Published online Sep 6, 2019. doi: 10.12998/wjcc.v7.i17.2450
Peer-review started: April 15, 2019
First decision: May 31, 2019
Revised: July 11, 2019
Accepted: July 20, 2019
Article in press: July 20, 2019
Published online: September 6, 2019
Processing time: 146 Days and 4.4 Hours
Transjugular intrahepatic portosystemic shunt (TIPS) is widely accepted as an alternative to surgery for management of complications of portal hypertension. TIPS has been used to treat portal vein thrombosis (PVT) in many centers since the 1990s. Although TIPS has good therapeutic effects on the formation of PVT, the effect of PVT on TIPS stenting has rarely been reported. Patients with splenectomy and pericardial devascu-larization have a high incidence of PVT, which can markedly affect TIPS stent patency and increase the risk of recurrent symptoms associated with shunt stenosis or occlusion.
To investigate the incidence of PVT after splenectomy and its influence on the patency rate of TIPS in patients with cirrhosis and portal hypertension.
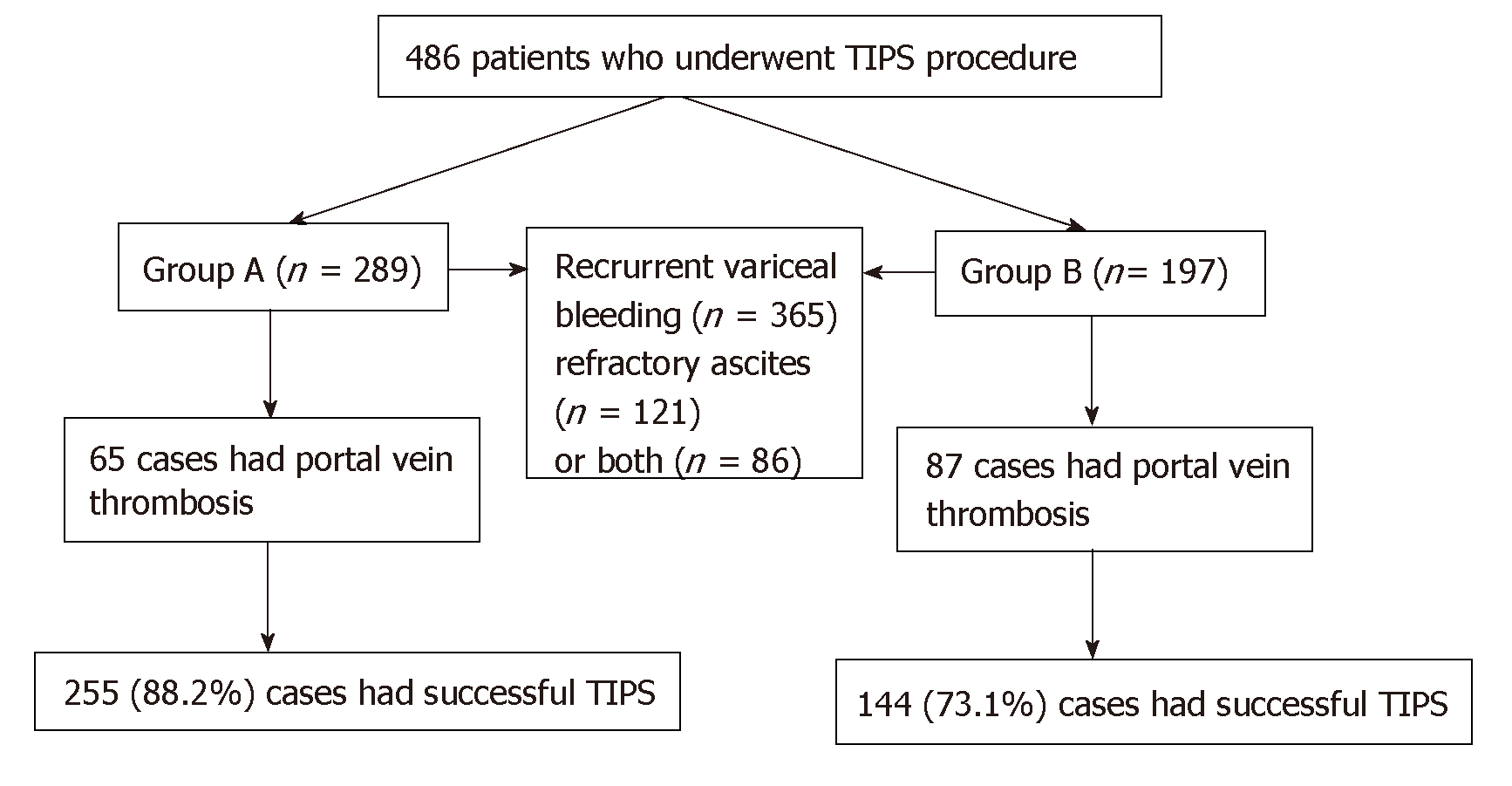
Four hundred and eighty-six patients with portal hypertension for refractory ascites and/or variceal bleeding who required TIPS placement between January 2010 and January 2016 were included in this retrospective analysis. Patients without prior splenectomy were defined as group A (n = 289) and those with prior splenectomy as group B (n = 197). The incidence of PVT before TIPS was compared between the two groups. After TIPS placement, primary patency rate was compared using Kaplan–Meier analysis at 3, 6, 9 and 12 mo, and 2 and 3 years. The clinical outcomes were analyzed.
