Published online Feb 16, 2026. doi: 10.12998/wjcc.v14.i5.117850
Revised: January 9, 2026
Accepted: January 26, 2026
Published online: February 16, 2026
Processing time: 55 Days and 3.3 Hours
Endoscopic vacuum therapy (EVT) has emerged as a secure and efficient organ-preserving option for gastrointestinal perforations, leaks, and fistulas, with high closure rates and low mortality.
A 41-year-old woman with a history of Roux-en-Y gastric bypass and diaphr
This case highlights the successful use of a staged multimodal EVT strategy to achieve definitive closure of a complex gastro-pleuro-broncho-cutaneous fistula.
Core Tip: This case demonstrates the successful resolution of a complex gastro-pleuro-broncho-cutaneous fistula using a staged endoscopic vacuum therapy (EVT) approach. Following failed surgical interventions, a sequential strategy involving initial percutaneous EVT followed by transluminal EVT was implemented. Concurrently, the bronchial defect was managed with argon plasma coagulation and a custom collagen-cellulose plug. This multidisciplinary technique achieved complete fistula closure and nutritional restoration, suggesting that staged EVT is a promising, organ-preserving salvage option for refractory, multi-system fistulas.
- Citation: Nunes BCM, Rocha RSP, Berzin TM, Franco MC, Kum AST. Staged multimodal endoscopic vacuum therapy for a complex gastro-pleuro-broncho-cutaneous fistula: A case report. World J Clin Cases 2026; 14(5): 117850
- URL: https://www.wjgnet.com/2307-8960/full/v14/i5/117850.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i5.117850
Gastrointestinal perforations, leaks, and fistulas are challenging complications for surgery and endoscopy, driving mortality rates up to 35%[1]. Endoscopic vacuum therapy (EVT), which applies continuous negative pressure to drain collections, accelerate granulation and foster defect contraction, achieves closure rates from 80% to 100%, with lower mortality than stenting or repeat surgery[1-3]. Here we report a gastro-pleuro-broncho-cutaneous fistula that