPRD patients” within the three last decades, had nonacid or mixed LPRD. With regard to the properties of anti-reflux drugs, alginate is a future candidate as single drug or additional drug to PPIs in the future studies. The concomitant use of twice daily PPIs and twice or thrice daily alginate or magaldrate could provide a consistent protection against the mucosal irritation of pepsin, trypsin and bile salts. Naturally, the administration of diet and behavioral changes is still required in all patients in order to improve the treatment efficacy. According to a recent management algorithm of LPRD (Figure 1)[1], MII-pH testing could be used as diagnostic and therapeutic control tool, providing better identification of the LPRD subtypes and better treatment. Because the compliance of LPRD patients to medical treatment and diet can be poor, the administration of a personalized treatment based on the patient MII-pH results and the lifestyle habits could improve the patient compliance to LPRD treatment.
For a long time, laryngopharyngeal reflux disease (LPRD) has been treated by proton pump inhibitors (PPIs) with an uncertain success rate.
The low success rate of PPIs as well as the cost of unsuccessful empirical therapeutic trials are important in otolaryngology. Many treatments of LPRD exist and we want to provide an analysis of the current therapeutic approach of this prevalent disease.
To shed light the current therapeutic strategies used for LPRD in order to analysis the rationale in the LPRD treatment.
Three authors conducted a PubMed systematic review respecting PRISMA statements.
The majority of studies consists of empirical therapeutic trials using PPIs as single drug. The success rate of PPIs ranges from 18% to 87% and there is an important heterogeneity between studies according to the diagnostic, the therapeutic outcomes and the duration of treatment.
The majority of treatments in LPRD are empirical and based on PPIs. The empirical therapeutic trial with PPIs is however associated with an uncertain success rate.
| 1. | Lechien JR, Akst LM, Hamdan AL, Schindler A, Karkos PD, Barillari MR, Calvo-Henriquez C, Crevier-Buchman L, Finck C, Eun YG, Saussez S, Vaezi MF. Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol Head Neck Surg. 2019;160:762-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 274] [Article Influence: 39.1] [Reference Citation Analysis (35)] |
| 2. | Sen P, Georgalas C, Bhattacharyya AK. A systematic review of the role of proton pump inhibitors for symptoms of laryngopharyngeal reflux. Clin Otolaryngol. 2006;31:20-4; discussion 24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 918] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 4. | Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA. 2005;294:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 341] [Article Influence: 16.2] [Reference Citation Analysis (35)] |
| 5. | Lechien JR, Saussez S, Schindler A, Karkos PD, Hamdan AL, Harmegnies B, De Marrez LG, Finck C, Journe F, Paesmans M, Vaezi MF. Clinical outcomes of laryngopharyngeal reflux treatment: A systematic review and meta-analysis. Laryngoscope. 2019;129:1174-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Lechien JR, Dapri G, Dequanter D, Rodriguez Ruiz A, Marechal MT, De Marrez LG, Saussez S, Fisichella PM. Surgical Treatment for Laryngopharyngeal Reflux Disease: A Systematic Review. JAMA Otolaryngol Head Neck Surg. 2019;145:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | DistillerSR software. Systematic and literature review resources 2011. Available from: http://distillercer.com/resources. |
| 8. | Hanson DG, Kamel PL, Kahrilas PJ. Outcomes of antireflux therapy for the treatment of chronic laryngitis. Ann Otol Rhinol Laryngol. 1995;104:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 120] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Jaspersen D, Weber R, Hammar CH, Draf W. Effect of omeprazole on the course of associated esophagitis and laryngitis. J Gastroenterol. 1996;31:765-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 10. | Shaw GY, Searl JP. Laryngeal manifestations of gastroesophageal reflux before and after treatment with omeprazole. South Med J. 1997;90:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Wo JM, Grist WJ, Gussack G, Delgaudio JM, Waring JP. Empiric trial of high-dose omeprazole in patients with posterior laryngitis: a prospective study. Am J Gastroenterol. 1997;92:2160-2165. [PubMed] |
