Published online Feb 16, 2026. doi: 10.12998/wjcc.v14.i5.117016
Revised: December 20, 2025
Accepted: January 16, 2026
Published online: February 16, 2026
Processing time: 76 Days and 4.6 Hours
Collision tumors of the thyroid are rare entities, defined by the coexistence of two histologically distinct tumors within the same organ, separated by intervening no
A 37-year-old female presented with palpitations, weight loss, and tremors. Thyroid function tests showed suppressed thyrotropin and slightly elevated free thyroxine. A thyroid ultrasound was performed with a report of a nodule in the right thyroid lobe. Thyroid scintigraphy revealed a goiter with increased ra
Collision tumors are rare entities but thyroid collision tumors in GD are even more infrequent despite the known association between GD and differentiated thyroid cancer. The mechanisms by which this clinical entity occurs are unclear. Because it is a rare pathology, there are currently no guidelines for its treatment. Treatment must be guided separately or based on the more aggressive neoplasm.
Core Tip: Collision tumors of the thyroid are rare entities, characterized by the presence of two histologically distinct tumors within the same organ, separated by normal intervening tissue. Graves’ disease is a well-established risk factor for papillary thyroid cancer but has not been associated with thyroid collision tumors or medullary thyroid carcinoma. In this review, we evaluate the available evidence on thyroid collision tumors.
- Citation: Alvarez M, Luna M, Suarez E, Rincon O, Guzman I, Mancera P. Thyroid collision tumor and Graves’ disease: A case report and review of literature. World J Clin Cases 2026; 14(5): 117016
- URL: https://www.wjgnet.com/2307-8960/full/v14/i5/117016.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i5.117016
The embryological origin of medullary thyroid carcinoma is distinct from that of differentiated thyroid carcinoma. C cells arise from the neural crest of ectodermal origin, while follicular cells are of endodermal origin[1,2]. Although rare, the coexistence of medullary thyroid cancer (MTC) and papillary thyroid cancer (PTC) has been reported as thyroid collision tumors[3,4]. Collision tumors are rare entities characterized by the presence of two distinct neoplastic histologies within the same organ, separated by normal tissue and lacking histological admixture[5,6]. Graves’ disease (GD) has been associated with an increased prevalence of PTC[7]. However, its association with MTC remains unclear, largely due to the extremely low incidence of this malignancy. While the increased detection of PTC may be attributed to intensified screening and incidental findings, MTC remains exceedingly rare in this setting. Consequently, reporting such cases is essential to better characterize this unique patient population. In this review, we evaluate the available evidence on thyroid collision tumor and in the context of GD and present the case of a 37-year-old female with GD and a thyroid collision tumor composed of both PTC and MTC.
A 37-year-old female Latin American female presented with palpitations, weight loss, and tremors. Thyroid function tests demonstrated suppressed thyrotropin (TSH) (0.0001 µU/mL) and a mildly elevated free thyroxine (FT4) (1.73 ng/dL, normal 0.92-1.70). Thyroid ultrasound revealed a 13 mm × 10 mm ovoid nodule in the right thyroid lobe, classified as TIRADS 3.
The diagnosis of GD was established based on positive TSH receptor antibodies (TRAb) and a thyroid scintigraphy demonstrating a diffuse goiter with increased radiotracer uptake. Ultrasound-guided fine needle aspiration (FNA) suggested MTC, Bethesda category VI. Immunohistochemistry was positive for synaptophysin and negative for thy
There was no history of past illness.
There was no personal history of thyroid disease. The family history was notable for PTC in her mother. She had no prior history of radiation exposure.
Physical examination revealed a grade 1 goiter.
An evaluation for multiple endocrine neoplasia (MEN) was performed. Chromogranin A was initially markedly elevated at 1870 ng/mL, while plasma fractionated metanephrines were within normal limits (20 pg/mL). Serum calcium (8.8 mg/dL) and parathyroid hormone (PTH) levels (54 pg/mL) were also normal. On follow-up, the chromogranin A level decreased to 770 ng/mL. Genetic testing for MEN, including sequencing of the RET oncogene, revealed no pathogenic variants. MEN was therefore ruled out.
