Published online Jun 16, 2014. doi: 10.12998/wjcc.v2.i6.211
Revised: March 26, 2014
Accepted: May 8, 2014
Published online: June 16, 2014
Processing time: 165 Days and 23.7 Hours
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder that almost exclusively involves motor neurons although autonomic dysfunction has also been reported. We present an 84-year-old female with no documented history of heart disease, who was admitted with negative T waves in the electrocardiogram precordial leads mimicking myocardial ischaemia. No other abnormalities were shown in the rest of the cardiologic evaluation, suggesting autonomic nervous system dysfunction. A neurophysiological study demonstrated acute and chronic denervation in multiple muscles with normal nerve conduction studies, confirming ALS diagnosis. Previous studies have shown that subclinical sympathetic hyperfunction and parasympathetic hypofunction might result in cardiovascular dysfunction in ALS patients. It is important to detect disturbances of autonomic cardiac control because this dysfunction may influence survival and quality of life, leading to a decrease in life expectancy in ALS patients. This Case Report may support the impairment of cardiac autonomic control in patients with ALS.
Core tip: A few cases showing electrocardiogram (ECG) abnormalities in amyotrophic lateral sclerosis (ALS) patients have been previously reported suggesting an autonomic disturbance in ALS. We present an ALS patient with abnormal ECG mimicking myocardial ischaemia, in whom both coronary disease and cardiac anatomic damage were ruled out supporting the autonomic nervous system involvement in this mainly motor neuron disease.
- Citation: Martínez J, Ramón C, Morís C, Pascual J, Morís G. Abnormal electrocardiogram in a patient with amyotrophic lateral sclerosis mimicking myocardial ischaemia. World J Clin Cases 2014; 2(6): 211-214
- URL: https://www.wjgnet.com/2307-8960/full/v2/i6/211.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i6.211
Amyotrophic lateral sclerosis (ALS) is an idiopathic, fatal neurodegenerative disease caused by degeneration of the first and second motoneurons. Recent advances indicate heterogeneity in phenotype, pathological substrate and genetic predisposition, suggesting that ALS should be considered a syndrome rather than a single disease entity. Therefore, the clinical presentation and progression of ALS may vary considerably. Cognitive and behavioural impairment is a frequent feature of ALS but other non-motor clinical features, such as autonomic nervous system (ANS) dysfunction, are underreported[1,2]. The affection of the ANS in ALS, as part of a complex degenerative process, has an increasing evidence and it is postulated that ALS patients develop dysautonomic dysfunction that may involve the heart[3].
Herein, we report an ALS patient with both negative T waves on the electrocardiogram (ECG) and no data of underlying coronary disease, supporting ANS dysfunction in ALS.
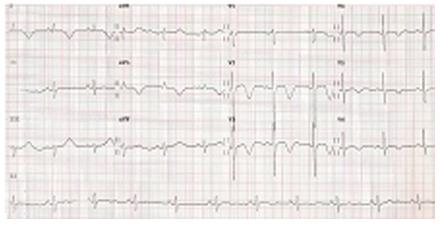
An 84-year-old female with no history of heart disease was referred to our hospital because of chest pain, dyspnoea and abnormal ECG showing negative T waves in precordial leads V2-V6 and I, aVL (Figure 1). Initially, the patient had been admitted to a different hospital due to chest pain and progressive dyspnoea. While performing an electromyogram, an episode of shortness of breath, rales on auscultation and desaturation was documented. A chest radiograph was performed suggesting cardiac failure and an ECG showed anterior and lateral subepicardial ischaemia. Diagnostic of acute coronary syndrome was done and the patient was transferred to our Hospital.
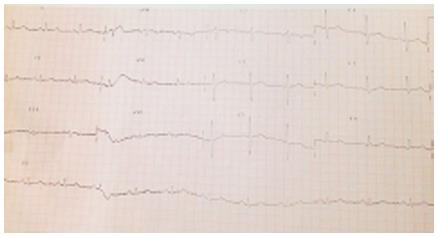
The patient had a diagnosis of hypertension made several years before, being under enalapril treatment since then. No other treatments had ever been prescribed. There was no history of myocardial infarction, myocarditis, cardiomyopathy, pericarditis, hyper- or hypothyroidism or calcium metabolism disturbances. An ECG performed 10 years ago showed no abnormalities (Figure 2); no other ECGs were performed before this episode. The patient was feeling well until six months ago, when she started experiencing painless and progressive weakness in her left hand. During the following weeks, weakness progressed to proximal and distal muscles in both upper limbs.