develops after an empyema in a patient with prior Roux-en-Y gastric bypass and diaphragmatic repair with biological mesh. We describe a stepwise application of EVT, initially percutaneous and later transluminal, supplemented by multimodal strategy, such as bronchial edge ablation and deployment of a collagen-cellulose plug, to cure a chronic, multisystem fistula after multiple failed thoracic operations, thereby underscoring EVT’s expanding role as a minimally invasive, organ-preserving solution for complex fistulas once deemed surgical dead-ends.
Although EVT has been increasingly reported for upper gastrointestinal leaks[2,3], its role in the management of complex fistulas involving the pleural and bronchial systems remains poorly described. Reports addressing a stepwise EVT strategy combined with bronchial endoscopic interventions in post-bariatric surgery patients are particularly scarce. This case aims to illustrate such an approach in a highly refractory clinical scenario.
A 41-year-old woman with a history of Roux-en-Y gastric bypass was hospitalized for a chronic gastro-pleuro-broncho-cutaneous fistula, associated with weight loss and malnutrition refractory to conventional thoracic surgical management.
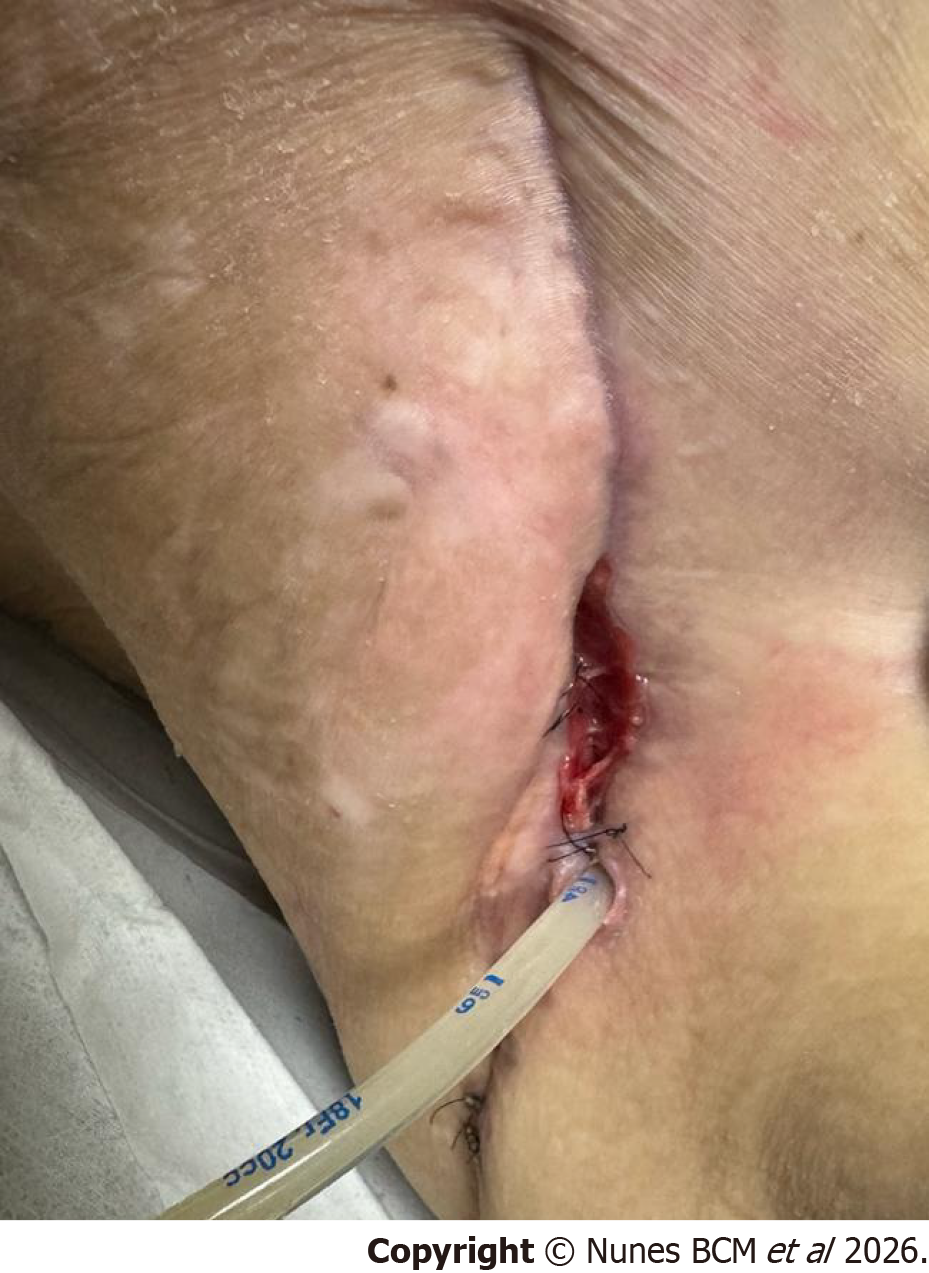
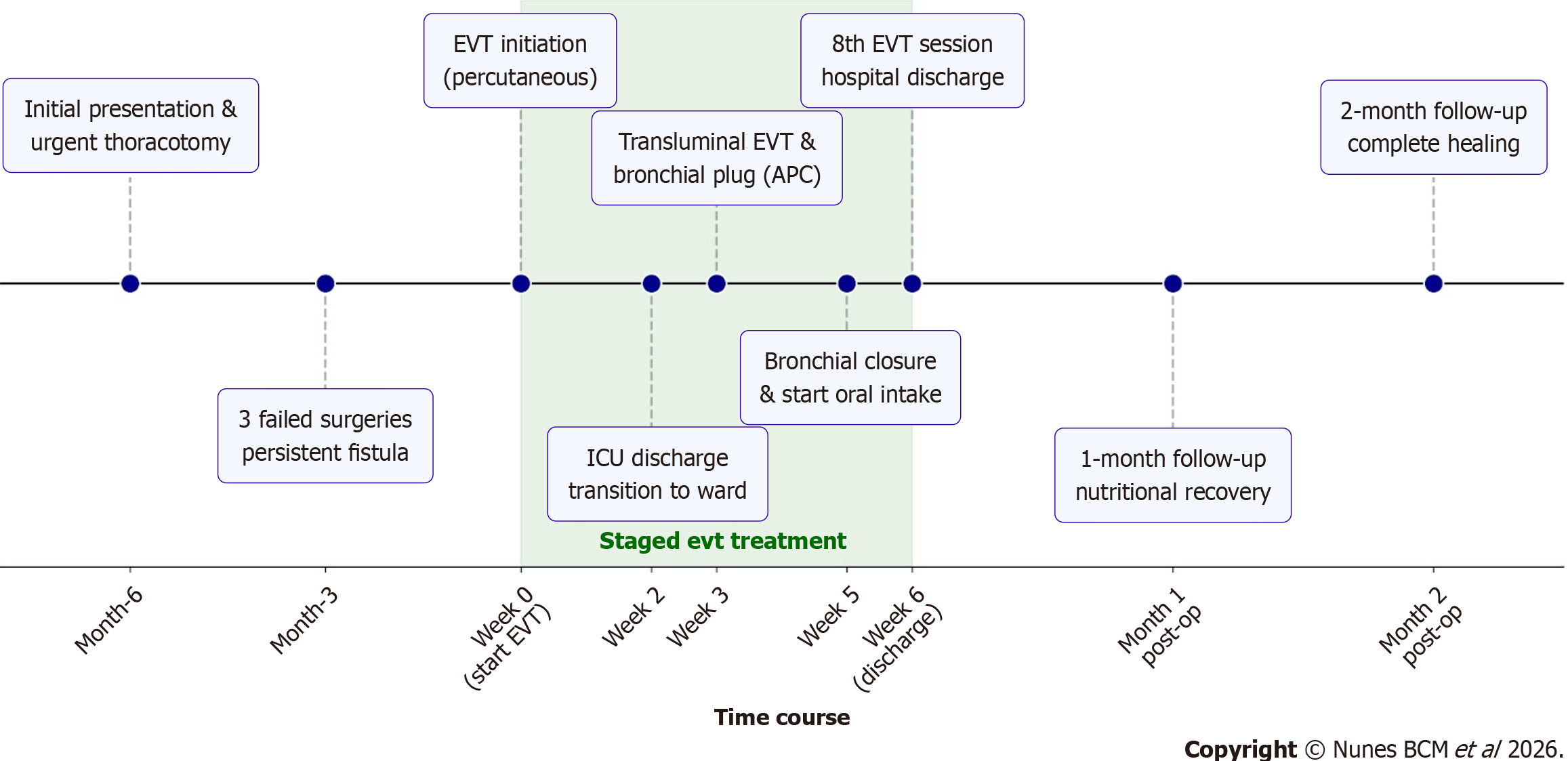
A 41-year-old woman with a history of Roux-en-Y gastric bypass (2007) and diaphragmatic repair with biological mesh (2007) presented in the emergency department with a 5-day history of cough, dyspnea, and fever. Chest computed tomography revealed a 131 mL heterogeneous pleural effusion with gas abutting the mesh in the left hemithorax, prompting urgent thoracotomy, which uncovered a new gastric pouch fistula draining into the pleural cavity and com
The patient underwent diaphragmatic repair with biological mesh in 2007 for complicated empyema secondary to pneumonia, three months after bariatric surgery.
The patient underwent Roux-en-Y gastric bypass in 2007.
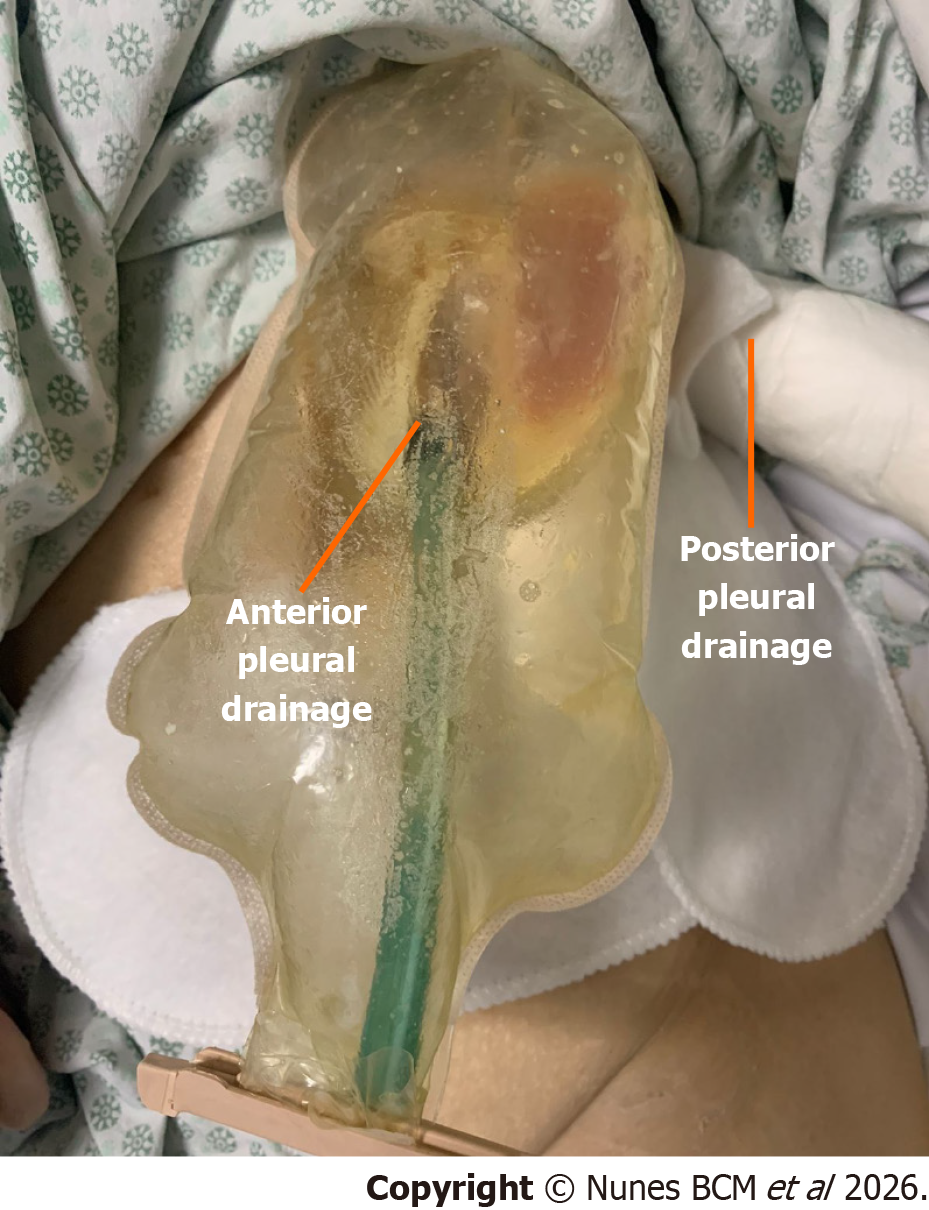