Before TIPS procedure, the incidence of PVT in group A was lower than in group B (P = 0.003), and TIPS technical success rate in group A was higher than in group B (P = 0.016). The primary patency rate in group A tended to be higher than in group B at 3, 6, 9 and 12 mo, 2 years and 3 years (P = 0.006, P = 0.011, P = 0.023, P = 0.032, P = 0.037 and P = 0.028, respectively). Recurrence of bleeding and ascites rate in group A was lower than in group B at 3 mo (P ≤ 0.001 and P = 0.001), 6 mo (P = 0.003 and P = 0.005), 9 mo (P = 0.005 and P = 0.012), 12 mo (P = 0.008 and P = 0.024), 2 years (P = 0.011 and P = 0.018) and 3 years (P = 0.016 and P = 0.017), respectively. During 3-years follow-up, the 1-, 2- and 3-year survival rate in group A were higher than in group B (P = 0.008, P = 0.021, P = 0.018, respectively), but there was no difference of the incidence of hepatic encephalopathy (P = 0.527).
Patients with prior splenectomy have a high incidence of PVT, which potentially increases the risk of recurrent symptoms associated with shunt stenosis or occlusion.
Core tip: There are several approaches for treatment of portal hypertension related varices and variceal hemorrhage, including drugs, endoscopic variceal ligation, transjugular intrahepatic portosystemic shunt, splenectomy with pericardial devascularization and liver transplantation. Transjugular intrahepatic portosystemic shunt is widely accepted as an alternative to surgery for management of complications of portal hypertension such as variceal bleeding, refractory ascites, Budd–Chiari syndrome, hepatorenal syndrome, hepatic hydrothorax and even hepatopulmonary syndrome. Patients with splenectomy with pericardial devascularization had a high incidence of portal vein thrombosis, which can markedly affect transjugular intrahepatic portosystemic shunt stent patency and potentially increase the risk of recurrent symptoms associated with shunt stenosis or occlusion.
- Citation: Dong F, Luo SH, Zheng LJ, Chu JG, Huang H, Zhang XQ, Yao KC. Incidence of portal vein thrombosis after splenectomy and its influence on transjugular intrahepatic portosystemic shunt stent patency. World J Clin Cases 2019; 7(17): 2450-2462
- URL: https://www.wjgnet.com/2307-8960/full/v7/i17/2450.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i17.2450
Portal hypertension secondary to liver cirrhosis is mainly due to chronic hepatitis and alcoholic liver disease[1]. Esophagogastric varices and ascites secondary to portal hypertension are common major complications of liver cirrhosis[2]. Esophagogastric varices are a serious, life-threatening complication and hypersplenism is often associated with portal hypertension in cirrhotic patients[3].
There are several approaches for treatment of portal hypertension related varices and variceal hemorrhage, including drugs, endoscopic variceal ligation, transjugular intrahepatic portosystemic shunt (TIPS), splenectomy with pericardial devascularization (SPD) and liver transplantation[4]. SPD without liver transplantation has been widely accepted as a surgical treatment for cirrhosis in patients with variceal bleeding and secondary hypersplenism in China for many years[5]. However, when compared with other treatments, simple splenectomy and SPD are associated with an increased incidence of postoperative complications, such as portal vein thrombosis (PVT)[6].
TIPS is widely accepted as an alternative to surgery for management of complications of portal hypertension such as variceal bleeding, refractory ascites, Budd–Chiari syndrome, hepatorenal syndrome, hepatic hydrothorax and even hepatopulmonary syndrome[7]. TIPS has been used to effectively treat PVT in many centers since the 1990s[8]. PVT is still considered a contraindication to the creation of a TIPS, however the advantages of TIPS for PVT in patients with cirrhosis are evident[9] as it addresses portal hypertension and reconstructs portal vein (PV) flow.
Despite its efficacy in treatment of complications of portal hypertension, TIPS is prone to shunt stenosis or occlusion leading to shunt failure, and approximately half of all patients with TIPS require shunt revision during follow-up[10], which makes close surveillance and frequent costly revisions mandatory[11] especially in patients with PVT despite the use of stent grafts covered with polytetrafluoroethylene. Even with these new stents, post-TIPS shunt obstruction and a high rate of clinical symptom recurrence remain problematic.
Although TIPS has good therapeutic effects on the formation of PVT, the effect of PVT on TIPS stenting has rarely been reported. The purpose of this study was to evaluate the incidence of PVT after splenectomy and its influence on the patency rate of TIPS in patients with cirrhosis of portal hypertension.
A total of 486 patients who underwent a TIPS procedure between January 2010 and January 2016 were enrolled retrospectively. The study protocol was approved by the Institutional Review Board and Ethics Committee, and all the patients provided a written consent at the time of operation in the hospital. All procedures were conducted according to the guidelines approved by the ethics committee. We reviewed the patients’ medical records and medical images to gather information regarding the underlying etiologies, clinical presentations, age, sex and severity of cirrhosis.
This was a multi-center, retrospective study. Patients with cirrhosis without prior splenectomy served as group A (n = 289), and those with cirrhosis and prior splenectomy served as group B (n = 197). Three hundred and sixty-five patients with portal-hypertension-related complications of recurrent variceal bleeding after band ligation and/or glue injection, 121 patients with refractory ascites and 86 with both who underwent TIPS were included. The incidence of PVT before TIPS was compared between the two groups. After TIPS placement, primary patency rate was compared at 3, 6, 9 and 12 mo, and 2 and 3 years. The clinical outcomes were analyzed.
Patients with acute PVT, variceal bleeding as an emergency indication, hepatic encephalopathy (HE), severe right-sided heart failure, severe liver failure, polycystic liver disease, dilated biliary ducts, age > 75 years, Child–Pugh score > 11, Model for End-stage Liver Disease score > 18, hepatic carcinoma, sepsis or spontaneous bacterial peritonitis and patients who underwent liver transplantation were excluded.
Color Doppler ultrasound was performed initially for the diagnosis of PVT. It revealed that the main PV was obstructed along with a reduction or absence of portal flow or disappearance of the native PV and formation of extensive collaterals. Contrast-enhanced computed tomography and/or magnetic resonance imaging showed stenosis, filling defects or complete occlusion of the PV with or without collaterals.