Imaging studies were performed to evaluate for metastatic disease and neuroendocrine tumors. Computed tomography of the thorax and abdomen was unremarkable, and gallium-68 positron emission tomography showed no abnormal uptake. Thyroid scintigraphy demonstrated a diffuse goiter with increased radiotracer uptake, consistent with GD. Thyroid ultrasound-guided FNA of the right thyroid nodule suggested a MTC, Bethesda category VI. Immunohistochemistry was positive for synaptophysin and negative for thyroglobulin, and the serum calcitonin level was elevated at 78 pg/mL.
The patient received comprehensive care through a specialized multidisciplinary approach. She was evaluated, managed, and monitored by a collaborative team including specialists in endocrine oncology, radiology, and head and neck surgery.
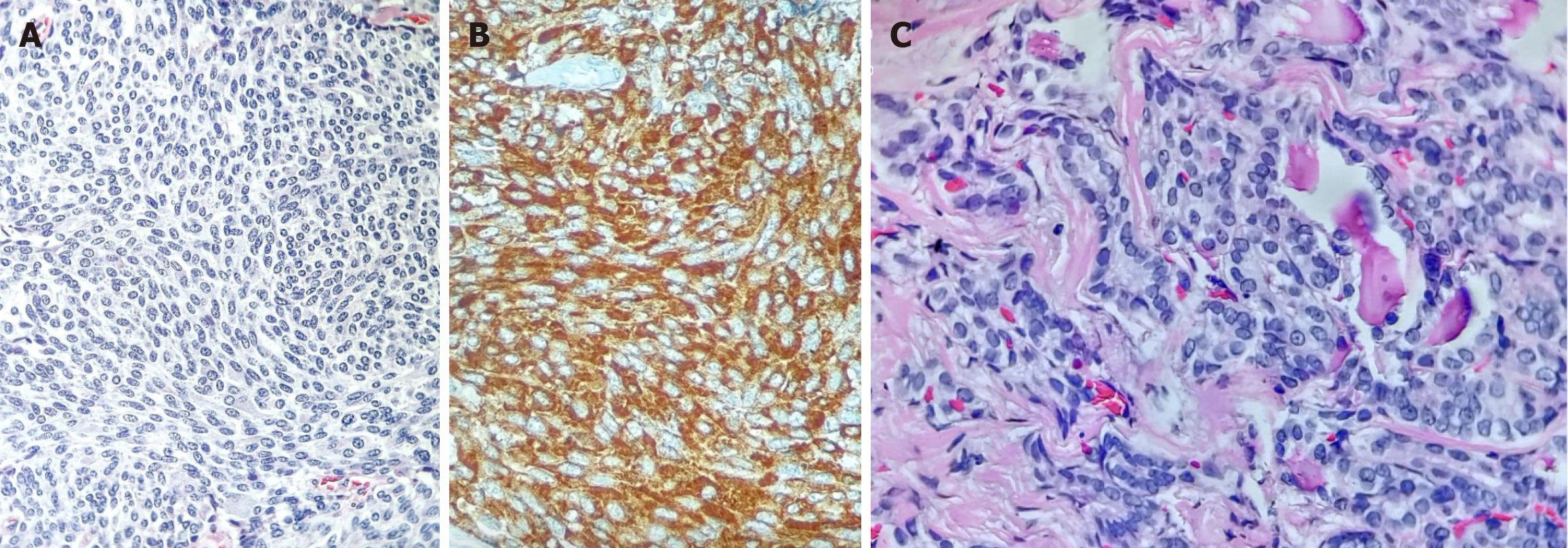
GD, medullary carcinoma in the right thyroid lobe and papillary microcarcinoma in the left lobe.
