Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2542
Peer-review started: May 21, 2019
First decision: July 30, 2019
Revised: August 11, 2019
Accepted: August 20, 2019
Article in press: August 20, 2019
Published online: September 6, 2019
Processing time: 116 Days and 3.9 Hours
Ovarian tumors are common gynecological diseases in children, and the most commonly seen ovarian tumors are germ cell tumors. Robotic surgery is the new access for children ovarian tumors.
From June to October 2017, 4 children with ovarian tumors were admitted and treated in the Department of Pediatric Surgery of People’s Liberation Army General Hospital. The mean age, height, and weight of these patients were 7.5 (1-13) years old, 123.75 (71-164) cm, and 36.8 (8.5-69.5) kg, respectively. Robotic-assisted resection of ovarian tumors was performed for all 4 patients. The 3-port approach was used for robotic manipulation. The surgical procedures were as follows. After creation of the pneumoperitoneum, the robotic scope was placed to explore and find the left ovarian tumor. The trocars for robotic arms 1 and 2 were placed at the sites to the lower right and left of the port of the scope. The tumor capsule in the fallopian tube was incised, and the tumor was completely stripped by an electric hook along the junction of the tumor and the capsule. The resected tumor was completely removed using an endobag. The average docking time of the robotic system was 18.5 min, the average operative time was 120 min, and the average blood loss was 20 mL. No drainage tube was placed except in one patient with a mucinous tumor of the ovary. No fever, pelvic fluid, or intestinal obstruction was reported after surgery. No antibiotics were used during the perioperative period, and the average length of hospital stay after surgery was 3 d.
Robotic-assisted resection of ovarian tumors is a simple, safe, and effective surgical procedure for selected patients.
Core tip: Ovarian tumors are common gynecological diseases in children, and 4 children with ovarian tumors were treated using a robotic surgical system in the Department of Pediatric Surgery of People's Liberation Army General Hospital. There are no reports available on the use of robotic surgery systems to treat ovarian tumors in children in China. We think that robotic-assisted resection of ovarian tumors in children is feasible and promising.
- Citation: Xie XX, Wang N, Wang ZH, Zhu YY, Wang JR, Wang XQ. Robotic-assisted resection of ovarian tumors in children: A case report and review of literature. World J Clin Cases 2019; 7(17): 2542-2548
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2542
Ovarian tumors are common gynecological diseases in children, and the most commonly seen ovarian tumors are germ cell tumors[1]. Ovarian mature cystic teratomas, also known as dermoid cysts, are the most common germ cell tumor, and they are also the most common benign tumors of the ovary[2]. Malignant germ cell tumors are relatively rare, but these malignant tumors usually a have high degree of malignancy. The proportion of malignancy is negatively associated with the age of the child (younger patients often have a greater likelihood of having malignant tumors)[3].
With the rapid development of minimally invasive surgery in recent years, robotic surgery systems have been widely used in many surgical procedures in adults[4-8]. However, due to the large age differences between pediatric patients, robotic surgery for children remains in the exploratory stage.
No reports are available on the use of robotic surgery systems to treat ovarian tumors in children in China. However, foreign medical centers have reported their experience in this field[9]. From June to October 2017, 4 children with ovarian tumors were treated using a robotic surgical system in the Department of Pediatric Surgery of People’s Liberation Army General Hospital. This study retrospectively analyzed the clinical data and surgical procedures of these patients and aimed to explore the feasibility and safety of robotic surgery systems in children with ovarian tumors, as well as to provide preliminary experience with its clinical application.
Four children were admitted to the hospital with mass in lower abdomen.