Acute PVT was defined by the absence of collateral vessels and any one of the following: (1) Aapid onset of abdominal pain due to PVT within 14 d and intestinal ischemia or infarction without chronic thrombosis; or (2) A high intraluminal density within the PV on non-contrast-enhanced computed tomography. Chronic PVT was defined by at least one of the following: (1) A low intraluminal density on contrast-enhanced computed tomography; (2) Replacement of the original main PV (MPV) with a fibrotic cord or no identifiable MPV; or (3) Presence of portal cavernoma. Thrombosis in the MPV was further classified as partial occlusion, complete occlusion and fibrotic cord in place of the original MPV.
Using standard local anesthesia, TIPS was performed through a transjugular approach, and the technique has been described previously[12]. For PVT patients with cirrhosis, the TIPS procedure was performed as described previously[13]. The entire length of the intrahepatic tract was covered by the 8-mm stent graft (Fluency; BARD, Voisins le Bretonneux, France or Viatorr; W.L. Gore and Associates, Flagstaff, AZ, United States). The shunts were dilated to full nominal diameter to reach a target portosystemic gradient of < 12 mmHg, and prominent gastroesophageal collateral vessels observed during the TIPS procedure were embolized with coils (Cook Inc., Bloomington, IL, United States).
Direct portography was then performed to confirm if the PV system was entirely patent. After the TIPS procedure, patients were treated with intravenous heparin (4000 U/d; Chase Sun Pharma Co. Ltd, Tianjing, China) and oral warfarin (2.5 mg/d; Orion Pharma Co. Ltd, Orionintie, Finland).
Patients underwent baseline duplex sonography on the day after TIPS. The results were compared with subsequent shunt velocities. After TIPS, patients were followed up using the same protocol for each group via outpatient visit 1 mo after the procedure and then every 3 mo or whenever needed. Clinical examination, blood chemistry test and assessment of HE were carried out during the follow-up period. Ultrasonography was also performed after TIPS or in case of recurrent bleeding or ascites.
Shunt dysfunction or significant recurrent symptoms were used as endpoints for the loss of primary unassisted patency. TIPS angiography was performed in these patients and TIPS revision was made when hemodynamically significant shunt stenosis was > 50% with recurrent variceal bleeding and ascites, and portosystemic pressure gradient was ≥ 15 mmHg without grade III/IV HE (West Haven Criteria)
Results are expressed as mean ± SD. Patency time was calculated by the Kaplan–Meier method, and the median time was compared by the log-rank test. Variables were subjected to logistic regression analysis. Differences between the groups were compared using one-way analysis of variance and least significant difference t test. P < 0.05 was considered statistically significant. SPSS version 20.0 (SPSS, Chicago, IL, United States) was used for the statistical analysis.
Between January 2010 and January 2016, there were 289 patients with cirrhosis with no prior splenectomy in group A, and 197 patients with cirrhosis who underwent splenectomy in group B. The etiology, clinical presentations, age, sex and severity of cirrhosis did not differ significantly (Table 1). In group A, the incidence of PVT was 11.0% (65/289). In group B, the incidence of PVT was 44.2% (87/197). The distribution of patients is shown in Figure 1. The incidence of PVT in group B was higher than in group A, and the difference was significant between the two groups (P = 0.003).
| Characteristics | Groups | P value | ||
| A | B | |||
| n | 486 | 289 | 197 | 0.614 |
| Gender | Male | 148 | 108 | 0.715 |
| Female | 141 | 89 | ||
| Age in yr | 36.25 ± 15.43 | 35.20 ± 14.14 | 0.710 | |
| Etiology | Hepatitis B | 184 | 131 | 0.743 |
| Hepatitis C | 51 | 32 | ||
| Ethanol consumption | 34 | 26 | ||
| Cryptogenic hepatitis | 10 | 8 | ||
| Child-Pugh score | A | 37 | 21 | 0.584 |
| B | 213 | 153 | ||
| C | 35 | 27 | ||
| Model for end-stage liver disease score | 9.49 ± 2.05 | 9.35 ± 1.99 | 0.508 | |
| Variceal bleeding | 365 | 215 | 150 | 0.329 |
| Refractory ascites | 121 | 76 | 45 | 0.672 |
| Both variceal bleeding and refractory ascites | 86 | 52 | 34 | 0.481 |
| Laboratory tests | ||||
| Alanine transaminase as U/L | 59.34 ± 11.41 | 63.05 ± 10.17 | 0.742 | |
| Aspartate transaminase as U/L | 72.36 ± 12.09 | 68.45 ± 13.23 | 0.689 | |
| Alkaline phosphatase as U/L | 193.43 ± 24.62 | 208.49 ± 32.54 | 0.893 | |
| Total bilirubin as μmol/L | 14.03 ± 5.15 | 16.21 ± 4.28 | 0.754 | |
| Albumin as g/L | 31.29 ± 1.46 | 29.19 ± 1.48 | 0.431 | |
| Prothrombin time in s | 14.72 ± 3.28 | 15.43 ± 3.17 | 0.638 | |
| Platelet count as × 109/L | 45.27 ± 12.38 | 38.39 ± 13.47 | 0.374 | |
| Clinical presentation | ||||
| Abdominal distention | 146 | 85 | 0.243 | |
| Abdominal pain | 78 | 49 | 0.217 | |
| Weakness | 46 | 37 | 0.158 | |
| Poor appetite | 163 | 117 | 0.362 | |
| Jaundice | 42 | 29 | 0.293 | |
| Lower limbs edema | 32 | 24 | 0.675 | |
| Endoscopic therapy | 538 | 372 | 0.427 | |
| Ascites paracentesis | 227 | 147 | 0.489 | |
Of the 289 patients in group A, 255 (88.2%) cases had technically successful TIPS compared with 144 (73.1%) cases in group B. TIPS technical success rate in group A was higher than in group B (P = 0.016). No patient died of severe procedure-related complications within 30 d after TIPS (Figure 2). After TIPS, the mean portosystemic pressure gradient decreased from 32.43 ± 6.64 to 11.15 ± 1.20 mmHg in group A (P = 0.027), and 31.90 ± 4.63 to 10.79 ± 1.18 mmHg in group B (P = 0.025). There were significant differences before and after TIPS (P < 0.05), but there was no difference before and after TIPS between the two groups (P = 0.447, P = 0.605, respectively) (Table 2).