During intensive care unit (ICU) evaluation, the patient was prostrated, afebrile, and eupneic on room air, with severe malnutrition (46 kg; BMI 17.9) despite full enteral and parenteral nutritional support, and had two left-sided thoracic drains with high output of approximately 300-500 mL/day.
Initial laboratory findings demonstrated hypoalbuminemia (3.0 g/dL). Other laboratory parameters were within normal limits, including complete blood count, metabolic panel, liver enzymes.
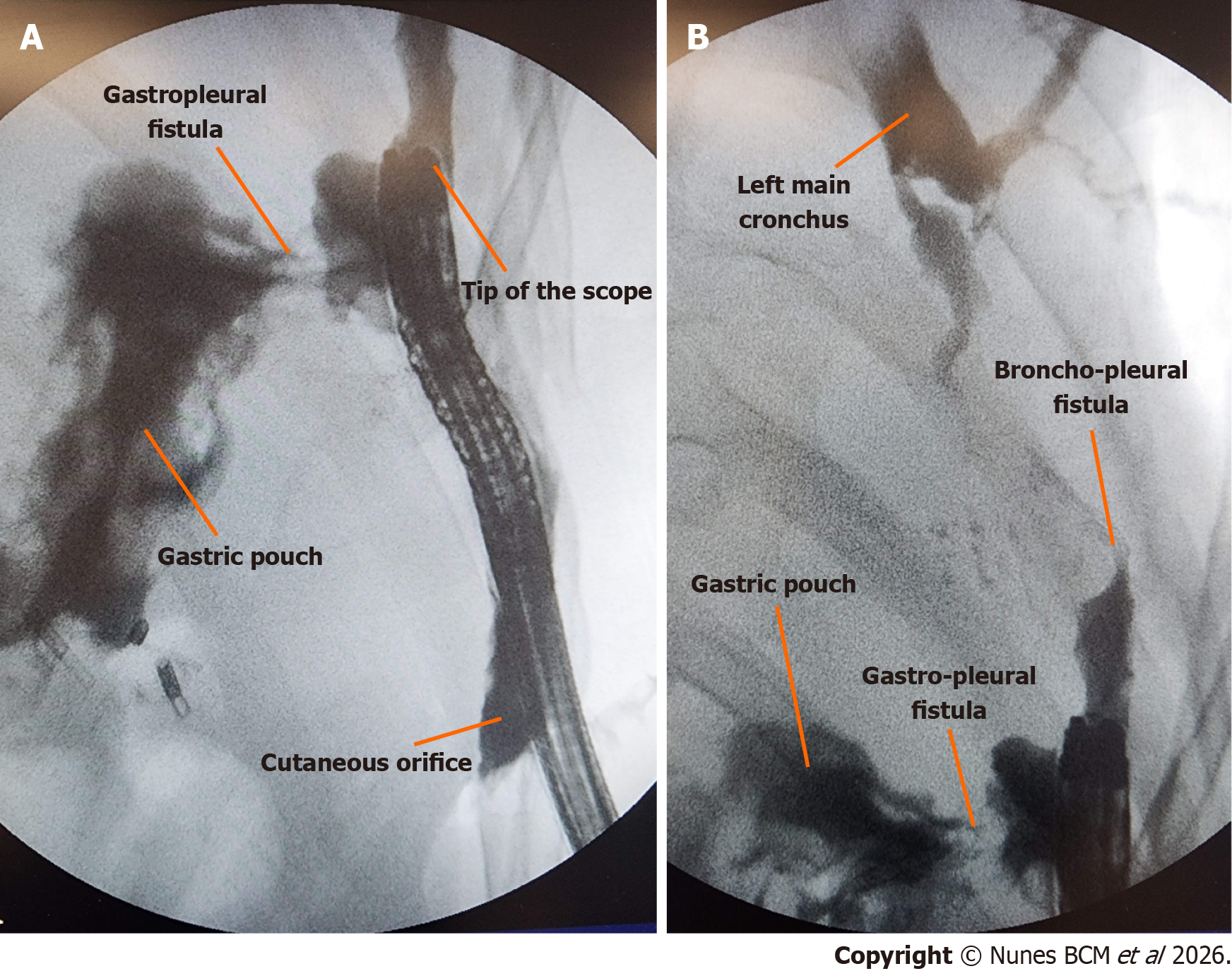
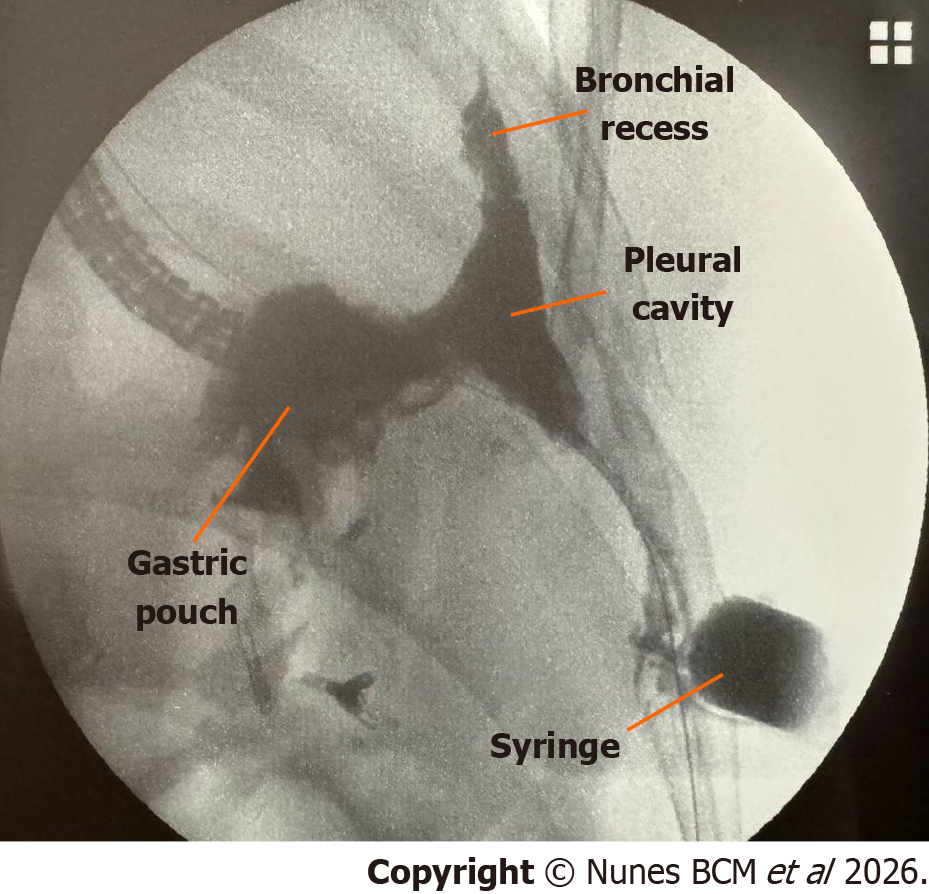
An upper gastrointestinal endoscopy was performed under general anesthesia in the operating room (Video 1), revealing the following findings: Roux-en-Y gastric bypass anatomy; 4 cm gastric pouch with visible 1 cm fistula at the cranial portion of the staple line; 2.5 cm marginal ulcer, involving 50% of the circumference of the gastrojejunal anastomosis with previous placed metal clips. The complex fistula was completely evaluated through the cutaneous orifice with the fluoroscopic guidance (Figure 2).