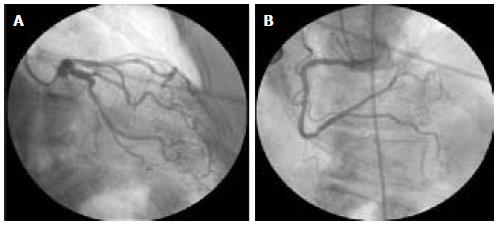
Neurological examination showed a normal mental status. Her speech was dysarthric, no facial paresis was noted but mild lingual weakness was observed. The visual fields were intact and ocular movements were full and smooth. Asymmetric muscle weakness and atrophy, involving the upper extremities, were present with severe atrophy of the left hand muscles. Mild proximal paresis was observed in both lower limbs. Fasciculations were noted in the tongue, upper, and lower extremities. Deep-tendon reflexes were brisk and there were bilateral ankle clonus. There was jaw hyperreflexia. Plantar responses were extensor. There were no sensory deficits. Bowel and bladder function remained normal. Complete blood cell count was normal, as were electrolyte, Troponin T, creatine kinase, creatinine, fasting plasma glucose and haemoglobin A1c concentrations. Liver function and thyroid function studies yielded normal results. A brain CT scan depicted no abnormalities and a cervical MRI showed no abnormal findings at the spinal cord or the nerve roots. A neurophysiological study demonstrated acute and ongoing chronic partial denervation in multiple muscles of bulbar region and both upper and lower extremities with normal nerve conduction studies. The diagnosis of definitive ALS was made according to the Awaji–Shima criteria[4]. Echocardiography showed 16 mm left ventricular symmetrical hypertrophy with normal wall motion and a coronary angiography showed no significant anomalies (Figure 3). The patient suffered a progressive respiratory failure and she died four days after hospital admission.
There is well known that various central nervous system disorders can cause ECG abnormalities that mimic coronary syndromes including S-T elevation, T wave inversion and Q-T prolongation. These findings have been well described in relation with subarachnoid or subdural haematomas. Central o peripheral autonomic dysfunction has been also described in patient with Parkinson′s disease, multiple system atrophy or Guillain-Barré syndrome which increases the risk for arrhythmias, therefore, ECG monitoring is essential[5].
The impact of ALS on the cardiovascular system is well known. Several studies have shown that subclinical sympathetic hyperfunction and parasympathetic hypofunction might result in cardiovascular dysfunction in ALS patients[3,6-10]. There are very few reports describing the ECG characteristics in relation with this pathology. ECG alterations in ALS have been presented as a pseudo-ischaemic pattern, however a pseudo-myocardial infarction pattern has also been described[11-13]. Moreover, an elevated Troponin T levels in a patient with ALS without underlying ischaemic cardiopathy has been reported as a consequence of hypoxic respiratory failure or as immune-mediated myocardial injury secondary to ALS[14].
In ALS, involvement of sympathetic neurons has been associated with neuron degeneration in the intermediolateral nucleus of the upper thoracic spinal cord, causing subclinical findings such as reduction in nocturnal blood pressure and loss of correlation between blood pressure and heart rate. In a study analyzing changes in the corrected QT interval (QTc) and QTc dispersion, ECG showed that both the average QTc and QTc dispersion was significantly higher in patients with ALS supporting sympathetic disturbances in this motoneuron disease[6]. Furthermore, Pavlovic et al[3], studied the cardiovascular autonomic control in 55 patients with ALS and compared it with 30 healthy controls. They found that patients with ALS have a significantly higher degree of both sympathetic and parasympathetic dysfunction with relative sympathetic predominance compared with controls. Disturbances of autonomic cardiac control in ALS patients may influence survival and quality of life predisposing to hypertensive crisis, sudden cardiac death, and cardiovascular collapse, all leading to a decrease in life expectancy[3,10,15]. In addition, recent studies have established the contribution of neuronal ion channel dysfunction to the pathophysiology of ALS, mainly Na+ and K+ channels; moreover, the modulation of ion channel function has been proposed as the mechanism by which riluzole exerts the neuroprotective effects in ALS[16-18]. Based on ion channels dysfunction implicated in heart disease, it could also be argued that ECG changes in ALS patients may be related with ion channel dysfunction[19].
We present an ALS patient with an ECG showing negative T waves in precordial leads mimicking myocardial ischaemia. Complementary tests ruled out systemic and cardiologic causes that might have been associated with these ECG disturbances; therefore, an association between the pseudo-ischaemic ECG and ALS was suspected. The underlying mechanism of these abnormalities in the ECG must be addressed although ANS dysfunction has been proposed. Unfortunately, neither a baseline ECG nor a follow-up ECG after the acute episode were performed and the only previous ECG was done 10 years before. However, we cannot completely rule out that other underlying processes not covered by our investigations were the cause. In conclusion, it is important to detect disturbances of autonomic cardiac dysfunction in ALS patients to avoid sudden death or other conditions leading to a decrease in life expectancy.
An 84-year-old female with a diagnosis of amyotrophic lateral sclerosis (ALS) presented with negative T waves in the electrocardiogram (ECG) mimicking myocardial ischaemia.
The patient exhibited classical clinical features of ALS and an ECG showed negative T waves in precordial leads I, aVL and V2-V6.