| Characteristics | Group | n | Portal vein thrombosis | Portal vein thrombo-sis rate | χ2 | P va-lue | ||||
| Yes | No | |||||||||
| Portal vein thrombosis | A | 289 | 65 | 224 | 11.0 | 25.60 | 0.003 | |||
| B | 197 | 87 | 110 | 44.2 | ||||||
| TIPS success | TIPS success rate (%) | |||||||||
| Yes | Not | |||||||||
| TIPS | A | 289 | 255 | 34 | 88.2 | 19.28 | 0.016 | |||
| B | 197 | 144 | 53 | 73.1 | ||||||
| Portosystemic gradient (mmHg) Pre-TIPS | A | 255 | 32.43 ± 6.64 | 0.447 | ||||||
| B | 144 | 31.90 ± 4.63 | ||||||||
| Portosystemic gradient (mmHg) Post-TIPS | A | 255 | 11.15 ± 1.20 | 0.027 | 0.605 | |||||
| 0.025 | ||||||||||
The primary patency rate for group A was 97.6% at 3 mo, 88.6% at 6 mo, 84.3% at 9 mo, 69.4% at 12 mo, 51.0% at 2 years and 30.6% at 3 years. In group B, the patency rate was 88.1% at 3 mo, 78.7% at 6 mo, 68.1% at 9 mo, 45.1% at 12 mo, 26.4% at 2 years and 12.5% at 3 years. Compared with the two groups, there were significant statistical differences at the time 3 mo, 6 mo, 9 mo, 12 mo, 2 year and 3 year (P = 0.006, P = 0.011, P = 0.023, P = 0.032, P = 0.037, P = 0.028, respectively). The median patency time of the total 3 years was 12 mo in group A (95% confidence interval (CI): 10-14) and 4 mo in group B (95%CI: 3-6), and a significant difference was observed in stent dysfunction times between groups A and B (P = 0.009, log-rank test) (Table 3).
| Time | Group | Patency | Patency rate, % | χ2 | P value | |||
| Yes | Not | |||||||
| 3 mo | A | 249 | 6 | 97.6 | 15.18 | 0.006 | ||
| B | 127 | 17 | 88.1 | |||||
| 6 mo | A | 226 | 29 | 88.6 | 10.34 | 0.011 | ||
| B | 109 | 35 | 75.7 | |||||
| 9 mo | A | 215 | 40 | 84.3 | 14.24 | 0.023 | ||
| B | 98 | 46 | 68.1 | |||||
| 12 mo | A | 177 | 78 | 69.4 | 25.93 | 0.032 | ||
| B | 65 | 79 | 45.1 | |||||
| 2 yr | A | 130 | 125 | 51.0 | 22.75 | 0.037 | ||
| B | 38 | 106 | 26.4 | |||||
| 3 yr | A | 78 | 177 | 30.6 | 21.39 | 0.028 | ||
| B | 18 | 126 | 12.5 | |||||
| Median stent patency in mo | Four quantile spacing in mo | |||||||
| Total | A | 12 | (10, 14) | 0.001 | ||||
Total shunt malfunction occurred 378 times of 289 patients in group A and 419 times of 197 patients in group B. There was a significant difference in stent dysfunction times between groups A and B (P = 0.006, log-rank test). The patients with stent dysfunction underwent balloon dilation. After stent revision, their symptoms disappeared.
Incidence of recurrent bleeding (Table 4) and ascites (Table 5) in group B was higher than in group A at 3 mo (14.6% vs 5.1%, P ≤ 0.001; 16.7% vs 5.9%, P = 0.001), 6 mo (25.7% vs 9.8%, P = 0.003; 24.3% vs 10.2%, P = 0.005), 9 mo (29.8% vs 15.3%, P = 0.005; 35.4% vs 20.0%, P = 0.012), 12 mo (39.6% vs 20.0%, P = 0.008; 43.8% vs 34.1%, P = 0.024), 2 years (45.1% vs 29.0%, P = 0.011; 39.6% vs 27.8%, P = 0.018) and 3 years (59.7% vs 40.0%, P = 0.016; 56.3% vs 40.8%, P = 0.017).
