Published online Jun 16, 2014. doi: 10.12998/wjcc.v2.i6.215
Revised: January 6, 2014
Accepted: May 8, 2014
Published online: June 16, 2014
Processing time: 189 Days and 13.6 Hours
We report the case of an urgent nephrectomy because of a pyonephrosis and sepsis due to an unsuspected sarcomatoid transitional cell carcinoma, an infrequent subtype with a bad oncological prognosis. We present a 58-year-old man assessed by internal medicine for a general syndrome and weakness many months previously. A pyonephrotic kidney was observed at abdominal computed tomography in the context of septic shock, without suspecting the underlying cause. The pathology report described a sarcomatoid transitional cell carcinoma. Sarcomatoid transitional cell carcinoma is an invasive and infrequent subtype of urothelial tumors. The symptoms are often the same as other renal masses; however, in this case, sepsis and pyonephrosis were the rare initial symptoms.
Core tip: Sarcomatoid transitional cell carcinoma is an invasive and rare subtype of urothelial tumors. The symptoms are often the same as other renal masses; however, in this case, sepsis and pyonephrosis were the rare initial symptoms.
- Citation: Fernández-Pello S, Venta V, González I, Gil R, Menéndez CL. Pyonephrosis as a sign of sarcomatoid carcinoma of the renal pelvis. World J Clin Cases 2014; 2(6): 215-218
- URL: https://www.wjgnet.com/2307-8960/full/v2/i6/215.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i6.215
Primary tumors of the renal pelvis account for approximately 7% of all renal tumors[1]; most of them are urothelial carcinoma. Urothelium can display a wide range of metaplastic changes and neoplasms arising from this epithelium can show several types of differentiation, especially in high-grade neoplasms. Unlike bladder urothelial carcinomas, the majority of primary urothelial carcinomas of the renal pelvis present with a high histological grade and show a tendency to display unusual morphological features with a metaplastic phenomena and aggressive behavior. Due to their location inside the kidney, a delay in diagnosis may happen with advanced stages, metastatic disease or massive infiltration of the kidney[2,3]. The sarcomatoid subtype is a kind of high grade urothelial carcinoma, a histological variant defined by a biphasic differentiation, epithelial and mesenchymal. Few cases have been reported in the literature since 1961 when Fauci and collegues reported the first case[4].
We present a 58-year-old man assessed for a general syndrome and isolated episode of hematuria a few months previously.
His medical history included being a heavy smoker and alcohol consumer, deep venous thrombosis and alcoholic chronic pancreatitis with partial pancreatectomy 10 years previously.
From the urological point of view, an isolated episode of gross hematuria was described and was concomitant with a high dosage of oral anticoagulants. At this time, ultrasound and cystoscopy were within the normal limits.
In hospital, he was afebrile with low levels of blood pressure. His blood analysis showed anemia (hemoglobin 8.6 g/dL), discrete leukocytosis and serum creatinine within normal limits.
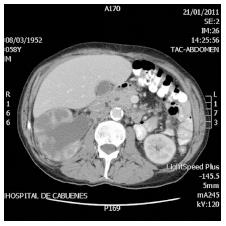
An abdominal computed tomography (CT) was requested which described a bizarre right kidney with intraparenchymatous levels of liquid with the renal pelvis and right ureter dilated up to the entrance of the bladder (Figure 1).
Initially, endovenous third generation cephalosporin treatment was administered and a ureteral stent was placed. After 12 h of observation, the clinical course worsened, with severe hypotension and elevated blood analysis parameters of lactic acid, C protein and procalcitonin. In this critical situation of septic shock, an emergency right nephrectomy was performed with an eleventh rib incision. The intraoperative description was of a dilated right kidney with plentiful purulent liquid linked with a pyonephrosis with no apparent cause. The patient had a normal postoperative course and was discharged on the seventh day.
