Published online Feb 6, 2026. doi: 10.12998/wjcc.v14.i4.117700
Revised: January 8, 2026
Accepted: January 23, 2026
Published online: February 6, 2026
Processing time: 53 Days and 23.7 Hours
Hospital-acquired functional decline (HAFD) is a poor prognostic factor in older patients who have undergone cardiovascular surgery.
To develop a model to predict HAFD and to identify its associated factors.
This retrospective observational study included 144 patients who underwent cardiovascular surgery between May 2019 and December 2023. HAFD was de
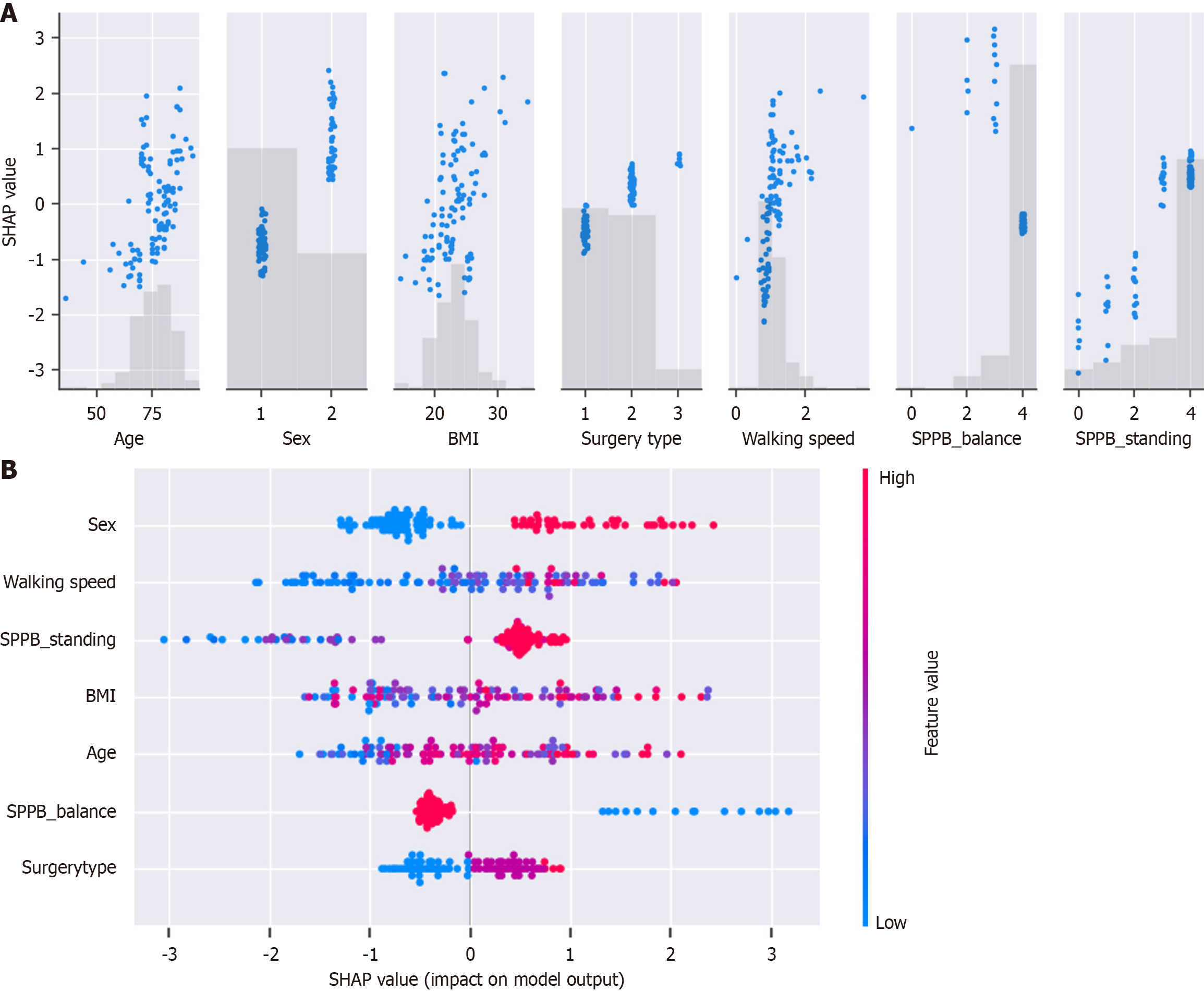