| Time | Group | Bleeding | Recurrence of bleeding, % | χ2 | P value | |
| Yes | No | |||||
| 3 mo | A | 13 | 242 | 5.1 | 12.13 | ≤ 0.001 |
| B | 21 | 123 | 14.6 | |||
| 6 mo | A | 25 | 230 | 9.8 | 19.53 | 0.003 |
| B | 37 | 107 | 25.7 | |||
| 9 mo | A | 39 | 216 | 15.3 | 13.18 | 0.005 |
| B | 43 | 101 | 29.8 | |||
| 12 mo | A | 51 | 204 | 20.0 | 20.90 | 0.008 |
| B | 57 | 87 | 39.6 | |||
| 2 yr | A | 74 | 181 | 29.0 | 12.10 | 0.011 |
| B | 65 | 79 | 45.1 | |||
| 3 yr | A | 102 | 153 | 40.0 | 16.20 | 0.016 |
| B | 86 | 58 | 59.7 | |||
| Median recurrent bleeding in mo | Four quantile spacing in mo | |||||
| Total | A | 10 | (8, 12) | |||
| B | 5 | (4, 7) | ≤ 0.001 | |||
| Time | Group | Ascites | Recurrence of ascites, % | χ2 | P value | |||
| Yes | No | |||||||
| 3 mo | A | 15 | 240 | 5.9 | 9.82 | 0.001 | ||
| B | 24 | 120 | 16.7 | |||||
| 6 mo | A | 26 | 229 | 10.2 | 16.15 | 0.005 | ||
| B | 35 | 109 | 24.3 | |||||
| 9 mo | A | 51 | 204 | 20.0 | 12.16 | 0.012 | ||
| B | 51 | 93 | 35.4 | |||||
| 12 mo | A | 87 | 168 | 34.1 | 4.50 | 0.024 | ||
| B | 63 | 81 | 43.8 | |||||
| 2 yr | A | 71 | 184 | 27.8 | 6.56 | 0.018 | ||
| B | 57 | 87 | 39.6 | |||||
| 3 yr | A | 104 | 151 | 40.8 | 10.02 | 0.017 | ||
| B | 81 | 63 | 56.3 | |||||
| Median recurrent of ascites in mo | Four quantile spacing in mo | |||||||
| Total 3 yr | A | 11 | (6, 16) | 0.009 | ||||
| B | 16 | (12, 19) | ||||||
The median time to recurrent bleeding was 10 mo in group A (95%CI: 8-12) and 5 mo in group B (95%CI: 4-7). The median time to recurrence of ascites was 11 mo in group A (95%CI: 6-16) and 16 mo in group B (95%CI: 12-19). There were significant differences in median time to recurrent bleeding and ascites between the two groups (P = 0.009, P ≤ 0.001, log-rank test).
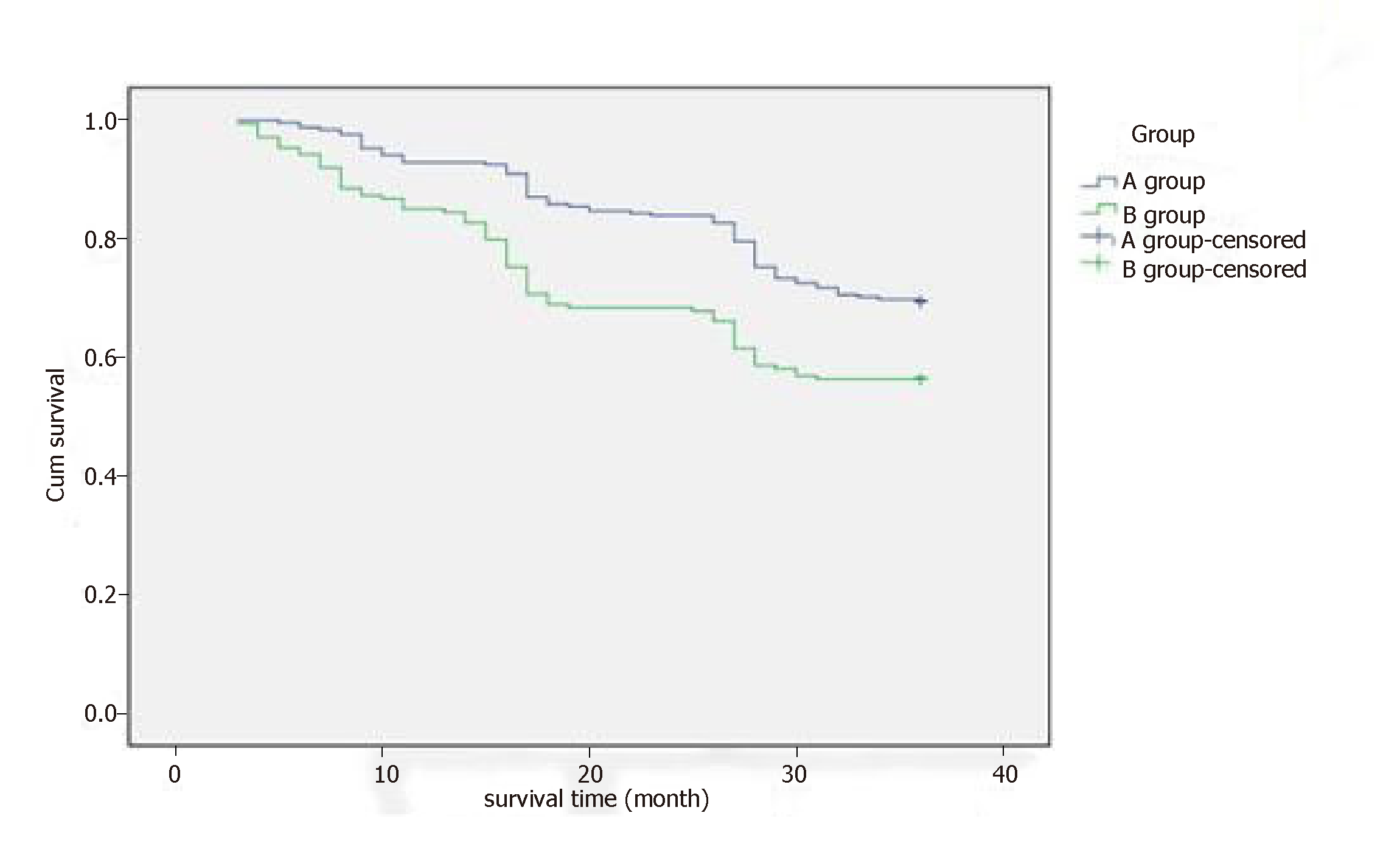
During the 3-year follow-up, the 1-year survival rate was 92.9% versus 85.4%, 2-year survival rate was 83.9% versus 68.1%, and 3-year survival rate was 69.4% versus 56.3% in group A and group B. Compared with group B, the 1-, 2-, and 3–year survival rates in group A were longer (P = 0.008, P = 0.021 and P = 0.011, respectively) (Table 6) (Figure 3).