| 12. | Metz DC, Childs ML, Ruiz C, Weinstein GS. Pilot study of the oral omeprazole test for reflux laryngitis. Otolaryngol Head Neck Surg. 1997;116:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 13. | Habermann W, Eherer A, Lindbichler F, Raith J, Friedrich G. Ex juvantibus approach for chronic posterior laryngitis: results of short-term pantoprazole therapy. J Laryngol Otol. 1999;113:734-739. [PubMed] |
| 14. | Havas T, Huang S, Levy M, Abi-Hanna D, Truskeyy P, Priestley J, Cox J, Wilson J. Posterior pharyngolaryngitis: double-blind randomised placebo-controlled trial of proton pump inhibitor therapy. Aust J Otolaryngol. 1999;3:243–246. |
| 15. | El-Serag HB, Lee P, Buchner A, Inadomi JM, Gavin M, McCarthy DM. Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo-controlled trial. Am J Gastroenterol. 2001;96:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 156] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 16. | Langevin S, Ngo H. GERD-Induced ENT Symptoms: A Prospective Placebo Controlled Study with omeprazole 40 mg a Day. Gastroenterology. 2001;120. [DOI] [Full Text] |
| 17. | Hamdan AL, Sharara AI, Younes A, Fuleihan N. Effect of aggressive therapy on laryngeal symptoms and voice characteristics in patients with gastroesophageal reflux. Acta Otolaryngol. 2001;121:868-872. [PubMed] |
| 18. | Rodríguez-Téllez M, Galera-Ruiz H, Argüelles-Arias F, Carmona I, Muñoz-Borje F, Herrerías JM. Posterior laryngitis: effects of treatment with omeprazole alone. Rev Esp Enferm Dig. 2002;94:123-130. [PubMed] |
| 19. | Habermann W, Kiesler K, Eherer A, Friedrich G. Short-term therapeutic trial of proton pump inhibitors in suspected extraesophageal reflux. J Voice. 2002;16:425-432. [PubMed] |
| 20. | DelGaudio JM, Waring JP. Empiric esomeprazole in the treatment of laryngopharyngeal reflux. Laryngoscope. 2003;113:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Bilgen C, Ogüt F, Kesimli-Dinç H, Kirazli T, Bor S. The comparison of an empiric proton pump inhibitor trial vs 24-hour double-probe Ph monitoring in laryngopharyngeal reflux. J Laryngol Otol. 2003;117:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Garrigues V, Gisbert L, Bastida G, Ortiz V, Bau I, Nos P, Ponce J. Manifestations of gastroesophageal reflux and response to omeprazole therapy in patients with chronic posterior laryngitis: an evaluation based on clinical practice. Dig Dis Sci. 2003;48:2117-2123. [PubMed] |
| 23. | Beaver ME, Stasney CR, Weitzel E, Stewart MG, Donovan DT, Parke RB, Rodriguez M. Diagnosis of laryngopharyngeal reflux disease with digital imaging. Otolaryngol Head Neck Surg. 2003;128:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 24. | Siupsinskiene N, Adamonis K. Diagnostic test with omeprazole in patients with posterior laryngitis. Medicina (Kaunas). 2003;39:47-55. [PubMed] |
| 25. | Williams RB, Szczesniak MM, Maclean JC, Brake HM, Cole IE, Cook IJ. Predictors of outcome in an open label, therapeutic trial of high-dose omeprazole in laryngitis. Am J Gastroenterol. 2004;99:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Issing WJ, Karkos PD, Perreas K, Folwaczny C, Reichel O. Dual-probe 24-hour ambulatory pH monitoring for diagnosis of laryngopharyngeal reflux. J Laryngol Otol. 2004;118:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Sereg-Bahar M, Jansa R, Hocevar-Boltezar I. Voice disorders and gastroesophageal reflux. Logoped Phoniatr Vocol. 2005;30:120-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Park W, Hicks DM, Khandwala F, Richter JE, Abelson TI, Milstein C, Vaezi MF. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope. 2005;115:1230-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Vaezi MF, Richter JE, Stasney CR, Spiegel JR, Iannuzzi RA, Crawley JA, Hwang C, Sostek MB, Shaker R. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope. 2006;116:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 225] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Dore MP, Pedroni A, Pes GM, Maragkoudakis E, Tadeu V, Pirina P, Realdi G, Delitala G, Malaty HM. Effect of antisecretory therapy on atypical symptoms in gastroesophageal reflux disease. Dig Dis Sci. 2007;52:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 31. | Qua CS, Wong CH, Gopala K, Goh KL. Gastro-oesophageal reflux disease in chronic laryngitis: prevalence and response to acid-suppressive therapy. Aliment Pharmacol Ther. 2007;25:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Oridate N, Takeda H, Asaka M, Nishizawa N, Mesuda Y, Mori M, Furuta Y, Fukuda S. Acid-suppression therapy offers varied laryngopharyngeal and esophageal symptom relief in laryngopharyngeal reflux patients. Dig Dis Sci. 2008;53:2033-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Reichel O, Dressel H, Wiederänders K, Issing WJ. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg. 2008;139:414-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | McGlashan JA, Johnstone LM, Sykes J, Strugala V, Dettmar PW. The value of a liquid alginate suspension (Gaviscon Advance) in the management of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol. 2009;266:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Vashani K, Murugesh M, Hattiangadi G, Gore G, Keer V, Ramesh VS, Sandur V, Bhatia SJ. Effectiveness of voice therapy in reflux-related voice disorders. Dis Esophagus. 2010;23:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Fass R, Noelck N, Willis MR, Navarro-Rodriguez T, Wilson K, Powers J, Barkmeier-Kraemer JM. The effect of esomeprazole 20 mg twice daily on acoustic and perception parameters of the voice in laryngopharyngeal reflux. Neurogastroenterol Motil. 2010;22:134-141, e44-e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 37. | Lam PK, Ng ML, Cheung TK, Wong BY, Tan VP, Fong DY, Wei WI, Wong BC. Rabeprazole is effective in treating laryngopharyngeal reflux in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol. 2010;8:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Ezzat WF, Fawaz SA, Fathey H, El Demerdash A. Virtue of adding prokinetics to proton pump inhibitors in the treatment of laryngopharyngeal reflux disease: prospective study. J Otolaryngol Head Neck Surg. 2011;40:350-356. [PubMed] |
| 39. | Chiba T, Kudara N, Abiko Y, Endo M, Suzuki K, Sugai T, Ishijima K, Fukuda K, Yamazaki K, Sato H. Effects of proton pump inhibitors in patients with laryngopharyngeal reflux disease. Hepatogastroenterology. 2011;58:1580-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 40. | Friedman M, Maley A, Kelley K, Pulver T, Foster M, Fisher M, Joseph N. Impact of pH monitoring on laryngopharyngeal reflux treatment: improved compliance and symptom resolution. Otolaryngol Head Neck Surg. 2011;144:558-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Lee YS, Choi SH, Son YI, Park YH, Kim SY, Nam SY. Prospective, observational study using rabeprazole in 455 patients with laryngopharyngeal reflux disease. Eur Arch Otorhinolaryngol. 2011;268:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Masaany M, Marina MB, Sharifa Ezat WP, Sani A. Empirical treatment with pantoprazole as a diagnostic tool for symptomatic adult laryngopharyngeal reflux. J Laryngol Otol. 2011;125:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Naiboglu B, Durmus R, Tek A, Toros SZ, Egeli E. Do the laryngopharyngeal symptoms and signs ameliorate by empiric treatment in patients with suspected laryngopharyngeal reflux? Auris Nasus Larynx. 2011;38:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Patigaroo SA, Hashmi SF, Hasan SA, Ajmal MR, Mehfooz N. Clinical manifestations and role of proton pump inhibitors in the management of laryngopharyngeal reflux. Indian J Otolaryngol Head Neck Surg. 2011;63:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Habermann W, Schmid C, Neumann K, Devaney T, Hammer HF. Reflux symptom index and reflux finding score in otolaryngologic practice. J Voice. 