Published online Feb 16, 2026. doi: 10.12998/wjcc.v14.i5.118498
Revised: January 24, 2026
Accepted: February 2, 2026
Published online: February 16, 2026
Processing time: 37 Days and 15.9 Hours
Melioidosis, caused by Burkholderia pseudomallei (B. pseudomallei), is endemic to Southeast Asia and northern Australia, with clinical manifestations ranging from localized infection to life-threatening disseminated disease. Mycotic aneurysm is a rare but serious complication of disseminated melioidosis, associated with high morbidity and mortality.
We describe a case of a 67-year-old man with diabetes mellitus who presented with B. pseudomallei bacteremia complicated by multiple deep-seated abscesses and multifocal mycotic aneurysms of the descending thoracic aorta. Despite ap
This case illustrates the aggressive nature of disseminated melioidosis with vascular involvement and highlights the need for early diagnosis and multidisciplinary management to improve outcomes.
Core Tip: Melioidosis is a potentially severe infectious disease caused by Burkholderia pseudomallei, with clinical manifestations ranging from localized infection to fulminant disseminated disease. Although vascular involvement is an uncommon complication, mycotic aneurysm represents a particularly severe manifestation. Involvement of the thoracic aorta is especially rare and carries a high risk of morbidity and mortality. This case highlights disseminated melioidosis complicated by multifocal mycotic aneurysms of the descending thoracic aorta, emphasizing the need for early recognition, serial imaging, and multidisciplinary management to improve patient outcomes.
- Citation: Chang CY, Nor Aswan FA, Muhamad Yazid MA. Disseminated melioidosis presenting with multifocal thoracic aortic mycotic aneurysms: A case report. World J Clin Cases 2026; 14(5): 118498
- URL: https://www.wjgnet.com/2307-8960/full/v14/i5/118498.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i5.118498
Melioidosis is an infectious disease caused by the Gram-negative bacterium Burkholderia pseudomallei (B. pseudomallei), which is endemic to Southeast Asia and northern Australia. The clinical manifestations of melioidosis are diverse, ranging from localized skin and soft tissue infections to pneumonia, genitourinary involvement, visceral abscesses, septic arthritis, and neurological melioidosis. The disease carries a high mortality rate, particularly in cases presenting with acute fulminant sepsis[1,2].
Individuals with frequent exposure to soil and surface water are at greatest risk, with diabetes mellitus, hazardous alcohol consumption, chronic kidney disease, immunosuppressive therapy, and thalassemia recognized as major predisposing factors[3]. The primary routes of transmission are believed to be percutaneous inoculation, inhalation, and ingestion. Person-to-person transmission is exceedingly rare, with sexual transmission proposed but not definitively established[4].
Melioidosis-related mycotic aneurysm is an uncommon but life-threatening complication, associated with high morbidity and mortality. In Malaysia, reports of mycotic aneurysms secondary to B. pseudomallei are limited[5]. Here, we describe a rare case of melioidosis-related mycotic aneurysm involving the descending thoracic aorta, accompanied by splenic and prostate abscesses.
A 67-year-old man presented with a two-week history of persistent fever, reduced oral intake, and vague lower abdo
Fever for two weeks associated with reduced oral intake and vague lower abdominal discomfort. He denied dysuria, urinary frequency, haematuria, chest pain, cough, or shortness of breath. There was no recent history of travel or contact with unwell individuals. He reported engaging in home gardening but denied recent soil exposure or skin injuries.
He was initially admitted to a district hospital, where blood cultures grew B. pseudomallei, identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Antimicrobial susceptibility testing demonstrated sensitivity to ceftazidime, meropenem, and trimethoprim–sulfamethoxazole. Intravenous ceft
The patient had underlying type 2 diabetes mellitus, hypertension, and a prior ischemic stroke. There was no history of chronic kidney disease, malignancy, or immunosuppressive therapy.
He was retired and lived independently. He denied smoking, alcohol consumption, and recreational drug use. There was no significant family history of infectious or hereditary diseases.
On admission, the patient was febrile with a temperature of 38.4 °C, heart rate of 96 beats per minute, blood pressure of 138/76 mmHg, and oxygen saturation of 98% on room air. He appeared comfortable at rest. Abdominal examination revealed mild lower abdominal tenderness without guarding or palpable masses. Cardiovascular and respiratory exami
Blood cultures were positive for B. pseudomallei, identified by MALDI-TOF MS. Antimicrobial susceptibility testing showed susceptibility to ceftazidime, meropenem, and trimethoprim-sulfamethoxazole.