| Characteristics | Group | Survival | Survival rate, % | χ2 | P value | |
| Yes | No | |||||
| 1 yr | A | 237 | 18 | 92.9 | 6.98 | 0.008 |
| B | 123 | 21 | 85.4 | |||
| 2 yr | A | 214 | 41 | 83.9 | 14.362 | 0.021 |
| B | 98 | 46 | 68.1 | |||
| 3 yr | A | 177 | 78 | 69.4 | 7.701 | 0.018 |
| B | 81 | 63 | 56.3 | |||
| HE | Morbidity, % | |||||
| Yes | Not | |||||
| HE | A | 70 | 185 | 27.5 | 0.40 | 0.527 |
| B | 35 | 109 | 24.3 | |||
HE occurred in 70 patients in group A and in 35 patients in group B during follow-up with an incidence of 27.5% and 24.3%, respectively. There were no significant differences between the groups (P = 0.527). After drug treatment, the symptoms disappeared in patients with grade overt and grade II of HE. In patients with grade III and grade IV of HE, the symptoms disappeared after 18 stents were implanted for shunt reduction.
Portal hypertension is a consequence of liver cirrhosis; the mechanisms by which it develops are complicated and associated with changes in the vascular architecture of the liver due to fibrosis and regenerative nodules[14]. Surgical treatments have been designed to prevent many complications. Currently, SPD is the most commonly used method in China[15]. SPD is one of the common treatment methods for patients with cirrhosis with portal-hypertension-related complications and hypersplenism[16] and can correct hypersplenism and reduce PV blood flow and pressure within a short period of time[17].
However, the availability of many treatment methods suggests that no one in particular yields entirely satisfactory outcomes for all patients or in all clinical situations[18]. Splenectomy in patients with portal hypertension does not resolve the risk of PVT, and it can further aggravate portal hypertension, cause PVT, increase the probability of rebleeding and ultimately affects quality of life[19,20]. In the study of resection of spleen, reduction of portal venous pressure depends on the splenic venous blood reflux and portal venous shunt. Resection of the communicating branches from the splenic hilus will cause increased portal venous pressure and portal venous thrombosis.
The incidence of PVT is mostly 12%-72% after splenectomy or SPD, and the risk factors for PVT after splenectomy have been studied[21,22]. Patients with portal hypertension were seeking TIPS treatment in our center. However, it has been found that the probability of PVT is significantly increased after splenectomy. This highlights the difficulties in the TIPS procedure, and it also affects the patient prognosis and the effect of liver transplantation. We found that the total incidence of PVT after splenectomy was 44.2%, which was higher in the splenectomy group than that in the group without splenectomy.
TIPS creation has been widely used in the treatment of patients with esophageal and gastric variceal bleeding secondary to portal hypertension and has achieved good results[23,24]. With the improvement of procedure methods and instruments, the incidence of complications after TIPS has greatly decreased. Although being effective in preventing such syndromes, TIPS may cause shunt stenosis or occlusion leading to shunt failure. Stent stenosis and occlusion are the main complications of TIPS placement and cause recurrent bleeding and ascites[25].
There are multiple causes of thrombosis after splenectomy. It is believed that splenectomy reduces synthesis of coagulation factors in patients with liver cirrhosis, and the scavenging activity of tissue plasminogen activator is decreased resulting in a high blood coagulation state[26]. In addition, the risk of PVT after splenectomy can be caused by lack of microcirculation, increased blood viscosity, blood stasis induced by splenic vein stump, decreased blind pouch postoperative PV pressure, slower blood flow and platelet count[27,28]. The presence of these factors can lead to the formation of PVT and have a continuous impact on the PV system despite treatment with TIPS. It is also easy to cause thrombosis in the TIPS shunt and PV system and to cause stenosis or occlusion of the TIPS shunt, and these factors promote each other.
PVT can develop in the trunk of the PV, including its right and left intrahepatic branches, or it can originate everywhere in the portal system and may even extend to the splenic or superior mesenteric veins or towards the liver involving the intrahepatic PV branches[29]. PVT leads to portal hypertension and cavernous transformation of the PV, which causes difficulty with TIPS creation[30]. Although TIPS has good therapeutic effects on the formation of PVT, the effect of PVT on TIPS stenting is rarely reported.
In our study, the incidence of PVT in group A was lower than in group B, and the success rate of TIPS placement was also higher in group A. Our results indicated that PVT easily forms after splenectomy as described previously, and it creates difficulty for treatment with TIPS and other methods. The patency rate after TIPS in group A was higher than in group B, the median unassisted patency time in group A was longer than in group B, and recurrent bleeding and ascites in group A were less than in group B. Our results confirmed that prior splenectomy is an important determinant of shunt patency.
It is reported[31] that after TIPS treatment, hypersplenism is relieved due to decreased PV pressure and splenic blood flow, which can improve quality of life. However, there are still some patients with hypersplenism with no satisfactory outcome of treatments, including partial splenic arterial embolization, which can improve the symptoms of hypersplenism[32,33]. In patients with cirrhosis who are prone to PVT, which can lead to difficulty with TIPS creation and stent stenosis or occlusion, we suggest that splenectomy should be considered carefully.
The present study has some limitations. TIPS was established by the left branch of the intrahepatic PV, which may affect the results of patency rate. This is only a retrospective study. Randomized controlled trials are needed to verify our results.
In conclusion, patients with portal hypertension with prior splenectomy had a high incidence of PVT, which is an important determinant of TIPS stent patency and potentially increases the risk of recurrent symptoms associated with shunt stenosis or occlusion. Patients with portal hypertension have the opportunity to avoid splenectomy if they are undergoing TIPS treatment.