Total thyroidectomy with central lymph node dissection was performed. Histopathological examination revealed a 1.2 cm × 1.0 cm × 0.7 cm medullary carcinoma in the right thyroid lobe (Figure 1A and B), without capsular invasion or ex
The patient has been followed by the endocrinology service for 5 years and remains in stable clinical remission. Serial follow-up evaluations, including measurements of tumor markers (CEA, calcitonin, thyroglobulin, and anti-thyroglobulin antibodies) and neck ultrasonography, have demonstrated no evidence of disease recurrence. She continues lev
MTC is a rare type of cancer, accounting for 1%-2% of all thyroid cancers[8]. About 25% of MTC cases are hereditary, while the remaining 75% are sporadic. The RET proto-oncogene plays a central role in its pathophysiology and is the primary driver of most cases[9]. RET mutations may occur as somatic events in up to 50% of sporadic MTC cases or as autosomal dominant germline mutations associated with multiple endocrine neoplasia type 2 (MEN2), including MEN2A, MEN2B, and familial MTC (FMTC) syndromes[1,8]. Sporadic MTC occurs more frequently in women, with a typical age of presentation between 40-60 years. Approximately 75% of cases present as a solitary nodule located in the upper part of the thyroid gland. At diagnosis, cervical lymph node involvement is observed in up to 70% of patients, and distant metastases are present in approximately 5%-10% of cases[1].
The coexistence of an MTC and PTC is very rare, initially reported by Lamberg et al[10] in 1981. It has been described in two scenarios; a synchronous occurrence of both tumors separated by normal thyroid tissue[11-13], or tumor collision or a mixed carcinoma showing dual differentiation[3,14]. In this case, we present a patient with concurrent MTC and PTC that has characteristics of a collision tumor. No mutations in the RET gene were detected. Notably, the patient presented with hyperthyroidism, and hyperfunctioning thyroid nodules are rarely associated with malignancy[2]. Previously, hy
Collision tumors have been described in several organs, but it is extremely rare in the thyroid, with a prevalence of less than 1% of all thyroid tumors[17]. The most frequently reported thyroid collision tumor is the coexistence of differentiated thyroid cancer with medullary cancer presenting in females between the fifth, sixth and seventh decades[14].
The concomitant presence of PTC and MTC is a rare event, as described in the literature in case series and reports[18,19]. There are case series in the literature where MTC components are separated from PTC by normal thyroid tissue. In 2004, Biscolla et al[20] reported one of the largest case series, with 27 patients with MTC and PTC, of which 77% were micropapillary.
Although the mechanisms by which this clinical situation occurs are not entirely clear, several hypotheses have been proposed: The theory that one tumor predisposes to another tumor; the stem cell theory and the theory of the random collision effect[17,21,22]. Among those, the theory of a coincident co-occurrence stands out, given that both neoplasms are separated by healthy thyroid tissue and have distinct embryological origins and pathogenesis mechanisms. While PTC is primarily associated with mutations in BRAF (v-raf murine sarcoma viral oncogene homolog B1), MTC is associated with mutations in RET[23,24].
Because it is a rare pathology, there are currently no standard treatment guidelines. Some authors suggest treating both neoplasms separately as if they were two primary tumors[25], while other authors suggest that treatment is based on the more aggressive neoplasm[26]. Total thyroidectomy is the treatment of choice for primary disease because of the aggressive nature of the medullary thyroid carcinoma component. The extent of lymph node dissection should be guided by preoperative calcitonin levels and the stage of disease[1]. In PTC, postoperative management should be guided by risk stratification, with radioactive iodine therapy considered according to the assigned risk category. Follow-up surveillance should include serial thyroglobulin measurements to assess for disease recurrence[2]. In cases of concomitant MTC and PTC, both calcitonin and thyroglobulin should be monitored to detect disease recurrence, while the degree of TSH suppression should be determined according to the risk stratification of the PTC component. The present case represents sporadic MTC with a coexisting papillary thyroid microcarcinoma, treated with total thyroidectomy and followed clinically with serial measurements of thyroglobulin, anti-thyroglobulin antibodies, calcitonin and CEA, with no evidence of disease recurrence.
GD is an organ-specific autoimmune disorder due to the presence of thyrotropin receptor-stimulating autoantibodies, with increased synthesis and release of thyroid hormones[27]. GD is the primary cause of hyperthyroidism in patients under 40 years of age with an incidence of 20 to 40 cases per 100000 population per year[28].