Given the severity and chronicity of the condition, as well as the patient’s inability to tolerate adequate nutritional support or further surgical intervention, a multidisciplinary team comprising gastroenterology, thoracic surgery, general surgery, infectious disease, and clinical nutrition specialists decided to pursue salvage EVT as a minimally invasive alternative aimed at promoting cavity granulation, fistula closure, and nutritional recovery.
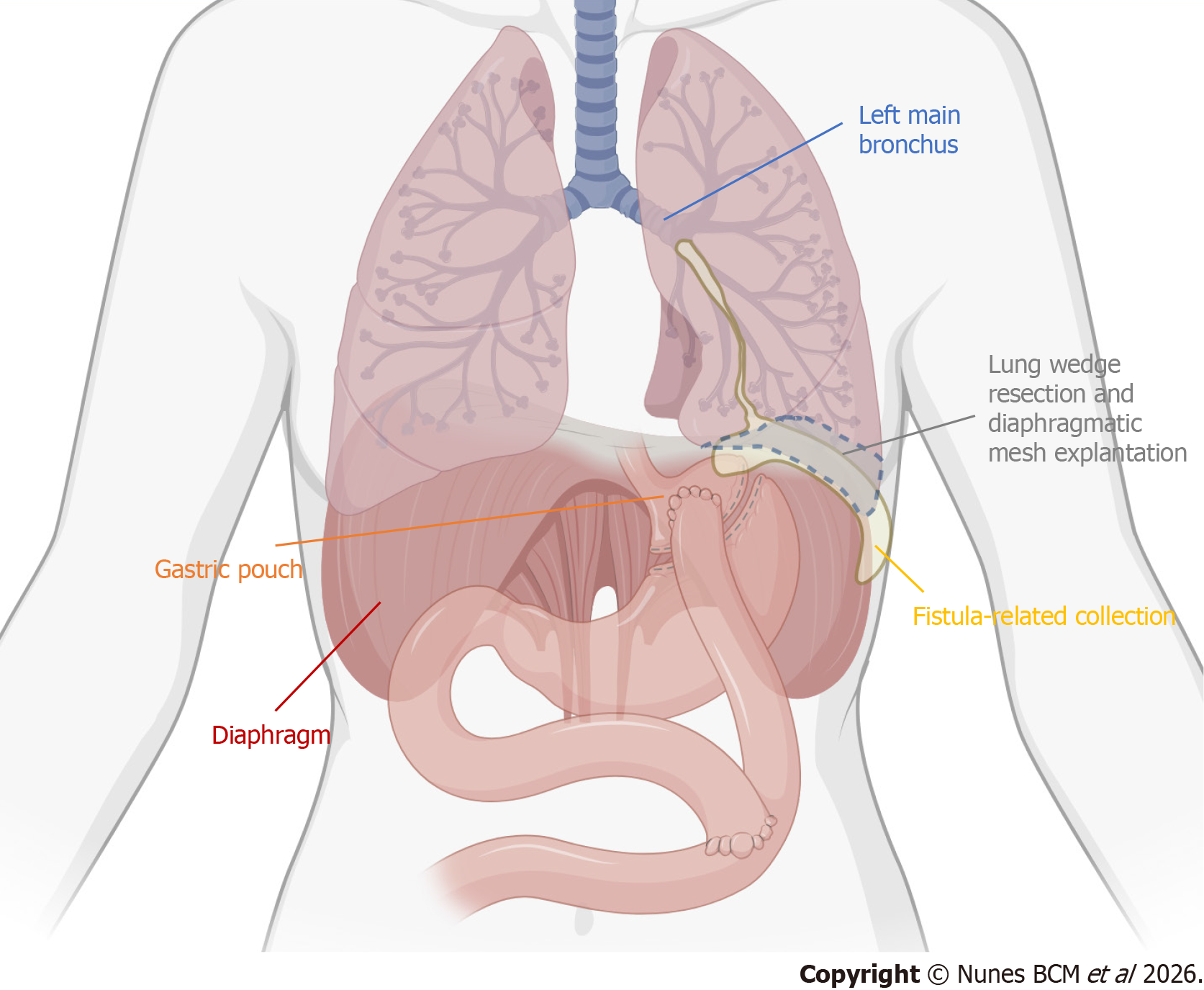
This constellation of findings confirmed a transmural fistulous tract originating from the excluded stomach, traversing the diaphragm into the left pleural cavity, with further extension into the bronchial tree and to the abdominal wall, consistent with a gastro-pleuro-broncho-cutaneous fistula, as illustrated in Figure 3.