Splenectomy with pericardial devascularization (SPD) without liver transplantation has been widely accepted for the treatment of cirrhosis in patients with variceal bleeding and secondary hypersplenism in China. However, when compared with other treatments, simple splenectomy and SPD are associated with an increased incidence of postoperative complications, such as portal vein thrombosis (PVT). Transjugular intrahepatic portosystemic shunt (TIPS), as an alternative to surgery, is now commonly used for management of complications of portal hypertension. Patients with SPD had a high incidence of PVT, which can markedly affect TIPS stent patency and increase the risk of recurrent symptoms associated with shunt stenosis or occlusion.
SPD is one of the common treatment methods used in China for patients with cirrhosis and portal-hypertension-related complications and hypersplenism. It can correct hypersplenism and reduce PV blood flow and pressure within a short period of time. However, it may aggravate the portal hypertension, cause PVT, increase the probability of rebleeding and ultimately affects quality of life. In this study, we evaluated the incidence of PVT after splenectomy and its influence on the patency rate of TIPS in patients with cirrhosis and portal hypertension.
The main objective of this study was to investigate the effects of high incidence of PVT in patients with portal hypertension and prior SPD on the TIPS stent patency and the risk of recurrent symptoms associated with shunt stenosis or occlusion.
We conducted a retrospective study to compare the incidence of PVT before TIPS for patients without prior SPD (group A) and those with prior SPD (group B). After TIPS placement, primary patency rate was compared using Kaplan-Meier analysis at 3, 6, 9 and 12 mo, and 2 and 3 years. The clinical outcomes were analyzed. Results are expressed as mean ± SD. Patency time was calculated using the Kaplan-Meier method, and the median time was compared by means of the log-rank test. Logistic regression analysis was performed on the variables. The differences between the groups were compared using one-way analysis of variance followed by least significant difference t tests. Differences were considered significant at P < 0.05. The statistical analysis was performed with SPSS version 20.0 (SPSS, Chicago, IL, United States).
The incidence of PVT in group B was higher than in group A, and the difference was significant between the two groups (P = 0.003). The success rate of TIPS in group A was higher than in group B, and the primary patency rate in group A tended to be higher than in group B at 3, 6, 9 and 12 mo, 2 years and 3 years. Recurrence of bleeding and ascites rate in group A were lower than in group B at 3 mo, 6 mo, 9 mo, 12 mo, 2 years and 3 years. During the 3-year follow-up, the 1-, 2- and 3-year survival rates in group A were higher than in group B, but there was no difference of the incidence of hepatic encephalopathy.
Patients with a SPD have a high incidence of PVT, which potentially increases the risk of recurrent symptoms associated with TIPS stenosis or occlusion.
This study showed that patients with portal hypertension with prior splenectomy had a high incidence of PVT, which is an important determinant of TIPS stent patency and potentially increases the risk of recurrent symptoms associated with shunt stenosis or occlusion. Patients with portal hypertension may avoid splenectomy when they are undergoing TIPS treatment. However, this is only a retrospective study, and randomized controlled trials are needed to verify our results.
We are grateful to all the patients who were involved in this study and our colleagues in the Department of Radiology of Air Force General Hospital of PLA for their contributions to the data collection.
| 1. | Augustin S, Pons M, Maurice JB, Bureau C, Stefanescu H, Ney M, Blasco H, Procopet B, Tsochatzis E, Westbrook RH, Bosch J, Berzigotti A, Abraldes JG, Genescà J. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66:1980-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 2. | Berzigotti A. Advances and challenges in cirrhosis and portal hypertension. BMC Med. 2017;15:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Keller EJ, Kulik L, Stankovic Z, Lewandowski RJ, Salem R, Carr JC, Schnell S, Markl M, Collins JD. JOURNAL CLUB: Four-Dimensional Flow MRI-Based Splenic Flow Index for Predicting Cirrhosis-Associated Hypersplenism. AJR Am J Roentgenol. 2017;209:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Costa Lacet CM, Neto JB, Ribeiro LT, Oliveira FS, Wyszomirska RF, Strauss E. Schistosomal portal hypertension: Randomized trial comparing endoscopic therapy alone or preceded by esophagogastric devascularization and splenectomy. Ann Hepatol. 2016;15:738-744. [PubMed] |
| 5. | Bao H, He Q, Dai N, Ye R, Zhang Q. Retrospective Study to Compare Selective Decongestive Devascularization and Gastrosplenic Shunt versus Splenectomy with Pericardial Devascularization for the Treatment of Patients with Esophagogastric Varices Due to Cirrhotic Portal Hypertension. Med Sci Monit. 2017;23:2788-2795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Wu S, Wu Z, Zhang X, Wang R, Bai J. The incidence and risk factors of portal vein system thrombosis after splenectomy and pericardial devascularization. Turk J Gastroenterol. 2015;26:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Luo SH, Chu JG, Huang H, Yao KC. Effect of initial stent position on patency of transjugular intrahepatic portosystemic shunt. World J Gastroenterol. 2017;23:4779-4787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Jiang TT, Luo XP, Sun JM, Gao J. Clinical outcomes of transcatheter selective superior mesenteric artery urokinase infusion therapy vs transjugular intrahepatic portosystemic shunt in patients with cirrhosis and acute portal vein thrombosis. World J Gastroenterol. 2017;23:7470-7477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Wang Z, Jiang MS, Zhang HL, Weng NN, Luo XF, Li X, Yang L. Is Post-TIPS Anticoagulation Therapy Necessary in Patients with Cirrhosis and Portal Vein Thrombosis? A Randomized Controlled Trial. Radiology. 