Differential diagnosis included myocardial infarction or ischaemia, myocarditis, cardiomyopathy, pericarditis, hyper- or hypothyroidism or calcium metabolism disturbances.
A neurophysiological study demonstrated acute and ongoing chronic partial denervation in multiple muscles of bulbar region and both upper and lower extremities with normal nerve conduction studies.
The cardiologic studies including echocardiogram and coronary angiography were normal.
The patient did not receive any treatment and she died in a few days.
A literature search revealed only a few cases of abnormal ECG in patients with ALS mimicking myocardial ischaemia or infarct.
The Awaji-Shima criteria in the diagnosis of ALS were proposed in 2008 to enable earlier diagnosis of ALS to obviate diagnostic delay and to promote earlier entry into clinical trials.
The affection of the autonomic nervous system in ALS has increasing evidence and it is postulated that ALS patients develop dysautonomic dysfunction that may involve the heart
This is a well-written, interesting case report with good images.
| 1. | Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 442] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 2. | Kiernan MC, Vucic1 S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet. 2011;377:942-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2163] [Cited by in RCA: 1904] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 3. | Pavlovic S, Stevic Z, Milovanovic B, Milicic B, Rakocevic-Stojanovic V, Lavrnic D, Apostolski S. Impairment of cardiac autonomic control in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Costa J, Swash M, de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis: a systematic review. Arch Neurol. 2012;69:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Marsh E, O’Callaghan P, Smith P. The humble electrocardiogram. Pract Neurol. 2008;8:46-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Asai H, Hirano M, Udaka F, Shimada K, Oda M, Kubori T, Nishinaka K, Tsujimura T, Izumi Y, Konishi N. Sympathetic disturbances increase risk of sudden cardiac arrest in sporadic ALS. J Neurol Sci. 2007;254:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Chida K, Sakamaki S, Takasu T. Alteration in autonomic function and cardiovascular regulation in amyotrophic lateral sclerosis. J Neurol. 1989;236:127-130. [PubMed] |
| 8. | Kandinov B, Korczyn AD, Rabinowitz R, Nefussy B, Drory VE. Autonomic impairment in a transgenic mouse model of amyotrophic lateral sclerosis. Auton Neurosci. 2011;159:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Baltadzhieva R, Gurevich T, Korczyn AD. Autonomic impairment in amyotrophic lateral sclerosis. Curr Opin Neurol. 2005;18:487-493. [PubMed] |
| 10. | Shimizu T, Hayashi H, Kato S, Hayashi M, Tanabe H, Oda M. Circulatory collapse and sudden death in respirator-dependent amyotrophic lateral sclerosis. J Neurol Sci. 1994;124:45-55. [PubMed] |
| 11. | Li AH, Hsu KL, Liau CS, Tseng YZ, Lee YT. Amyotrophic lateral sclerosis with a ‘pseudo-infarction’ pattern on the electrocardiograph. A case report. Cardiology. 2000;93:133-136. [PubMed] |
| 12. | Rodriguez-Castro CE, Elfar A, Gonzalez-Ibarra FP, Siddiqui T, Abbas A. Amyotrophic lateral sclerosis and pseudo-infarct pattern on the electrocardiogram. Am J Med. 2013;126:e3-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Zhang J, Yang SW, Wang Z, Wei GR, Zhou YJ. Pseudo-ischaemic ECG in a patient with amyotrophic lateral sclerosis surviving for a decade. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Von Lueder TG, Melsom MN, Atar D, Agewall S. Amyotrophic lateral sclerosis (ALS), a novel rare cause of elevated plasma troponin T levels. Clin Lab. 2011;57:615-618. [PubMed] |
| 15. | Gil J, Funalot B, Verschueren A, Danel-Brunaud V, Camu W, Vandenberghe N, Desnuelle C, Guy N, Camdessanche JP, Cintas P. Causes of death amongst French patients with amyotrophic lateral sclerosis: a prospective study. Eur J Neurol. 2008;15:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 118] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 16. | Kanai K, Kuwabara S, Misawa S, Tamura N, Ogawara K, Nakata M, Sawai S, Hattori T, Bostock H. Altered axonal excitability properties in amyotrophic lateral sclerosis: impaired potassium channel function related to disease stage. Brain. 2006;129:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Krishnan AV, Lin CS, Park SB, Kiernan MC. Axonal ion channels from bench to bedside: a translational neuroscience perspective. Prog Neurobiol. 2009;89:288-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Vucic S, Lin CS, Cheah BC, Murray J, Menon P, Krishnan AV, Kiernan MC. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain. 2013;136:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Chockalingam P, Wilde A. The multifaceted cardiac sodium channel and its clinical implications. Heart. 2012;98:1318-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
P- Reviewers: Chu D, Hirose H, Riva N, Schoenhagen P, Trohman RG S- Editor: Song XX L- Editor: A E- Editor: Wu HL