2012;26:e123-e127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 46. | Park JO, Shim MR, Hwang YS, Cho KJ, Joo YH, Cho JH, Nam IC, Kim MS, Sun DI. Combination of voice therapy and antireflux therapy rapidly recovers voice-related symptoms in laryngopharyngeal reflux patients. Otolaryngol Head Neck Surg. 2012;146:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 47. | Becker V, Graf S, Schlag C, Schuster T, Feussner H, Schmid RM, Bajbouj M. First agreement analysis and day-to-day comparison of pharyngeal pH monitoring with pH/impedance monitoring in patients with suspected laryngopharyngeal reflux. J Gastrointest Surg. 2012;16:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Hunchaisri N. Treatment of laryngopharyngeal reflux: a comparison between domperidone plus omeprazole and omeprazole alone. J Med Assoc Thai. 2012;95:73-80. [PubMed] |
| 49. | Chung JW, Chun HJ, Lee MS, Ahn KR, Kim CS, Kang KS, Yoo SH, Chung JH, Kim NS, Seo YH, Gong HY, Lee YM. Effect of stellate ganglion block on laryngopharyngeal reflux disease. Korean J Anesthesiol. 2013;64:439-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Oridate N, Tokashiki R, Watanabe Y, Taguchi A, Kawamura O, Fujimoto K. Endoscopic laryngeal findings in Japanese patients with laryngopharyngeal reflux symptoms. Int J Otolaryngol. 2012;2012:908154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Chun BJ, Lee DS. The effect of itopride combined with lansoprazole in patients with laryngopharyngeal reflux disease. Eur Arch Otorhinolaryngol. 2013;270:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Beech TJ, Campbell G, McDermott AL, Batch AJ. The effect of anti-reflux treatment on subjective voice measurements of patients with laryngopharyngeal reflux. J Laryngol Otol. 2013;127:590-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Vailati C, Mazzoleni G, Bondi S, Bussi M, Testoni PA, Passaretti S. Oropharyngeal pH monitoring for laryngopharyngeal reflux: is it a reliable test before therapy? J Voice. 2013;27:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Lee JS, Lee YC, Kim SW, Kwon KH, Eun YG. Changes in the quality of life of patients with laryngopharyngeal reflux after treatment. J Voice. 2014;28:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Chappity P, Kumar R, Deka RC, Chokkalingam V, Saraya A, Sikka K. Proton Pump Inhibitors Versus Solitary Lifestyle Modification in Management of Laryngopharyngeal Reflux and Evaluating Who is at Risk: Scenario in a Developing Country. Clin Med Insights Ear Nose Throat. 2014;7:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Wan Y, Yan Y, Ma F, Wang L, Lu P, Maytag A, Jiang JJ. LPR: how different diagnostic tools shape the outcomes of treatment. J Voice. 2014;28:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 57. | Semmanaselvan K, Mukaddam QI, Naik M. An Open Label, Prospective, Single Centre Study to Evaluate the Efficacy and Safety of Fixed Dose Combination of Rabeprazole (Enteric-Coated, EC) 20 mg + Domperidone (Sustained Release, SR) 30 mg Capsule in Treatment of Patients with Laryngopharyngeal Reflux Disease. J Assoc Physicians India. 2015;63:27-32. [PubMed] |
| 58. | Ozturan O, Dogan R, Yenigun A, Veyseller B, Yildirim YS. Photographic Objective Alterations for Laryngopharyngeal Reflux Diagnosis. J Voice. 2017;31:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Gupta N, Green RW, Megwalu UC. Evaluation of a laryngopharyngeal reflux management protocol. Am J Otolaryngol. 2016;37:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Nennstiel S, Andrea M, Abdelhafez M, Haller B, Schmid RM, Bajbouj M, Becker V. pH/multichannel impedance monitoring in patients with laryngo-pharyngeal reflux symptoms - Prediction of therapy response in long-term follow-up. Arab J Gastroenterol. 