2016;279:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Rösch J, Keller FS. Transjugular intrahepatic portosystemic shunt: present status, comparison with endoscopic therapy and shunt surgery, and future prospectives. World J Surg. 2001;25:337-45; discussion 345-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Rossi P, Salvatori FM, Fanelli F, Bezzi M, Rossi M, Marcelli G, Pepino D, Riggio O, Passariello R. Polytetrafluoroethylene-covered nitinol stent-graft for transjugular intrahepatic portosystemic shunt creation: 3-year experience. Radiology. 2004;231:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Weber CN, Nadolski GJ, White SB, Clark TW, Mondschein JI, Stavropoulos SW, Shlansky-Goldberg RD, Trerotola SO, Soulen MC. Long-Term Patency and Clinical Analysis of Expanded Polytetrafluoroethylene-Covered Transjugular Intrahepatic Portosystemic Shunt Stent Grafts. J Vasc Interv Radiol. 2015;26:1257-65; quiz 1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Han G, Qi X, He C, Yin Z, Wang J, Xia J, Yang Z, Bai M, Meng X, Niu J, Wu K, Fan D. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Choy TY, Simoens C, Thill V, Mboti F, Vandaele S, Mendes da Costa P. Results of surgical treatment of uncontrollable upper gastrointestinal hemorrhage using endoscopy. Hepatogastroenterology. 2011;58:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Wang RY, Wang JF, Liu Q, Ma N, Chen WX, Li JL. Combined Rex-bypass shunt with pericardial devascularization alleviated prehepatic portal hypertension caused by cavernomatous transformation of portal vein. Postgrad Med. 2017;129:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Zhang YB, Lu Y, Wu WD, Zhang CW, Shen GL, Hong dF. Indocyanine green retention is a potential prognostic indicator after splenectomy and pericardial devascularization for cirrhotic patients. Hepatobiliary Pancreat Dis Int. 2016;15:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 401] [Article Influence: 50.1] [Reference Citation Analysis (1)] |
| 19. | Rottenstreich A, Kleinstern G, Spectre G, Da'as N, Ziv E, Kalish Y. Thromboembolic Events Following Splenectomy: Risk Factors, Prevention, Management and Outcomes. World J Surg. 2018;42:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Wang Y, Yu M, Huang J, Deng D, Xue H. Effective Prevention for Portal Venous System Thrombosis After Splenectomy: A Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2017;27:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Zhang N, Yao Y, Xue W, Wu S. Early prophylactic anticoagulation for portal vein system thrombosis after splenectomy: A systematic review and meta-analysis. Biomed Rep. 2016;5:483-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Zhang W, Zhou DM, Li Y. [Clinical effect of low-molecular-weight heparin in prevention and treatment of liver cirrhosis and portal vein thrombosis after splenectomy: a systematic review and meta-analysis]. Zhonghua Gan Zang Bing Za Zhi. 2016;24:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Trebicka J. Emergency TIPS in a Child-Pugh B patient: When does the window of opportunity open and close? J Hepatol. 2017;66:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Miraglia R, Maruzzelli L, Tuzzolino F, Petridis I, D'Amico M, Luca A. Transjugular Intrahepatic Portosystemic Shunts in Patients with Cirrhosis with Refractory Ascites: Comparison of Clinical Outcomes by Using 8- and 10-mm PTFE-covered Stents. Radiology. 2017;284:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Marticorena Garcia SR, Langmann M, Schnorr B, Günther RW, Hamm B, Althoff CE. Use of Paclitaxel-Coated Balloon Catheter Dilation to Reduce In-Stent Restenosis in Transjugular Intrahepatic Portosystemic Shunt (TIPS). Rofo. 2016;188:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Ruiz-Tovar J, Priego P. Portal Vein Thrombosis After Splenic and Pancreatic Surgery. Adv Exp Med Biol. 2017;906:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 27. | Matsui T, Usui M, Wada H, Iizawa Y, Kato H, Tanemura A, Murata Y, Kuriyama N, Kishiwada M, Mizuno S, Sakurai H, Isaji S. Platelet Activation Assessed by Glycoprotein VI/Platelet Ratio Is Associated With Portal Vein Thrombosis After Hepatectomy and Splenectomy in Patients With Liver Cirrhosis. Clin Appl Thromb Hemost. 2018;24:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | de'Angelis N, Abdalla S, Lizzi V, Esposito F, Genova P, Roy L, Galacteros F, Luciani A, Brunetti F. Incidence and predictors of portal and splenic vein thrombosis after pure laparoscopic splenectomy. Surgery. 2017;162:1219-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Lombardo S, Espejo JJ, Pérez-Montilla ME, Zurera LJ, González-Galilea Á. The keys to successful TIPS in patients with portal vein thrombosis and cavernous transformation. Radiologia. 2018;60:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Qi X, He C, Guo W, Yin Z, Wang J, Wang Z, Niu J, Bai M, Yang Z, Fan D, Han G. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with variceal bleeding in liver cirrhosis: outcomes and predictors in a prospective cohort study. Liver Int. 2016;36:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Massoud OI, Zein NN. The Effect of Transjugular Intrahepatic Portosystemic Shunt on Platelet Counts in Patients With Liver Cirrhosis. Gastroenterol Hepatol (N Y). 2017;13:286-291. [PubMed] |
| 32. | Karasu Z, Gurakar A, Kerwin B, Hulagu S, Jazzar A, McFadden R, Nour B, Sebastian A, Cassidy F, Stokes K, Van Thiel DH, Wright H. Effect of transjugular intrahepatic portosystemic shunt on thrombocytopenia associated with cirrhosis. Dig Dis Sci. 2000;45:1971-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Jabbour N, Zajko A, Orons P, Irish W, Fung JJ, Selby RR. Does transjugular intrahepatic portosystemic shunt (TIPS) resolve thrombocytopenia associated with cirrhosis? Dig Dis Sci. 1998;43:2459-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng TH S-Editor: Dou Y L-Editor: Filipodia E-Editor: Zhou BX