The mean age, height, and weight of these patients were 7.5 (1-13) years old, 123.75 (71-164) cm, and 36.8 (8.5-69.5) kg, respectively. The Basic data of patients see Table 1.
| Case | Age | Height | Weight | Tumor | Side | Pathology |
| cm | kg | cm | ||||
| 1 | 8 | 120 | 22.3 | 13.4 | Right | Ovarian mature cystic teratoma |
| 2 | 1 | 71 | 8.5 | 5.4 | Right | Ovarian mature cystic teratoma |
| 3 | 13 | 164 | 69.5 | 21 | Left | Mucinous tumor of the ovary |
| 4 | 8 | 140 | 47 | 11.6 | Right | Ovarian teratoma |
The patient exhibited mass in lower abdomen.
Ultrasonography revealed cystic mass in the lower abdomen.
Ovarian tumors.
For the treatment of ovarian tumors, Robotic-assisted resection was performed under general anesthesia. All of the patients were indicated for robotic surgery without contraindications. And the patients were fully informed and signed an informed consent form.
Routine bowel preparation was performed before surgery. Total intravenous anesthesia was administered with tracheal intubation. The end-tidal CO2 concentration was conventionally monitored. The patient was placed in the supine position and was restrained with tape or bandages. The direct trocar entry technique was used to create the pneumoperitoneum. The conventional CO2 pneumoperi-toneum pressure was maintained at 8 mm-10 mm Hg, but the recommended pressure was 6 mm-8 mm Hg in the neonates. The upper limit of pneumoperitoneum pressure was used to create the pneumoperitoneum to fully expose the abdominal cavity. After introducing robotic instruments, the pressure was reduced to the lower limit, and excellent exposure was achieved.
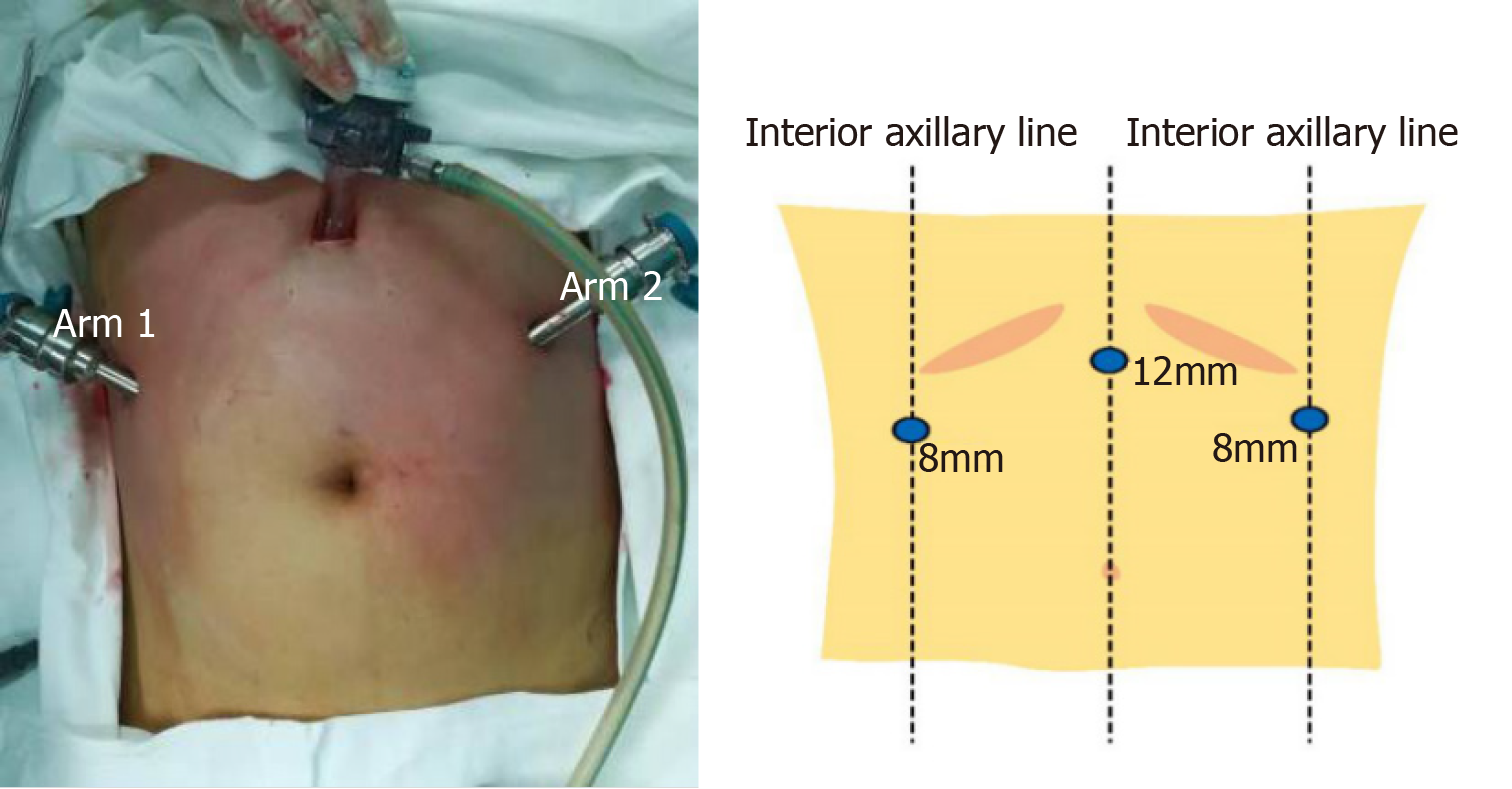
A 12 mm trocar was directly inserted into the abdominal cavity at a site 5 cm to 10 cm above the umbilicus to create a pneumoperitoneum with placement of the robotic scope. An 8 mm trocar was placed at a site 1 cm below the right costal margin in the anterior axillary line and was used for arm 1 (Figure 1). An 8 mm trocar was placed at a site 1 cm below the left costal margin in the anterior axillary line and used for arm 2 (Figure 1).