2016;17:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 61. | Batıoğlu-Karaaltın A, Develioğlu ÖN, Tarhan Ö, Külekçi M. The importance of voice analysis in evaluating the effectiveness of reflux treatment. Kulak Burun Bogaz Ihtis Derg. 2016;26:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Dulery C, Lechot A, Roman S, Bastier PL, Stoll D, de Gabory L, Zerbib F. A study with pharyngeal and esophageal 24-hour pH-impedance monitoring in patients with laryngopharyngeal symptoms refractory to proton pump inhibitors. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 63. | Joshi AA, Chiplunkar BG, Bradoo RA. Assessment of treatment response in patients with laryngopharyngeal reflux. Indian J Otolaryngol Head Neck Surg. 2017;69:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Pullarat AN, Attakkil A, Sibin. MA, Nayak DR, Pillai S. Hoarseness and Laryngopharyngeal Reflux: A Prospective Study. Iosr J Dent Med Sci. 2017;16:41-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Zalvan CH, Hu S, Greenberg B, Geliebter J. A Comparison of Alkaline Water and Mediterranean Diet vs Proton Pump Inhibition for Treatment of Laryngopharyngeal Reflux. JAMA Otolaryngol Head Neck Surg. 2017;143:1023-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 66. | Carroll TL, Werner A, Nahikian K, Dezube A, Roth DF. Rethinking the laryngopharyngeal reflux treatment algorithm: Evaluating an alternate empiric dosing regimen and considering up-front, pH-impedance, and manometry testing to minimize cost in treating suspect laryngopharyngeal reflux disease. Laryngoscope. 2017;127 Suppl 6:S1-S13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Lechien JR, Finck C, Khalife M, Huet K, Delvaux V, Picalugga M, Harmegnies B, Saussez S. Change of signs, symptoms and voice quality evaluations throughout a 3- to 6-month empirical treatment for laryngopharyngeal reflux disease. Clin Otolaryngol. 2018;43:1273-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 68. | Mozzanica F, Robotti C, Lechien JR, Pizzorni N, Pirola F, Mengucci A, Dell'Era A, Ottaviani F, Schindler A. Vocal Tract Discomfort and Dysphonia in Patients Undergoing Empiric Therapeutic Trial with Proton Pump Inhibitor for Suspected Laryngopharyngeal Reflux. J Voice. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Wilkie MD, Fraser HM, Raja H. Gaviscon® Advance alone versus co-prescription of Gaviscon® Advance and proton pump inhibitors in the treatment of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol. 2018;275:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 70. | Yang J, Dehom S, Sanders S, Murry T, Krishna P, Crawley BK. Treating laryngopharyngeal reflux: Evaluation of an anti-reflux program with comparison to medications. Am J Otolaryngol. 2018;39:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 71. | Kirti YK. Reflux Finding Score (RFS) a Quantitative Guide for Diagnosis and Treatment of Laryngopharyngeal Reflux. Indian J Otolaryngol Head Neck Surg. 2018;70:362-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Suzuki M, Yokota M, Matsumoto T, Nakayama M, Takemura M, Kanemitsu Y, Niimi A, Nakamura Y, Murakami S. Proton Pump Inhibitor Ameliorates Taste Disturbance among Patients with Laryngopharyngeal Reflux: A Randomized Controlled Study. Tohoku J Exp Med. 2019;247:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 73. | Noordzij JP, Khidr A, Desper E, Meek RB, Reibel JF, Levine PA. Correlation of pH probe-measured laryngopharyngeal reflux with symptoms and signs of reflux laryngitis. Laryngoscope. 2002;112:2192-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 74. | Belafsky PC, Postma GN, Koufman JA. The validity and reliability of the reflux finding score (RFS). Laryngoscope. 2001;111:1313-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 705] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 75. | Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. 2002;16:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1030] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 76. | Eherer AJ, Habermann W, Hammer HF, Kiesler K, Friedrich G, Krejs GJ. Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol. 2003;38:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 77. | Steward DL, Wilson KM, Kelly DH, Patil MS, Schwartzbauer HR, Long JD, Welge JA. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg. 2004;131:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 78. | Wo JM, Koopman J, Harrell SP, Parker K, Winstead W, Lentsch E. Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol. 2006;101:1972-8; quiz 2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Jin bJ, Lee YS, Jeong SW, Jeong JH, Lee SH, Tae K, Chang CS, Liang WM. Change of acoustic parameters before and after treatment in laryngopharyngeal reflux patients. Laryngoscope. 2008;118:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 80. | Lien HC, Wang CC, Lee SW, Hsu JY, Yeh HZ, Ko CW, Chang CS, Liang WM. Responder Definition of a Patient-Reported Outcome Instrument for Laryngopharyngeal Reflux Based on the US FDA Guidance. Value Health. 2015;18:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Waxman J, Yalamanchali S, Valle ES, Pott T, Friedman M. Effects of Proton Pump Inhibitor Therapy for Laryngopharyngeal Reflux on Posttreatment Symptoms and Hypopharyngeal pH. Otolaryngol Head Neck Surg. 2014;150:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Tseng WH, Tseng PH, Wu JF, Hsu YC, Lee TY, Ni YH, Wang HP, Hsiao TY, Hsu WC. Double-blind, placebo-controlled study with alginate suspension for laryngopharyngeal reflux disease. Laryngoscope. 2018;128:2252-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 83. | Agrawal N, Yadlapati R, Shabeeb N, Price CP, Lidder A, Shintani-Smith S, Bové M, Pandolfino J, Tan B. Relationship between extralaryngeal endoscopic findings, proton pump inhibitor (PPI) response, and pH measures in suspected laryngopharyngeal reflux. Dis Esophagus. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Koufman J, Sataloff RT, Toohill R. Laryngopharyngeal reflux: consensus conference report. J Voice. 1996;10:215-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 151] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Lechien JR, Muls V, Dapri G, Mouawad F, Eisendrath P, Schindler A, Nacci A, Barillari MR, Finck C, Saussez S, Akst LM, Sataloff RT. The management of suspected or confirmed laryngopharyngeal reflux patients with recalcitrant symptoms: A contemporary review. Clin Otolaryngol. 2019;44:784-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 86. | Savarino E, de Bortoli N, Zentilin P, Martinucci I, Bruzzone L, Furnari M, Marchi S, Savarino V. Alginate controls heartburn in patients with erosive and nonerosive reflux disease. World J Gastroenterol. 2012;18:4371-4378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Johnston N, Dettmar PW, Ondrey FG, Nanchal R, Lee SH, Bock JM. Pepsin: biomarker, mediator, and therapeutic target for reflux and aspiration. Ann N Y Acad Sci. 2018;1434:282-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 88. | Johnston N, Ondrey F, Rosen R, Hurley BP, Gould J, Allen J, DelGaudio J, Altman KW. Airway reflux. Ann N Y Acad Sci. 2016;1381:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 89. | Johnston N, Wells CW, Samuels TL, Blumin JH. Pepsin in nonacidic refluxate can damage hypopharyngeal epithelial cells. Ann Otol Rhinol Laryngol. 2009;118:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 90. | Vela MF, Camacho-Lobato L, Srinivasan R, Tutuian R, Katz PO, Castell DO. Simultaneous intraesophageal impedance and pH measurement of acid and nonacid gastroesophageal reflux: effect of omeprazole. Gastroenterology. 2001;120:1599-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 426] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 91. | Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534 [DOI: 10.1007/s11894-008-0098-4]. |