Thyroid carcinoma in GD was initially considered a rare phenomenon, and GD was even proposed to have a protective effect through autoimmune-mediated antitumor activity[29]. However, subsequent studies have reported a higher prevalence of thyroid cancer compared to the general population, with an even greater incidence observed among patients with GD, particularly those managed surgically[7,30,31]. The routine use of thyroid ultrasound in this population has possibly led to overdiagnosis of cancer, and it has been suggested that thyrotropin receptor-stimulating auto
In 2016, Staniforth et al[34] published a meta-analysis of surgically resected specimens and found that the rate of GD-associated thyroid carcinoma was 0.07 (95%CI: 0.04-0.12), with a thyroid cancer rate 2.5 times higher in patients with GD than in the general population. In contrast, the American Thyroid Association states that thyroid cancer occurs in GD with a frequency of 2% or less[35]. In this meta-analysis, 88% were papillary carcinoma, 10% follicular, 0.6% mixed (papillary-follicular), 0.6% medullary, and 0.3% anaplastic. Of the papillary carcinomas, 29% were micropapillary, con
In 2019, a meta-analysis by Mekraksakit et al[36] evaluated the association between GD and prognosis in patients with differentiated thyroid cancer. They found an increased risk of multifocality/multicentricity (odds ratio = 1.45, 95%CI: 1.04-2.02, I2 = 6.5%, P = 0.381) and distant metastasis at the time of cancer diagnosis (odds ratio = 2.19, 95%CI: 1.08-4.47, I2 = 0.0%, P = 0.497) in differentiated thyroid cancer patients with GD than those without GD.
The reported case describes a patient with GD and a collision tumor of the thyroid gland with PTC and MTC. MTC is rarely associated with GD or other forms of hyperthyroidism[37,38]. One of the earliest reported cases was described by McFarland et al[39] in 1980, involving a 30-year-old female with hyperthyroidism and an associated goiter who un
To our knowledge, only one prior case of a thyroid collision tumor involving MTC and GD has been reported. Mazziotti et al[44] described in 2001 a Caucasian woman with MTC, a concomitant papillary thyroid microcarcinoma, and GD, with no detectable mutations in the RET proto-oncogene. At present, no established pathophysiological mechanism explains the association between MTC and GD, and their coexistence has been suggested to be incidental.
Collision tumors are rare entities characterized by the coexistence of two distinct neoplastic histologies within the same organ, separated by normal tissue and without histological admixture. Thyroid collision tumors in the setting of GD are even more uncommon, despite the known association between GD and differentiated thyroid cancer. The mechanisms underlying this clinical entity remain unclear, and due to its rarity, no specific management guidelines currently exist. Consequently, treatment should be individualized and guided by the more aggressive neoplasm or addressed separately for each tumor.
| 1. | Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1559] [Article Influence: 141.7] [Reference Citation Analysis (0)] |
| 2. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 10288] [Article Influence: 1028.8] [Reference Citation Analysis (1)] |
| 3. | Pastolero GC, Coire CI, Asa SL. Concurrent medullary and papillary carcinomas of thyroid with lymph node metastases. A collision phenomenon. Am J Surg Pathol. 1996;20:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Dikbas O, Duman AA, Guvendi GF. Medullary Thyroid Carcinoma and Papillary Thyroid Carcinoma in the Same Patient as a Collision Tumour. Case Rep Endocrinol. 2019;2019:4038628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Gadong LC, Crisostomo T. Simultaneous Occurrence of Papillary Carcinoma and Medullary Carcinoma. J ASEAN Fed Endocr Soc. 2019;34:226-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Negura I, Ianole V, Danciu M, Preda C, Iosep DG, Dănilă R, Grigorovici A, Ciobanu Apostol DG. Thyroid Collision Tumors: The Presence of the Medullary Thyroid Carcinoma Component Negatively Influences the Prognosis. Diagnostics (Basel). 2023;13:285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Gabriele R, Letizia C, Borghese M, De Toma G, Celi M, Izzo L, Cavallaro A. Thyroid cancer in patients with hyperthyroidism. Horm Res. 2003;60:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388:2783-2795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1075] [Article Influence: 107.5] [Reference Citation Analysis (1)] |