Preparation of the robotic surgical system: First, the surgical robotic arms of the robotic surgery system were placed on the patient’s leg side, and the connection between the surgical robotic arms and the trocar is described as follows: (1) The robotic arm of the scope was connected to the 12 mm trocar above the umbilicus, and the robotic scope was inserted; (2) The two robotic arms were connected to the left and right 8 mm trocars, respectively. A monopolar electrocoagulation hook or a robotic ultrasonic knife was mounted on one side, and a bipolar electrocoagulation hook was mounted on the other side.
After successful anesthesia, the anesthesiologist placed the radial artery catheter. The urinary catheter was placed. The patient was placed in the lithotomy position with the feet elevated above the head. The operative field was routinely disinfected with iodine and draped. The pneumoperitoneum needle was inserted above the umbilicus to create a pneumoperitoneum with pressure of 13 cm H2O. A 12 mm trocar and the robotic scope were placed. The exploration showed that the diameter of the left ovarian tumor was approximately 10 cm, and the right ovary was unremarkable. No pelvic or abdominal metastasis or malignant ascites was observed. The trocars for robotic arms 1 and 2 were placed to the lower right and lower left to the port of the scope, respectively. The robotic scope and the instrument arm were inserted and connected from the foot side.
The tumor capsule on the fallopian tube was circularly incised at a site approximately 3 cm from the infundibulum of the fallopian tube, and the tumor was completely stripped with an electric hook along the junction of the tumor and the capsule. The examination was repeated to confirm no active bleeders in the fallopian tube stump and residual ovarian capsule. Robotic arms 1 and 2 were withdrawn. The incision for arm 1 was extended. The resected tumor of the left ovary was placed in an endobag, in which the tumor was punctured to rupture. Clear liquid flowed out, and hair and fat were visible inside. The resected tumor was completely removed using an endobag.
The procedure went well. The patient returned to the ward after surgery. No blood transfusion was needed.
All 4 patients successfully underwent the robotic-assisted surgery, and there was no conversion to open surgery. The average docking time of the robotic system was 18.5 min, the average operative time was 120 min, and the average blood loss was 20 mL. No drainage tubes were placed except in a patient with a mucinous tumor of the ovary.