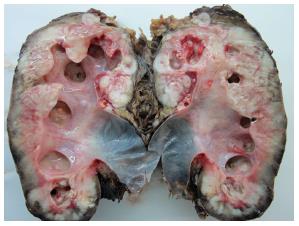
The surgery specimen weighed 500 g and the volume was 15 cm × 9 cm × 7 cm, with thick parenchyma with pyelocalyceal dilation with purulent content at the sagittal section. Necrotic areas spread outside the renal tissue, the perinephric fatty tissue (Figure 2).
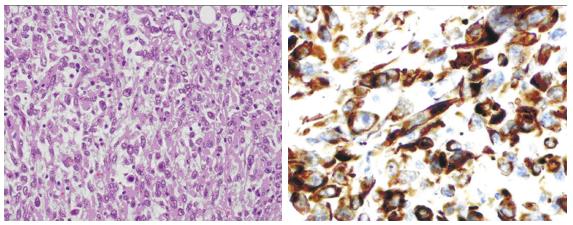
The pathology report described a sarcomatoid urothelial carcinoma with invasion into the parenchyma, renal sinus, perinephric fatty, lymphovascular and neural tissue. Carcinoma in situ was also associated with the upper calyceal system according to the American joint committee of cancer (AJCC), seventh edition, pT4NX. Immunochemistry studies revealed positivity to CK7, CD10 and vimentin. Moreover, the report described intense proliferative activity Ki67, 80% (Figure 3).
Chemotherapy was rejected due to the clinical situation of the patient and the patient refused a new surgical intervention in order to eliminate the ureteral remnant. The patient was followed up periodically and 18 mo after nephrectomy he was asymptomatic with no signs of relapse on imaging techniques.
Transitional cell carcinoma of the renal pelvis and ureter are relatively rare and are less than 1% of all genitourinary cancers, likewise 5% and 7% of urinary tract tumors[5]. Tumors arising from the renal pelvis are morphologically similar to those from the bladder. Their incidence varies from 0.7 to 1.1 per 100000 with a male to female ratio of 1.7 to 1, but with an increasing trend in women. They more frequently appear in the elderly (mean age 70 years). Over 90% of tumors arising from the renal pelvis and ureter are urothelial carcinomas. Hematuria and back pain are the most common signs and symptoms[6]. Hematuria, either gross or microscopic, is present in 75%-90% of cases. Back pain occurs in 20%-40% of cases, usually secondary to obstruction by the tumor that can mimic a ureteral calculus. Urinary symptoms, such as dysuria, urinary frequency, nocturia or urinary retention, can be found in up to 25%-50% of patients. The physical examination is usually normal, with the exception of a lumbar palpable mass in less than 10% of patients[5].
Sarcomatoid transitional cell carcinoma (sarcomatoid carcinoma) of the renal pelvis should not be confused with sarcomatoid renal cell carcinoma, an undifferentiated high grade epithelial tumor whose origin is at the parenchyma. Another tumor with a bad prognosis is the collecting duct carcinoma (Bellini duct carcinoma) which is centered in the medulla, develops a tubulopapillary architecture and is surrounded by a desmoplastic reaction[7]. Radiologically, some researchers reported that sarcomatoid carcinomas and renal cell carcinomas are indistinguishable from each other.
The term “transitional cell carcinoma with sarcomatoid differentiation” should be used in solid tumors with biphasic epithelial and mesenchymal differentiation (with the presence or absence of heterologous components).
Another neoplasm considered at the differential diagnosis is carcinosarcoma. Carcinosarcomas and sarcomatoid carcinomas are difficult to distinguish in hematoxylin-eosin samples. Carcinosarcomas are composed of two components, epithelial and sarcomatous, and sarcomatoid carcinomas are malignant epithelial tumors which show sarcomatoid changes[8]. There is too much confusion and disagreement in the literature regarding the nomenclature and histogenesis of these tumors. In some series, both terms, carcinosarcoma and sarcomatoid carcinoma, are included under the term “sarcomatoid carcinoma” and in others they are listed as two different entities[3,4,9,10].