EVT was initiated and consisted of every 5-7 days endoscopic sessions for vacuum catheter exchange. Initially, a cu
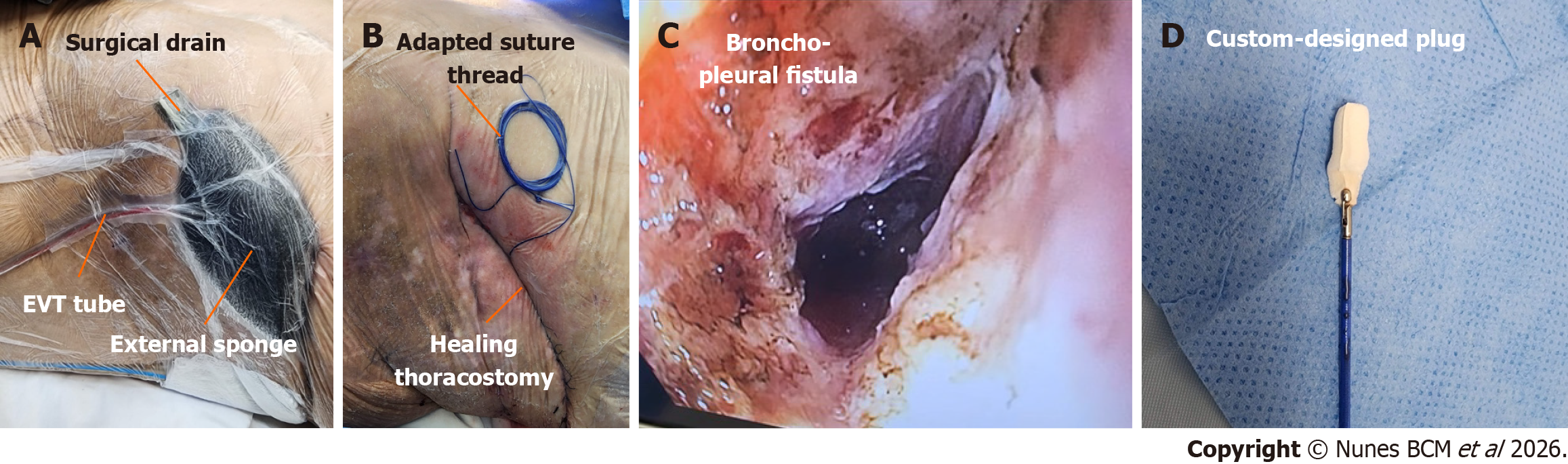
The distal tip sponge was positioned in the cavity and the proximal tip into the thoracotomy wound with an extra external sponge, to enable simultaneous internal and external closure of the fistulous tract, with a continuous negative pressure of -125 mmHg (Figure 4A). A negative pressure of -125 mmHg was selected based on previous EVT protocols, aiming to optimize granulation while minimizing tissue ischemia[1,3,4].
During the first two weeks of EVT, the patient remained in the ICU, receiving intravenous meropenem due to polymicrobial secretion cultures with prolonged hospitalization. The treatment resulted in significant cutaneous orifice closure, switching to a single tip sponge EVT (Video 3 and Supplementary Figure 1), which was positioned into the cavity by guidewire, followed by transfer to the general ward once clinically stable.
After three weeks of therapy, the approach was transitioned to a transluminal route, with a nasal insertion of the EVT catheter through the gastric fistula tract into the pleural cavity (Video 4). The thoracotomy site was managed with a sealed sterile occlusive dressing with an external surgical thread, serving as guidewire for the next approach (Figure 4B).
At the same time, bronchial fistula edge ablation was performed using argon plasma coagulation (APC) via endoscopy through the cutaneous orifice (Figure 4C), followed by the placement of a custom-designed conical plug composed of Surgicel® and Surgisis® (Figure 4D), manually molded to seal the bronchial opening. This plug was inserted in the bro
Two weeks later, bronchial fistula closure was confirmed through fluoroscopy, with the formation of a bronchial residual cavity (Figure 5). At six weeks, the gastrocutaneous fistula tract had become linear and narrowed to a 10 mm diameter. As cavity control was achieved and the fistula progressively collapsed, enteral nutrition was gradually re
Final management involved the placement of an 18 Fr gastrostomy balloon catheter, with the balloon anchored intragastrically to achieve effective occlusion of the tract (Video 6 and Supplementary Figure 2). After a total of eight EVT sessions conducted over approximately six weeks, the patient was discharged with tolerance of a soft oral diet and close outpatient follow-up.