On the first day after surgery, the patients were allowed to ambulate and were provided with a liquid diet. On the second day, the patients were offered a semiliquid diet and were allowed to perform normal daily activities. No fever, pelvic fluid, or intestinal obstructions were reported after surgery. No antibiotics were used, and no complications (Clavien III or higher) occurred. The average length of hospital stay after surgery was 3 d.
No serious complications or recurrences were observed after the 6-mo follow-up.
In 2005, the United States Food and Drug Administration approved robotic surgery systems for gynecological surgery. Over the past 10 years, robotic surgery systems have been widely used in the surgical treatment of adult gynecologic tumors[10-12]. Robotic gynecological surgery in the treatment of benign and malignant gynecological tumors has been highly praised by gynecologists at home and abroad[13-16]. Robotic surgery in children was first used in urology[17], and since then, an increasing number of surgeons have reported the use of robotic surgery in various fields of pediatric surgery.
In this group of patients, the robotic arms were used to accurately complete exposure of the ovary, dissection of blood vessels, resection of the tumor, hemostasis of the wound, and removal of the specimen. During stripping of the cysts, the cysts were not ruptured in the 4 patients, and the blood loss was small. The morphology of the ovary was maintained, which is beneficial to the recovery of postoperative ovarian function.
In gynecological treatment, laparoscopic ovarian cystectomy is one of the most widely used procedures[18]. Laparoscopic ovarian cystectomy is a toilless procedure for separation of the tumor, and surgeons have sufficient understanding of the anatomy. Therefore, laparoscopic ovarian cystectomy is an ideal procedure for using robotic surgery systems. In this study, the procedures were performed over a period of 4 months. In these 4 procedures, the docking time of the robotic system and the operative time of the robotic surgery decreased as the surgeons accumulated more experience. The procedure was prolonged in a few cases, but a prolonged operation time can be accepted, considering the benefits of minimally invasive surgery. Some studies have shown that[19-21], in the treatment of complex gynecological diseases, the learning curve with robotic surgery is shorter and easier than that with laparoscopic surgery, and the operator can complete the surgical task quickly and accurately.
We summarize the experience of using the Da Vinci system in pediatric gynecological surgery as follows. First, Chang et al[22] reported that the successful operation with robotic surgery requires four elements: A good understanding of the surgical procedure, superb surgical skills and frequent training, teamwork, and trocar placement. We agree with this statement and suggest that the most critical step is the placement of trocars, especially for children. In the early stage of this study, we noted that improper placement of the trocars would limit robotic manipulation in the abdominal cavity and increase the chances of instrument conflicts due to the small surface area of the abdominal wall of children and the relatively small space in the abdominal cavity. Therefore, the rational placement of the trocars is essential for a smooth operation. Second, unlike adult gynecological surgery, in which 4 robotic arms can be used, surgeons often only use 3 robotic arms in the majority of pediatric gynecological surgeries. Most pediatric gynecological surgeries can be completed using two operating arms. If the fourth arm is added, it will require more space and interfere with the operation of other arms in the pelvis, thereby increasing difficulty of the manipulation of the other arms. We suggest that, according to the experience of Finkelstein et al[23], the distance between the anterior superior iliac spine (> 13 cm) should first be evaluated before surgery to confirm that there is sufficient space to operate smoothly. Third, the standard operation of the robotic surgical system is to insert the trocar for the scope first and then insert the trocars for the robotic arms. The improvement that we made is to insert the trocars for the robotic arms on both sides first and then insert the trocar for the scope. This operation order is simple, safe, and consistent with the experience in previous reports. Fourth, children’s abdominal walls are relatively thin and mobile. In the first few cases in this study, trocar detachment occurred and affected the operation of robotic surgery. The experience in some centers is to directly suture the trocar to the abdominal wall. We do not usually use this method. According to the position and degree of movement of the robotic arm, the position of the trocar’s remote center is adjusted appropriately. The trocar should be placed in the abdominal cavity deeply or at least reach the remote center if the robotic arm needs to be moved in a large range, or it should reach the edge of the remote center if the robotic arm requires moving in a small range. Fifth, less pressure for the pneumoperitoneum was required for children’s robotic surgery than with conventional laparoscopic surgery to achieve the same exposure effect. The main reason is that the abdominal wall can be retracted outward by the robotic arm to enlarge the space in the abdominal cavity. The principle of this method is to create a pneumoperitoneum with the abdominal wall hanging up. Therefore, the requirements of the body position are not as strict as those of conventional laparoscopic surgery[22].