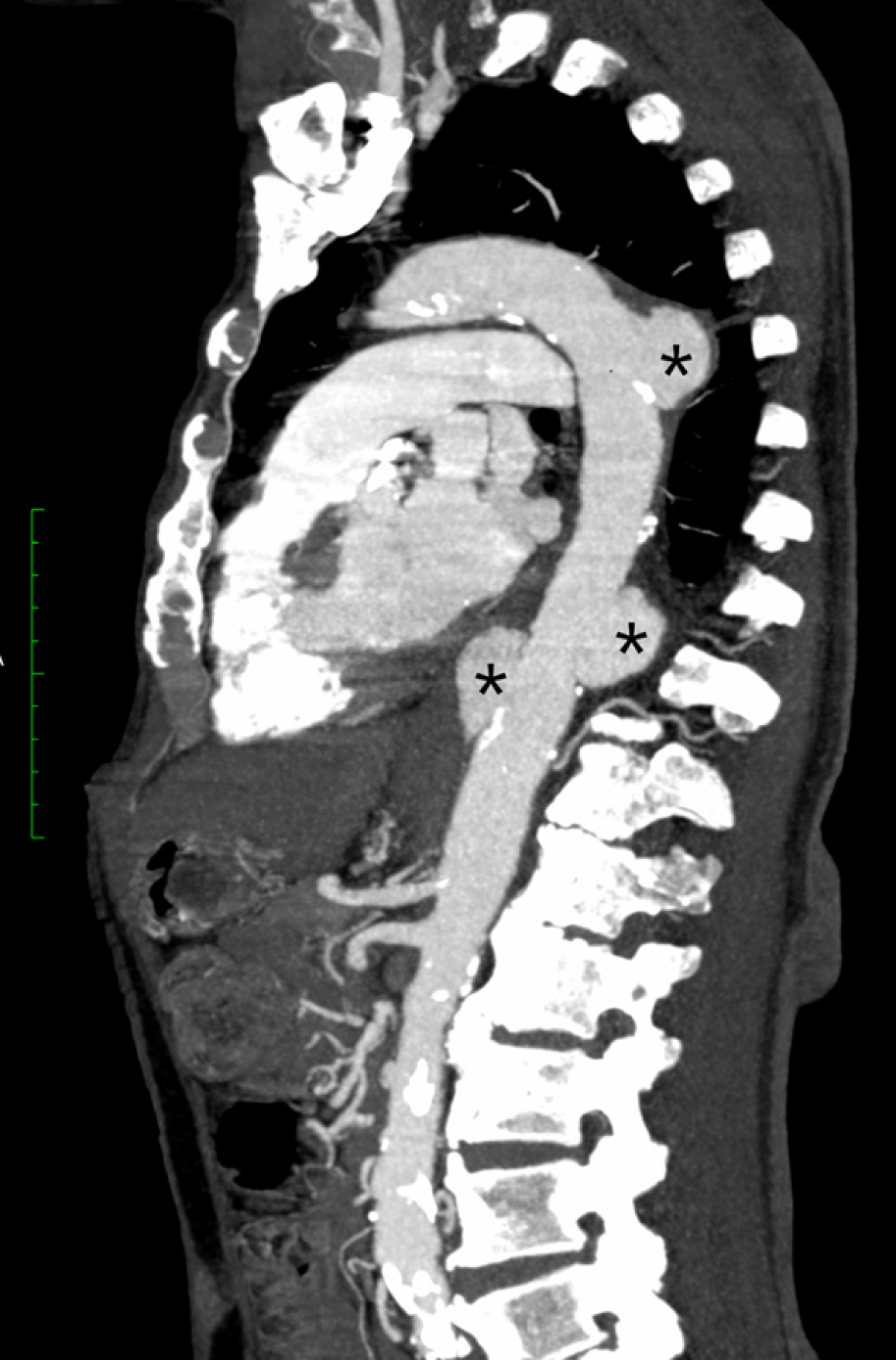
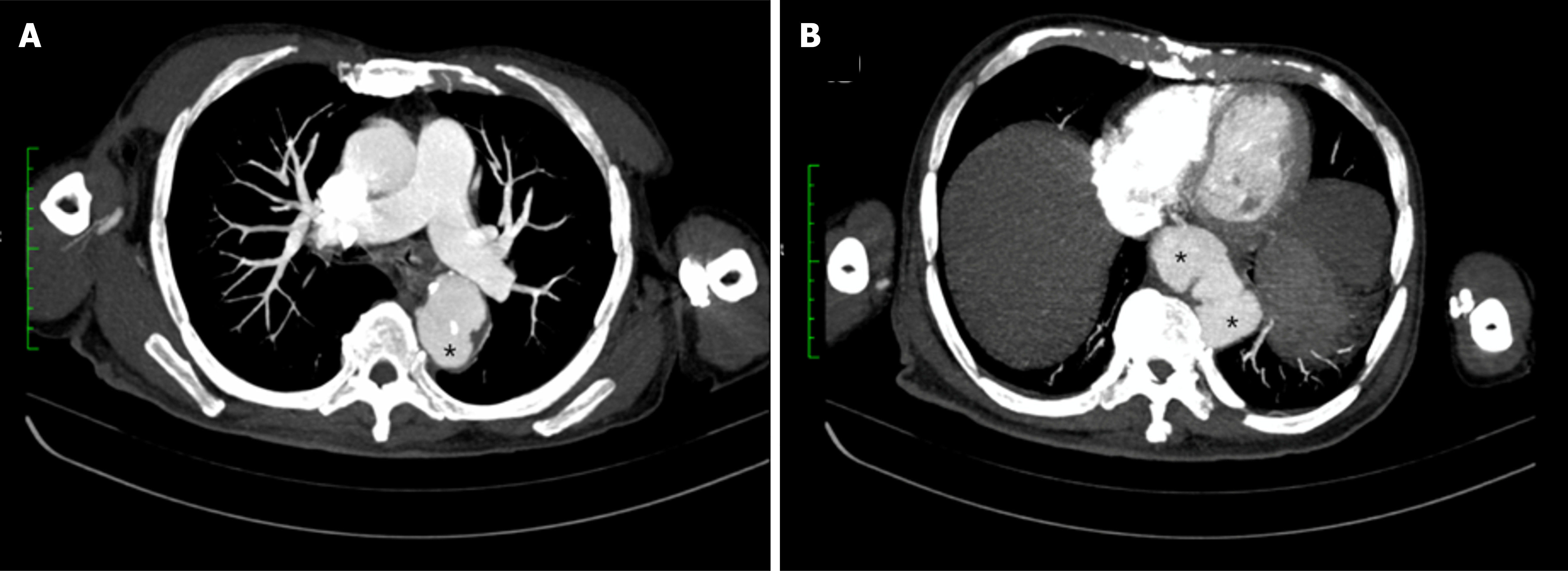
Abdominal ultrasonography demonstrated no intra-abdominal collections. In view of persistent fever and the high risk of deep-seated infection associated with B. pseudomallei bacteraemia, a contrast-enhanced computed tomography (CT) scan of the thorax, abdomen, and pelvis was performed. This revealed multifocal saccular aneurysmal dilatations of the descending thoracic aorta at the T5 and T11 vertebral levels, with associated periaortic inflammatory changes and collections, consistent with mycotic aneurysms (Figures 1 and 2). Additional findings included multiple splenic abscesses, a prostatic abscess, and a thick-walled cavitary lesion in the left upper lobe consistent with pulmonary melioidosis.
Disseminated melioidosis complicated by multifocal mycotic aneurysms of the descending thoracic aorta, splenic abs
Intravenous ceftazidime 2 g every six hours was continued. Oral trimethoprim–sulfamethoxazole was added during the second week of therapy. Cardiothoracic surgical consultation initially recommended conservative management with antimicrobial therapy. Despite radiological progression of the aortic aneurysms, the patient declined surgical inter
Repeat contrast-enhanced CT imaging demonstrated progressive enlargement of the multifocal thoracic aortic mycotic aneurysms despite interval reduction in the size of the splenic and prostatic abscesses. Clinically, the patient showed symptomatic improvement with resolution of fever and stabilization of vital signs. Surgical intervention was again recommended; however, he declined operative management. He was discharged with plans for close outpatient follow-up and serial imaging. Unfortunately, the patient did not attend scheduled follow-up appointments and remained uncontactable despite multiple attempts.