At the 1-month follow-up, the patient reported good oral intake tolerance, with a weight gain of 6 kg, progressing from 49 kg to 55 kg (BMI 19.1 to 21.5) since hospital discharge. Follow-up endoscopic evaluation demonstrated further nar
Considering the favorable evolution, the decision was made to remove the gastrostomy catheter and transition to exclusive external management with a sealed occlusive curative. The patient was advised to progress to a full oral diet as tolerated. During the 2-month follow-up, she continued to show excellent dietary acceptance, with a total weight gain of 15 kg from discharge, reaching 64 kg (BMI of 25.0) and normalization of serum albumin level (3.8 g/dL). Physical ex
The management of complex gastro-pleuro-broncho-cutaneous fistulas, particularly in patients with altered gas
EVT has emerged as a promising minimally invasive alternative for managing transmural defects of the gas
In the presented case, EVT was innovatively applied in a stepwise manner. Initially, a percutaneous approach was utilized, enabling external drainage and progressive removal of surgical drains. Subsequently, a transluminal route was adopted, inserting the vacuum catheter nasally into the gastric fistula tract. This transition facilitated internal drainage and significantly promoted cavity healing.
The management of the bronchial component involved endoscopic APC of the fistula edges, which disrupts the epithelialized margins, combined with the placement of a custom-designed collagen and cellulose plug to facilitate tissue ingrowth and stabilization. This strategy aligns with previously described literature, demonstrating successful outcomes using endoscopic interventions, including biological plugs and sealants, for broncho-pleural fistulas[5,6].
The patient’s clinical evolution was highly favorable, characterized by significant weight gain and improved nu
Importantly, the success achieved in this complex clinical scenario highlights the fundamental role of multidisciplinary management. Complex fistulas often necessitate coordinated interventions across various medical specialties, including gastroenterology, thoracic surgery, general surgery, infectious disease management, clinical nutrition, and wound care specialists. This collaborative approach facilitates comprehensive care, optimizes patient outcomes, and significantly enhances the management efficacy of severe and refractory gastrointestinal conditions[3,4].
This case demonstrates the successful utilization of EVT as the central component with supportive interventions in treating a complex gastro-pleuro-broncho-cutaneous fistula refractory to surgery. The progressive staged and multimodal approach, transitioning from percutaneous to transluminal EVT, combined with targeted endoscopic interventions for the bronchial fistula, resulted in complete fistula closure and significant clinical improvement. This case contributes valuable evidence supporting EVT as a viable and minimally invasive alternative to traditional surgical interventions for complex gastrointestinal fistulas, expanding therapeutic options in critically ill patients previously considered unsuitable for further intervention.
| 1. | Han S, Girotra M, Abdi M, Akshintala VS, Chen D, Chen Y, Das KK, Desilets DJ, Vinsard DG, Leung G, Mishra G, Muthusamy VR, Onyimba FU, Pawa S, Rustagi T, Sakaria S, Shahnavaz N, Law RJ. Endoscopic vacuum therapy. iGIE. 2024;3:333-341. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Gensthaler L, Stauffer M, Jedamzik J, Bichler C, Nixdorf L, Richwien P, Eichelter J, Langer FB, Prager G, Felsenreich DM. Endoluminal Vacuum Therapy as Effective Treatment for Patients with Postoperative Leakage After Metabolic Bariatric Surgery-A Single-Center Experience. Obes Surg. 2024;34:3306-3314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Pattynama LMD, Eshuis WJ, Seewald S, Pouw RE. Multi-modality management of defects in the gastrointestinal tract: Where the endoscope meets the scalpel: Endoscopic vacuum therapy in the upper gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2024;70:101901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | de Moura DTH, Hirsch BS, Ribas PHBV, Silveira SQ, Guedes HG, Bestetti AM. Endoscopic vacuum therapy: pitfalls, tips and tricks, insights, and perspectives. Transl Gastroenterol Hepatol. 2024;9:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 5. | Tao H, Araki M, Sato T, Morino S, Kawanami R, Yoshitani M, Nakamura T. Bronchoscopic treatment of postpneumonectomy bronchopleural fistula with a collagen screw plug. J Thorac Cardiovasc Surg. 2006;132:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Matsumoto A, Shoji T, Katakura H. Vacuum-assisted closure therapy for an empyema with bronchopleural fistula with artificial dermis covering the bronchial stump. Gen Thorac Cardiovasc Surg Cases. 2023;2:38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/