| 9. | Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on Fundamental Mechanisms of Thyroid Cancer. Front Endocrinol (Lausanne). 2020;11:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 10. | Lamberg BA, Reissel P, Stenman S, Koivuniemi A, Ekbolm M, Mäkinen J, Franssila K. Concurrent medullary and papillary thyroid carcinoma in the same thyroid lobe and in siblings. Acta Med Scand. 1981;209:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Abdullah AM, Ali RM, Salih KM, Mohammed KK, Kakamad FH, Salih AM. Synchronous occurrence of papillary thyroid microcarcinoma, medullary thyroid carcinoma and Hashimoto thyroiditis in a single thyroid: A case report with literature review. Int J Surg Case Rep. 2022;93:106888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | SamieeRad F, Emami A. Synchronous Occurrence of Papillary Thyroid Carcinoma and Medullary Carcinoma in the Setting of Hashimoto's Thyroiditis and Multi Nodular Goiter. Iran J Pathol. 2022;17:91-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Tang PY, Khor LY, Takano A. Synchronous papillary thyroid carcinoma and medullary thyroid carcinoma - a pitfall waiting to happen. Malays J Pathol. 2017;39:171-174. [PubMed] |
| 14. | Bojoga A, Stănescu L, Badiu C. Collision tumors of the thyroid. A special clinical and pathological entity. Arch Clin Cases. 2021;8:84-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 15. | Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, Pinchera A, Vitti P. Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer. 2009;16:1251-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Medas F, Erdas E, Canu GL, Longheu A, Pisano G, Tuveri M, Calò PG. Does hyperthyroidism worsen prognosis of thyroid carcinoma? A retrospective analysis on 2820 consecutive thyroidectomies. J Otolaryngol Head Neck Surg. 2018;47:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | V GK, Kajamohideen S, M A, Joseph LD, Parthasarathy I. A Rare Collision in Thyroid and Lymph Node: A Case Report and Review of Literature. Cureus. 2025;17:e86190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Kobayashi K, Teramoto S, Maeta H, Ishiguro S, Mori T, Horie Y. Simultaneous occurrence of medullary carcinoma and papillary carcinoma of the thyroid. J Surg Oncol. 1995;59:276-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kim WG, Gong G, Kim EY, Kim TY, Hong SJ, Kim WB, Shong YK. Concurrent occurrence of medullary thyroid carcinoma and papillary thyroid carcinoma in the same thyroid should be considered as coincidental. Clin Endocrinol (Oxf). 2010;72:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Biscolla RP, Ugolini C, Sculli M, Bottici V, Castagna MG, Romei C, Cosci B, Molinaro E, Faviana P, Basolo F, Miccoli P, Pacini F, Pinchera A, Elisei R. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid. 2004;14:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Yao J, Li CF, Wang JJ, Zhang SY, Li YL, Yang J, Zheng H. Medullary thyroid carcinoma combined with papillary thyroid carcinoma: case report and literature review. Int J Clin Exp Pathol. 2020;13:2710-2717. [PubMed] |
| 22. | Younes N, Shomaf M, Al Hassan L. Simultaneous medullary and papillary thyroid carcinoma with lymph node metastasis in the same patient: case report and review of the literature. Asian J Surg. 2005;28:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Hellmann AR, Patel A, Śledziński M, Obołończyk Ł, Bieńkowski M, Mikaszewski B, Kaska Ł. Medullary thyroid carcinoma of unknown primary origin with synchronous finding of papillary thyroid carcinoma. Endokrynol Pol. 2020;71:200-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Fallahi P, Patrizio A, Stoppini G, Elia G, Ragusa F, Paparo SR, Balestri E, Mazzi V, Botrini C, Varricchi G, Ulisse S, Ghionzoli M, Antonelli A, Ferrari SM. Simultaneous Occurrence of Medullary Thyroid Carcinoma and Papillary Thyroid Carcinoma: A Case Series with Literature Review. Curr Oncol. 2023;30:10237-10248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 25. | Thomas A, Mittal N, Rane SU, Bal M, Patil A, Ankathi SK, Vaish R. Papillary and Medullary Thyroid Carcinomas Presenting as Collision Tumors: A Case Series of 21 Cases at a Tertiary Care Cancer Center. Head Neck Pathol. 