Melioidosis is a potentially life-threatening infection caused by B. pseudomallei, which is endemic to Southeast Asia and northern Australia. The disease is associated with diverse clinical manifestations, ranging from localized abscesses to severe pneumonia, septicaemia, and disseminated infection with visceral abscess formation. Among its rarer but recog
Melioidosis-related mycotic aneurysm is an uncommon entity, but its true incidence is likely underestimated, particularly in endemic areas where awareness and routine screening may be limited. This complication results from hematogenous seeding of the vascular endothelium by B. pseudomallei, leading to inflammation, destruction of the arterial wall, and subsequent aneurysm formation[6,7]. The abdominal aorta is the most common site of mycotic aneurysm in melioidosis, as demonstrated in a case series from China, where six of eight patients had abdominal aortic involvement, while the remaining cases involved the left iliac artery and the mesenteric artery, respectively[8]. The descending thoracic aorta, as seen in our patient, is a less commonly reported site of involvement.
Several case series have highlighted the severe morbidity and mortality associated with melioidosis-related mycotic aneurysm. Azizi et al[9] described three cases of pseudoaneurysm secondary to melioidosis, all of whom underwent aneurysmectomy and graft placement. Despite surgical intervention, one patient died, presumably due to post-operative complications. In a larger series, Wu et al[8] reported eight cases of melioidosis-related mycotic aneurysm in Hainan province, China. Six patients survived following a combination of surgical repair and antimicrobial therapy, resulting in a mortality rate of 25%.
In our case, the patient presented with B. pseudomallei bacteremia complicated by multiple deep-seated abscesses and descending thoracic aortic mycotic aneurysms. Despite initial appropriate antimicrobial therapy, follow-up imaging demonstrated progressive enlargement of the aneurysms, reflecting the aggressive nature of the infection. Surgical repair was indicated; however, the patient declined operative intervention after extensive discussion regarding the risks and benefits.
The management of melioidosis-related mycotic aneurysm typically involves prolonged antimicrobial therapy alongside timely surgical or endovascular intervention. The recommended intensive phase includes intravenous cefta
Melioidosis-related mycotic aneurysm remains a rare but serious complication. The descending thoracic aorta is an uncommon site for vascular involvement in melioidosis. Multidisciplinary involvement, including infectious disease specialists, radiologists, and vascular or cardiothoracic surgeons, is critical in guiding individualized treatment strategies.
| 1. | Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, Limmathurotsakul D. Melioidosis. Nat Rev Dis Primers. 2018;4:17107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 478] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 2. | Dance DA, Limmathurotsakul D. Global Burden and Challenges of Melioidosis. Trop Med Infect Dis. 2018;3:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, Budhsarawong D, Mootsikapun P, Wuthiekanun V, Teerawatasook N, Lulitanond A. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Chang CY, Lau NLJ, Currie BJ, Podin Y. Disseminated melioidosis in early pregnancy - an unproven cause of foetal loss. BMC Infect Dis. 2020;20:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (3)] |
| 5. | Nathan S, Chieng S, Kingsley PV, Mohan A, Podin Y, Ooi MH, Mariappan V, Vellasamy KM, Vadivelu J, Daim S, How SH. Melioidosis in Malaysia: Incidence, Clinical Challenges, and Advances in Understanding Pathogenesis. Trop Med Infect Dis. 2018;3:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 6. | Boyle R, Withey G, Smith S, Hanson J. Mycotic aneurysms due to Burkholderia pseudomallei in Far North Queensland, tropical Australia: A case series and review of the literature. Acta Trop. 2024;260:107480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Cham CY, Choo KSY, Rajahram GS, Chang CY. Mycotic Aneurysm Due to Burkholderia pseudomallei: A Case Report From Malaysia. Cureus. 2025;17:e77177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Wu H, Wang X, Zhou X, Wu Z, Wang Y, Pan M, Lu B. Mycotic aneurysm secondary to melioidosis in China: A series of eight cases and a review of literature. PLoS Negl Trop Dis. 2020;14:e0008525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Azizi ZA, Yahya M, Lee SK. Melioidosis and the vascular surgeon: Hospital Kuala Lumpur experience. Asian J Surg. 2005;28:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Sullivan RP, Marshall CS, Anstey NM, Ward L, Currie BJ. 2020 Review and revision of the 2015 Darwin melioidosis treatment guideline; paradigm drift not shift. PLoS Negl Trop Dis. 2020;14:e0008659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/