| 92. | Lew EA. Review article: pharmacokinetic concerns in the selection of anti-ulcer therapy. Aliment Pharmacol Ther. 1999;13 Suppl 5:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Savarino E, Zentilin P, Marabotto E, Bodini G, Della Coletta M, Frazzoni M, de Bortoli N, Martinucci I, Tolone S, Pellegatta G, Savarino V. A review of pharmacotherapy for treating gastroesophageal reflux disease (GERD). Expert Opin Pharmacother. 2017;18:1333-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 94. | Castell DO. Rationale for high-dose H2-receptor blockade in the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1991;5 Suppl 1:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 95. | Postma GN, Johnson LF, Koufman JA. Treatment of laryngopharyngeal reflux. Ear Nose Throat J. 2002;81:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 96. | Ren LH, Chen WX, Qian LJ, Li S, Gu M, Shi RH. Addition of prokinetics to PPI therapy in gastroesophageal reflux disease: a meta-analysis. World J Gastroenterol. 2014;20:2412-2419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 97. | Mikami H, Ishimura N, Fukazawa K, Okada M, Izumi D, Shimura S, Okimoto E, Aimi M, Ishihara S, Kinoshita Y. Effects of Metoclopramide on Esophageal Motor Activity and Esophagogastric Junction Compliance in Healthy Volunteers. J Neurogastroenterol Motil. 2016;22:112-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 98. | Quigley EM. Cisapride: what can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011;12:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Glicksman JT, Mick PT, Fung K, Carroll TL. Prokinetic agents and laryngopharyngeal reflux disease: Prokinetic agents and laryngopharyngeal reflux disease: a systematic review. Laryngoscope. 2014;124:2375-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 100. | Vardar R, Sweis R, Anggiansah A, Wong T, Fox MR. Upper esophageal sphincter and esophageal motility in patients with chronic cough and reflux: assessment by high-resolution manometry. Dis Esophagus. 2013;26:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Benjamin T, Zackria S, Lopez R, Richter J, Thota PN. Upper esophageal sphincter abnormalities and high-resolution esophageal manometry findings in patients with laryngopharyngeal reflux. Scand J Gastroenterol. 2017;52:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Lechien JR, Bobin F, Muls V, Thill MP, Horoi M, Ostermann K, Huet K, Harmegnies B, Dequanter D, Dapri G, Maréchal MT, Finck C, Rodriguez Ruiz A, Saussez S. Validity and reliability of the reflux symptom score. Laryngoscope. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 103. | Lee JS, Jung AR, Park JM, Park MJ, Lee YC, Eun YG. Comparison of Characteristics According to Reflux Type in Patients With Laryngopharyngeal Reflux. Clin Exp Otorhinolaryngol. 2018;11:141-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (35)] |
| 104. | Lee YC, Kwon OE, Park JM, Eun YG. Do laryngoscopic findings reflect the characteristics of reflux in patients with laryngopharyngeal reflux? Clin Otolaryngol. 2018;43:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Sereg-Bahar M, Jerin A, Jansa R, Stabuc B, Hocevar-Boltezar I. Pepsin and bile acids in saliva in patients with laryngopharyngeal reflux - a prospective comparative study. Clin Otolaryngol. 2015;40:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 106. | Woodland P, Lee C, Duraisamy Y, Farré R, Dettmar P, Sifrim D. Assessment and protection of esophageal mucosal integrity in patients with heartburn without esophagitis. Am J Gastroenterol. 2013;108:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 107. | Zentilin P, Dulbecco P, Savarino E, Parodi A, Iiritano E, Bilardi C, Reglioni S, Vigneri S, Savarino V. An evaluation of the antireflux properties of sodium alginate by means of combined multichannel intraluminal impedance and pH-metry. Aliment Pharmacol Ther. 2005;21:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 108. | Lechien JR, Huet K, Khalife M, De Marrez LG, Finck C, Harmegnies B, Saussez S. Alkaline, protein, low-fat and low-acid diet in laryngopharyngeal reflux disease: Our experience on 65 patients. Clin Otolaryngol. 2019;44:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Belgium
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciuman RR, Noussios GI S-Editor: Dou Y L-Editor: A E-Editor: Qi LL