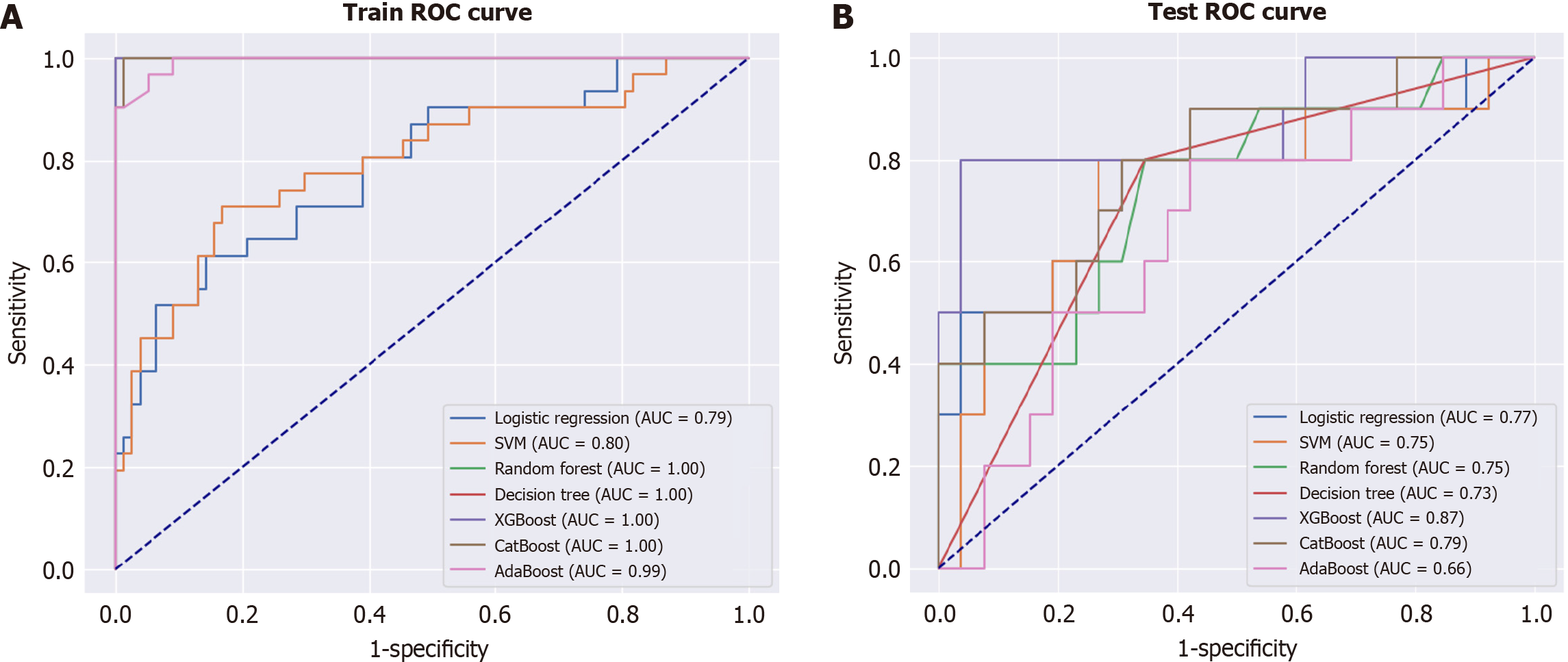
Among the 144 participants, 41 (28.5%) experienced HAFD. Of the 7 machine learning models, the extreme gradient boosting model (XGBoost) achieved the best performance, with an AUC of 0.87. SHAP analysis revealed that being female and having a slower preoperative walking speed markedly impacted HAFD oc
We developed a high-accuracy model to predict HAFD in older patients who have undergone cardiovascular surgery and identified key associated factors, informing preoperative evaluations and interventions in clinical practice.