In addition, the differential diagnosis should also include locally aggressive and benign conditions, such as postoperative spindle cell nodes and pseudotumors as inflammatory myofibroblastic tumors.
Histologically, sarcomatoid areas may be combined with foci of transitional cell carcinoma, squamous cell carcinoma, adenocarcinoma or small cell carcinoma. Heterologous differentiation may be present but has no prognostic significance. All sarcomatoid carcinomas are high-grade and have a poor prognosis. These tumors show no difference in survival when compared stage by stage with conventional urothelial carcinoma. In the absence of invasive urothelial carcinoma or obvious epithelial differentiation, a prior history of urothelial carcinoma, the coexistence of urothelial carcinoma in situ, or immunoreactivity for cytokeratin or epithelial membrane antigen (EMA) in the sarcomatous areas are useful for the diagnosis of sarcomatoid carcinoma. The immunohistochemistry is the key to the diagnosis.
The immunoreactivity for keratins and EMA are specific for epithelial cells and the presence of epithelial markers in mesenchymal areas and/or the presence in sarcomatoid elements of ultrastructural features of epithelial differentiation (desmosomes or tonofilaments) suggest the diagnoses of sarcomatoid carcinoma. Likewise, the mesenchymal elements in carcinosarcomas do not stain with epithelial markers and have no desmosomes or tonofilaments[3].
The gold standard treatment for urothelial carcinoma is surgery, in this case nephroureterectomy, but a nephrectomy was only performed because the presence of an aggressive tumour was unsuspected. Neoadjuvant chemotherapy was not an option for this patient. On the one hand, it was an emergency operation and on the other hand, contrary to what has been demonstrated for bladder cancer, there have been no reported effects of neoadjuvant therapy for upper urinary tract cancer[11].
Adjuvant chemotherapy can somehow achieve a recurrence free rate of up to 50% but clearly has no impact on survival and no data are currently available to provide any recommendations[12]. At our institution, chemotherapy is only considered as palliative in cases of metastatic evolution or with the presence of symptoms.
Adjuvant radiotherapy may improve local control of the disease and can be combined with a cisplatinum regimen[13] but they are no longer considered at our center as a standard care for this kind of tumors.
This pT4Nx tumor is included in the IV stage according to AJCC classification (remember IV stage includes all pT4 with N+ or N0 and M+ or M0), the fact of pT4 being directly included in IV prognostic stage in spite of no pathological node report and no signs of metastatic disease. The observed overall survival with this classification with data taken from National Cancer Data Base for the year 2000 to 2002 is: 43.3% in 1 year, 24% in 2 years, 16.4% in 3 years, 12.4% in 4 years and 10.2% in 5 years.
An interesting paper describes the local relapse rate during a mean follow-up of 58 mo in 14% of the patients, with the overall mortality of 14% and the mean survival of 109 mo. Stage T3 and T4 were significantly linked with survival[14].
We report the case of a 58-year-old man with the radiological finding of pyonephrosis and an emergency nephrectomy being performed with the worsening of the clinical condition. The pathology examination suggested a renal pelvis urothelial neoplasm with sarcomatoid differentiation. This neoplasm widely spread though perirenal fatty tissue and lymphatic vascular tissue.
Sarcomatoid subtype is a rare presentation of urothelial carcinoma and is linked with a bad prognosis. In the same sample, zones of epithelial carcinoma, adenocarcinoma and small cell carcinoma are usually described.
A 58-year-old man assessed for a general syndrome and isolated episode of hematuria.
The radiological finding of pyonephrosis and worsening of the clinical condition, with an emergency nephrectomy being performed.
Ultrasound, cystoscopy, computed tomography (CT).