2021;15:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Ryan N, Walkden G, Lazic D, Tierney P. Collision tumors of the thyroid: A case report and review of the literature. Head Neck. 2015;37:E125-E129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 27. | Antonelli A, Fallahi P, Elia G, Ragusa F, Paparo SR, Ruffilli I, Patrizio A, Gonnella D, Giusti C, Virili C, Centanni M, Shoenfeld Y, Ferrari SM. Graves' disease: Clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract Res Clin Endocrinol Metab. 2020;34:101388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 28. | Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, Eckstein AK, Stagnaro-Green A, Kahaly GJ. Graves' disease. Nat Rev Dis Primers. 2020;6:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 29. | Imam S, Dar P, Paparodis R, Almotah K, Al-Khudhair A, Hasan SA, Salim N, Jaume JC. Nature of coexisting thyroid autoimmune disease determines success or failure of tumor immunity in thyroid cancer. J Immunother Cancer. 2019;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Pazaitou-Panayiotou K, Michalakis K, Paschke R. Thyroid cancer in patients with hyperthyroidism. Horm Metab Res. 2012;44:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Keskin C, Sahin M, Hasanov R, Aydogan BI, Demir O, Emral R, Gullu S, Erdogan MF, Gedik V, Uysal AR, Baskal N, Corapcioglu D. Frequency of thyroid nodules and thyroid cancer in thyroidectomized patients with Graves' disease. Arch Med Sci. 2020;16:302-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Belfiore A, Russo D, Vigneri R, Filetti S. Graves' disease, thyroid nodules and thyroid cancer. Clin Endocrinol (Oxf). 2001;55:711-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Yoon JH, Jin M, Kim M, Hong AR, Kim HK, Kim BH, Kim WB, Shong YK, Jeon MJ, Kang HC. Clinical Characteristics and Prognosis of Coexisting Thyroid Cancer in Patients with Graves' Disease: A Retrospective Multicenter Study. Endocrinol Metab (Seoul). 2021;36:1268-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Staniforth JUL, Erdirimanne S, Eslick GD. Thyroid carcinoma in Graves' disease: A meta-analysis. Int J Surg. 2016;27:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26:1343-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2006] [Cited by in RCA: 1628] [Article Influence: 162.8] [Reference Citation Analysis (0)] |
| 36. | Mekraksakit P, Rattanawong P, Karnchanasorn R, Kanitsoraphan C, Leelaviwat N, Poonsombudlert K, Kewcharoen J, Dejhansathit S, Samoa R. Prognosis of differentiated thyroid carcinoma in patients with Graves disease: a systematic review and meta-analysis. Endocr Pract. 2019;25:1323-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Khan SH, Rather TA, Makhdoomi R, Malik D. Nodular Graves' disease with medullary thyroid cancer. Indian J Nucl Med. 2015;30:341-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Ahmed SR, Ball DW. Clinical review: Incidentally discovered medullary thyroid cancer: diagnostic strategies and treatment. J Clin Endocrinol Metab. 2011;96:1237-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | McFarland KF, Hawksley VC, Reynolds JC. Hyperthyroidism and medullary carcinoma of the thyroid. South Med J. 1980;73:1661-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 40. | Sapalidis K, Papanastasiou A, Michalopoulos N, Mantalovas S, Giannakidis D, Koimtzis GD, Florou M, Poulios C, Mantha N, Kesisoglou II. A Rare Coexistence of Medullary Thyroid Cancer with Graves Disease: A Case Report and Systematic Review of the Literature. Am J Case Rep. 2019;20:1398-1401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Nakamura S, Saio Y, Ishimori M, Shima H. Incidental medullary thyroid carcinoma in a case of Graves' disease. Intern Med. 2002;41:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Schwartz RW, Kenady DE, Bensema M, McGrath PC, Flueck J. Medullary thyroid cancer and Graves' disease. Surgery. 1989;105:804-807. [PubMed] |
| 43. | Akin R, Gilley D, Tassone P. "Incidentally discovered medullary thyroid carcinoma in the post-operative Graves' patient - A case report". Am J Otolaryngol. 2022;43:103450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Mazziotti G, Rotondi M, Manganella G, Franco R, Capone PFRS, Colantuoni V, Amato G, Carella C. Medullary thyroid cancer, papillary thyroid microcarcinoma and Graves' disease: an unusual clinical coexistence. J Endocrinol Invest. 2001;24:892-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/