Core Tip: Hospital-acquired functional decline (HAFD) is a critical yet underrecognized complication in older patients undergoing cardiovascular surgery. We developed and validated a machine learning–based prediction model for HAFD using preoperative clinical and physical function data. Among seven models, the extreme gradient boosting model de
- Citation: Hiramatsu R, Imaoka S, Minata S, Sako H, Sato N. Machine learning model for predicting hospital-acquired functional decline in older patients with postoperative cardiovascular surgery. World J Clin Cases 2026; 14(4): 117700
- URL: https://www.wjgnet.com/2307-8960/full/v14/i4/117700.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i4.117700
Cardiac rehabilitation following postoperative cardiovascular surgery in older patients effectively improves physical function and reduces postoperative complications and hospital stays[1]. It has been actively implemented in the early stages of hospitalization in many hospitals, partly owing to reported improvements in long-term prognosis[2] and the growing emphasis on shorter hospital stays in acute care settings[3].
However, despite postoperative cardiac rehabilitation, some patients experience limited physical recovery and require prolonged hospital stays owing to complications or other factors. Many of these patients present with frailty and pre
Recently, hospital-acquired functional decline (HAFD) has gained attention as a new predictive index in postoperative cardiovascular surgery in older patients[6,7]. Furthermore, HAFD is an independent predictor of poor prognosis two years after surgery[8]. Therefore, preoperative frailty and postoperative HAFD may have predictive value. Although HAFD is associated with poor prognosis, no studies have developed models to predict HAFD and its related factors.
Therefore, in this study, we aimed to develop a model to predict HAFD in postoperative cardiovascular surgery in older patients and to investigate the factors associated with its occurrence.
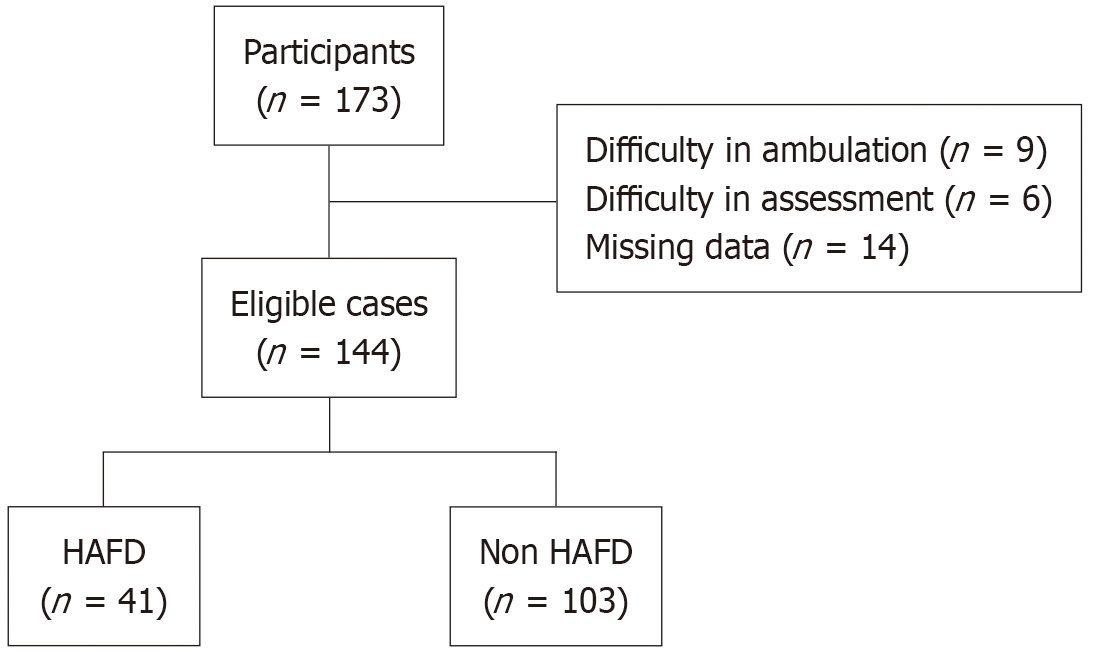
The study included 144 of the 173 patients who underwent standby cardiac surgery (coronary artery bypass, valvular, or mixed surgery) at our cardiovascular surgery center between May 2019 and December 2023. A total of 29 patients were excluded because they met at least one of the following predefined exclusion criteria: Difficulty in walking at admission, inability to undergo preoperative physical function assessment, or missing data. This study was approved by the ethical review committee of the Oita Oka Hospital. Patients were excluded based on the following criteria: (1) Difficulty in walking at the time of admission; (2) Difficulty in undergoing preoperative evaluation; and (3) Missing data.