There have been some controversies with robotic surgery in children. The population is relatively smaller in children than in adults. The number of accumulated procedures in children is difficult to compare with that in adults, which could lead to an imbalance between the efficiency and the cost. Cundy et al[24] suggested that robotic surgery in children is driven more by technology and industry than by clinical demand. Currently, there is no literature directly demonstrating the applicability of robotic surgery in children. As Peters[25] commented, “similar to children, new technologies, do not emerge in a mature form but, rather, require time for development and refinement”.
Robotic surgery is undoubtedly a trend in minimally invasive surgery, and its emergence is not limited to itself. With the emergence of robotic surgery, a series of technological innovations will follow. Pediatric surgery-specific robotic techniques are on the rise, and the pediatric cardiac bioengineering laboratory led by Damian et al[26] is working on the invention of a small implantable robot; microrobots and nanorobots will be able to enter the human body to complete surgical tasks under wireless control without any awareness of patients[27].
Robotic-assisted resection of ovarian tumors is a simple, safe, and effective surgical procedure for selected pediatric patients. In centers where robotic surgery services are newly available, robotic-assisted resection of ovarian tumors is a suitable entry-level procedure.
| 1. | Vaysse C, Delsol M, Carfagna L, Bouali O, Combelles S, Lemasson F, Le Mandat A, Castex MP, Pasquet M, Moscovici J, Guitard J, Pienkowski C, Rubie H, Galinier P, Vaysse P. Ovarian germ cell tumors in children. Management, survival and ovarian prognosis. A report of 75 cases. J Pediatr Surg. 2010;45:1484-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21:475-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 320] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 3. | Kokoska ER, Keller MS, Weber TR. Acute ovarian torsion in children. Am J Surg. 2000;180:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 144] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Yao Y. Current status and prospects of robotic surgery for gynecologic tumor. Zhongguofuqiangjing Waike Zazhi: Electronic Edition. 2013;6:330-333. |
| 5. | Tseng SI, Huang CW, Huang TY. Robotic-assisted transanal repair of rectourethral fistula. Endoscopy. 2019;51:E96-E97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Tinelli A, Malvasi A, Gustapane S, Buscarini M, Gill IS, Stark M, Nezhat FR, Mettler L. Robotic assisted surgery in gynecology: current insights and future perspectives. Recent Pat Biotechnol. 2011;5:12-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Marengo F, Larraín D, Babilonti L, Spinillo A. Learning experience using the double-console da Vinci surgical system in gynecology: a prospective cohort study in a University hospital. Arch Gynecol Obstet. 2012;285:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Wong J, Murtaugh T, Lakra A, Cooper HJ, Shah RP, Geller JA. Robotic-assisted unicompartmental knee replacement offers no early advantage over conventional unicompartmental knee replacement. Knee Surg Sports Traumatol Arthrosc. 2019;27:2303-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Nakib G, Calcaterra V, Scorletti F, Romano P, Goruppi I, Mencherini S, Avolio L, Pelizzo G. Robotic assisted surgery in pediatric gynecology: promising innovation in mini invasive surgical procedures. J Pediatr Adolesc Gynecol. 2013;26:e5-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Matanes E, Lauterbach R, Boulus S, Amit A, Lowenstein L. Robotic laparoendoscopic single-site surgery in gynecology: A systematic review. Eur J Obstet Gynecol Reprod Biol. 2018;231:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Aloisi A, Tseng JH, Sandadi S, Callery R, Feinberg J, Kuhn T, Gardner GJ, Sonoda Y, Brown CL, Jewell EL, Barakat RR, Leitao MM. Is Robotic-Assisted Surgery Safe in the Elderly Population? An Analysis of Gynecologic Procedures in Patients ≥ 65 Years Old. Ann Surg Oncol. 