Blood analysis showed anemia (hemoglobin 8.6 g/dL). The surgery specimen weighed 500 g and volume was 15 cm × 9 cm × 7 cm, with thick parenchyma with pyelocalyceal dilation with purulent content at the sagittal section.
An abdominal CT was requested and described a bizarre right kidney with intraparenchymatous levels of liquid, with the renal pelvis and right ureter dilated up to the entrance of the bladder.
The pathology report described a sarcomatoid urothelial carcinoma with invasion into the parenchyma, renal sinus, perinephric fatty, lymphovascular and neural tissue.
The clinical evolution with an emergency nephrectomy.
Sarcomatoid subtype is a rare presentation of urothelial carcinoma and is linked with a bad prognosis. In the same sample, zones of epithelial carcinoma, adenocarcinoma and small cell carcinoma are usually described.
The authors describe a case of pyonephrosis as a sign of sarcomatoid carcinoma of the renal pelvis. This is an interesting paper.
| 1. | Droller MJ. Transitional Cell Carcinoma: upper tracts and bladder. Campbells Urology. WB Saunders: Philadelphia 1986; 1343-1440. |
| 2. | Perez-Montiel D, Wakely PE, Hes O, Michal M, Suster S. High-grade urothelial carcinoma of the renal pelvis: clinicopathologic study of 108 cases with emphasis on unusual morphologic variants. Mod Pathol. 2006;19:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 139] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Lopez-Beltran A, Pacelli A, Rothenberg HJ, Wollan PC, Zincke H, Blute ML, Bostwick DG. Carcinosarcoma and sarcomatoid carcinoma of the bladder: clinicopathological study of 41 cases. J Urol. 1998;159:1497-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 165] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Lopez-Beltran A, Escudero AL, Cavazzana AO, Spagnoli LG, Vicioso-Recio L. Sarcomatoid transitional cell carcinoma of the renal pelvis. A report of five cases with clinical, pathological, immunohistochemical and DNA ploidy analysis. Pathol Res Pract. 1996;192:1218-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Ozsahin M, Ugurluer G, Zouhair A. Management of transitional-cell carcinoma of the renal pelvis and ureter. Swiss Med Wkly. 2009;139:353-356. [PubMed] |
| 6. | Lopez-Beltran A, Montironi R, Vidal-Jimenez A, Cheng L. Pathology of tumors of the renal pelvis and ureter, and the urethra. Clinical Pathology of Urologic Tumors. London: Informa Healthcare 2007; 235-242. [DOI] [Full Text] |
| 7. | Skinnider BF, Folpe AL, Hennigar RA, Lim SD, Cohen C, Tamboli P, Young A, de Peralta-Venturina M, Amin MB. Distribution of cytokeratins and vimentin in adult renal neoplasms and normal renal tissue: potential utility of a cytokeratin antibody panel in the differential diagnosis of renal tumors. Am J Surg Pathol. 2005;29:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Yilmaz E, Birlik B, Arican Z, Guney S. Carcinosarcoma of the renal pelvis and urinary bladder: a case report. Korean J Radiol. 2003;4:255-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Lopez-Beltran A, Sauter G, Gasser T, Hartmann A. Urothelial tumors: infiltrating urothelial carcinoma. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. Lyon: IARC Press 2004; . |
| 10. | Lopez-Beltran A, Cheng L. Histologic variants of urothelial carcinoma: differential diagnosis and clinical implications. Hum Pathol. 2006;37:1371-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Matin SF, Margulis V, Kamat A, Wood CG, Grossman HB, Brown GA, Dinney CP, Millikan R, Siefker-Radtke AO. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer. 2010;116:3127-3134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Vassilakopoulou M, de la Motte Rouge T, Colin P, Ouzzane A, Khayat D, Dimopoulos MA, Papadimitriou CA, Bamias A, Pignot G, Nouhaud FX. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer. 2011;117:5500-5508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
P- Reviewers: Ansari MS, Di Lorenzo G, Maurer T, Papatsoris AG S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wu HL