This study was designed as a retrospective, observational study. Data were collected retrospectively from patients’ me
Postoperative rehabilitation was conducted according to the attending physician’s instructions, based on the “Criteria for the Start of Postoperative Weaning after Cardiac Surgery” and the “Criteria for Judging the Exercise Load Test (Step-up Criteria)” outlined in the Guidelines for Rehabilitation in Cardiovascular Disease (revised edition, 2021)[1], published by the Japanese Circulation Society.
Rehabilitation sessions were conducted twice daily for 40-60 minutes per session under the direction of the attending physician. In the rehabilitation room, patients primarily performed aerobic exercise using a bicycle ergometer and resistance training with equipment such as therabands and weights, in accordance with established rehabilitation guidelines.
HAFD was defined as a decrease of one or more points in the short physical performance battery (SPPB) score before surgery and before hospital discharge[9]. The SPPB is a comprehensive physical function assessment tool for older individuals, consisting of balance, gait, and standing tests[10]. It is a validated and reliable measure commonly used to evaluate physical function in older populations[11].
The minimal clinically important difference for the SPPB is 1 point[12]. Accordingly, in this study, HAFD was defined as a decrease of 1 or more points in the preoperative SPPB score prior to hospital discharge.
Data on age, sex, body mass index (BMI), comorbidities, and laboratory findings, including left ventricular ejection fraction as well as levels of brain natriuretic peptide, creatinine, urea nitrogen, hemoglobin, and C-reactive protein, were obtained from electronic medical records. Preoperative physical function was measured from admission through the day before surgery. Surgical procedure data were collected from surgical records.
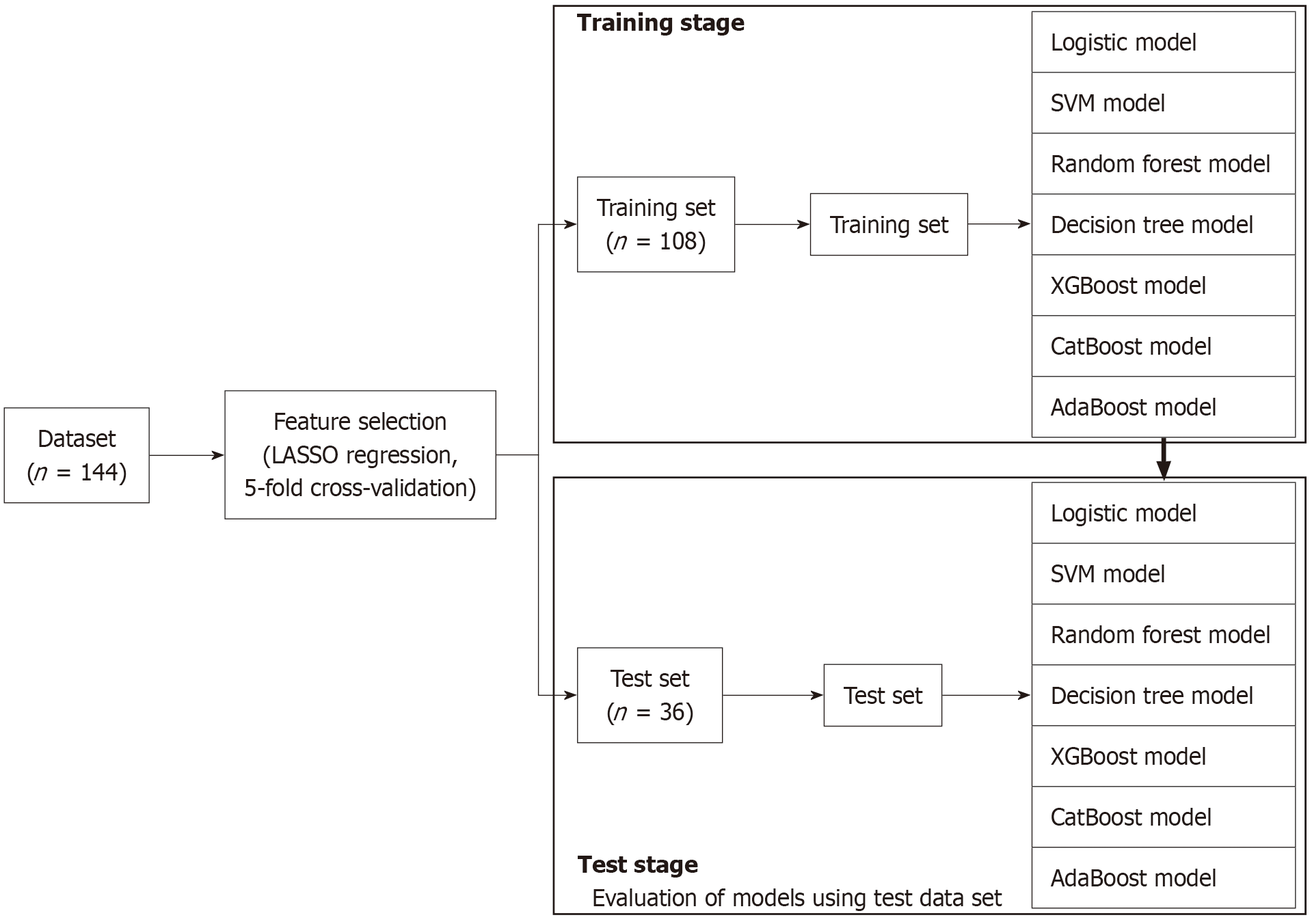
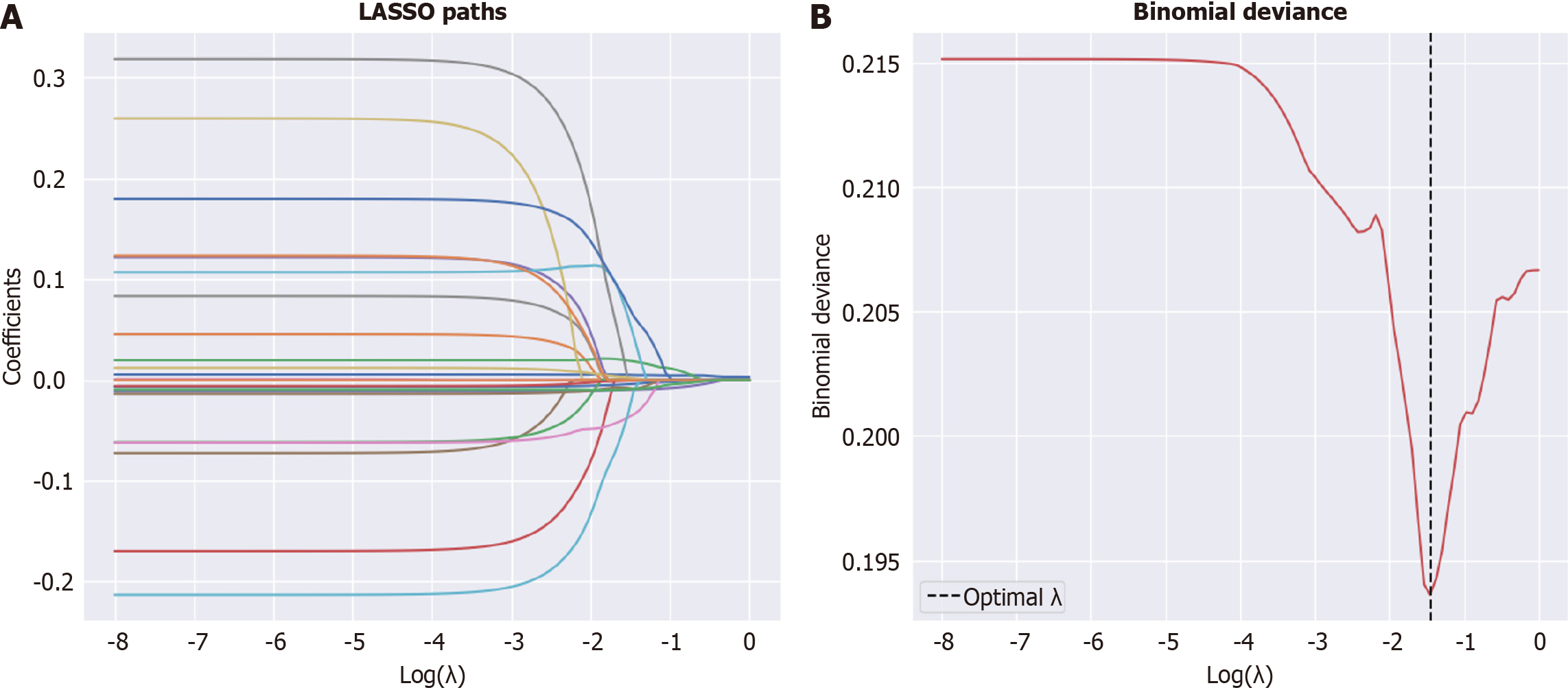
Data analysis was conducted in three steps. First, feature selection was performed using LASSO regression with 5-fold cross-validation to improve generalization. Second, we split the dataset into training and test data, and we constructed predictive models using the features selected in the first step. These models included a logistic regression model, support vector machine (SVM), random forest, decision tree, the extreme gradient boosting (XGBoost), category boosting (CatBoost), and adaptive boosting (AdaBoost). Third, for the best-performing models, SHapley additive exPlanations (SHAP) values were calculated to investigate the effects of individual variables on the predictions made by the models (Figure 1). All analyses were performed using Python 3.913 software.