2019;26:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Das D, Propst K, Wechter ME, Kho RM. Evaluation of Positioning Devices for Optimization of Outcomes in Laparoscopic and Robotic-Assisted Gynecologic Surgery. J Minim Invasive Gynecol. 2019;26:244-252.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Yao Y, Li X, Yang Y, Liu Z, Yan H, Yan Z, Chen L, Wang J. [Robotic surgery in the management of early ovarian malignancy tumors]. Zhonghua Fu Chan Ke Za Zhi. 2015;50:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Zanagnolo V, Garbi A, Achilarre MT, Minig L. Robot-assisted Surgery in Gynecologic Cancers. J Minim Invasive Gynecol. 2017;24:379-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Conrad LB, Ramirez PT, Burke W, Naumann RW, Ring KL, Munsell MF, Frumovitz M. Role of Minimally Invasive Surgery in Gynecologic Oncology: An Updated Survey of Members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Gala RB, Margulies R, Steinberg A, Murphy M, Lukban J, Jeppson P, Aschkenazi S, Olivera C, South M, Lowenstein L, Schaffer J, Balk EM, Sung V; Society of Gynecologic Surgeons Systematic Review Group. Systematic review of robotic surgery in gynecology: robotic techniques compared with laparoscopy and laparotomy. J Minim Invasive Gynecol. 2014;21:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Heller K, Gutt C, Schaeff B, Beyer PA, Markus B. Use of the robot system Da Vinci for laparoscopic repair of gastro-oesophageal reflux in children. Eur J Pediatr Surg. 2002;12:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Raicevic M, Saxena AK. Review of Laparoscopic Management of Mature Cystic Teratoma of Ovaries in Children. J Indian Assoc Pediatr Surg. 2019;24:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 1216] [Article Influence: 121.6] [Reference Citation Analysis (0)] |
| 20. | Hassan SO, Dudhia J, Syed LH, Patel K, Farshidpour M, Cunningham SC, Kowdley GC. Conventional Laparoscopic vs Robotic Training: Which is Better for Naive Users? A Randomized Prospective Crossover Study. J Surg Educ. 2015;72:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Moore LJ, Wilson MR, Waine E, Masters RS, McGrath JS, Vine SJ. Robotic technology results in faster and more robust surgical skill acquisition than traditional laparoscopy. J Robot Surg. 2015;9:67-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Chang C, Steinberg Z, Shah A, Gundeti MS. Patient positioning and port placement for robot-assisted surgery. J Endourol. 2014;28:631-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Finkelstein JB, Levy AC, Silva MV, Murray L, Delaney C, Casale P. How to decide which infant can have robotic surgery? Just do the math. J Pediatr Urol. 2015;11:170.e1-170.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Cundy TP, Marcus HJ, Hughes-Hallett A, Khurana S, Darzi A. Robotic surgery in children: adopt now, await, or dismiss? Pediatr Surg Int. 2015;31:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Peters CA. Pediatric robotic-assisted surgery: too early an assessment? Pediatrics. 2009;124:1680-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Damian DD, Arabagi S, Fabozzo A, Ngo P, Jennings R, Manfredi M, Dupont PE. Robotic implant to apply tissue traction forces in the treatment of esophageal atresia. ICRA. 2014;786-782. [DOI] [Full Text] |
| 27. | Bergeles C, Yang GZ. From passive tool holders to microsurgeons: safer, smaller, smarter surgical robots. IEEE Trans Biomed Eng. 2014;61:1565-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma RA, Mayer RJ, Yoon DH S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Qi LL