A total of 144 patients were enrolled in the study, of whom 41 (28.5%) developed HAFD (Figure 2). Table 1 presents a comparison of the preoperative clinical characteristics of the HAFD and non-HAFD groups. The HAFD group was significantly older than the non-HAFD group (79.1 ± 6.6 years vs 74.9 ± 9.2 years, P = 0.009). Additionally, the HAFD group had a significantly higher proportion of female patients (53.7% vs 32.0%, P = 0.026) and faster walking speed (1.2 ± 0.5 milliseconds vs 1.1 ± 0.3 milliseconds, P = 0.020) compared with the non-HAFD group. No significant differences were observed between the two groups in terms of BMI, surgery type, comorbidities (hypertension, dyslipidemia, stroke, diabetes, orthopedic disorders, chronic obstructive pulmonary disease), laboratory data (hemoglobin, estimated glo
| Overall (n = 144) | HAFD group (n = 41) | Non-HAFD group (n = 103) | P value | |
| Age (years) | 76.1 ± 8.7 | 79.1 ± 6.6 | 74.9 ± 9.2 | 0.009 |
| Sex (female) | 55 (38.2) | 22 (53.7) | 33 (32.0) | 0.026 |
| BMI (kg/m2) | 22.9 ± 3.2 | 23.6 ± 3.3 | 22.7 ± 3.1 | 0.105 |
| Surgery type | 0.056 | |||
| CABG | 63 (43.8) | 12 (29.3) | 51 (49.5) | |
| Valve replacement | 73 (50.7) | 25 (61.0) | 48 (46.6) | |
| Combined | 8 (5.6) | 4 (9.8) | 4 (3.9) | |
| Comorbidity | ||||
| Hypertension | 110 (76.4) | 30 (73.2) | 80 (77.7) | 0.722 |
| Dyslipidemia | 91 (63.2) | 26 (63.4) | 65 (63.1) | 1.000 |
| Stroke | 16 (11.1) | 3 (7.3) | 13 (12.6) | 0.535 |
| Diabetes | 59 (41.0) | 14 (34.1) | 45 (43.7) | 0.388 |
| Orthopedic disorders | 40 (27.8) | 13 (31.7) | 27 (26.2) | 0.647 |
| COPD | 5 (3.5) | 2 (4.9) | 3 (2.9) | 0.939 |
| Laboratory data | ||||
| BNP (pg/mL) | 246.1 ± 285.4 | 294.0 ± 321.6 | 227.1 ± 268.9 | 0.205 |
| Creatinine (mg/dL) | 1.2 ± 1.3 | 1.2 ± 1.1 | 1.3 ± 1.4 | 0.623 |
| eGFR (mL/minute/1.73 m2) | 54.0 ± 20.2 | 52.4 ± 20.2 | 54.7 ± 20.3 | 0.528 |
| BUN (mg/dL) | 21.0 ± 10.0 | 20.9 ± 9.8 | 21.1 ± 10.1 | 0.917 |
| Hemoglobin (g/dL) | 12.7 ± 2.1 | 12.3 ± 2.0 | 12.9 ± 2.1 | 0.148 |
| CRP (mg/dL) | 0.6 ± 1.3 | 0.4 ± 0.8 | 0.6 ± 1.5 | 0.423 |
| EF (%) | 55.3 ± 13.1 | 54.0 ± 14.5 | 55.8 ± 12.5 | 0.471 |
| %VC (%) | 91.6 ± 12.2 | 92.8 ± 9.4 | 91.2 ± 13.2 | 0.465 |
| FEV1.0% (%) | 78.7 ± 9.2 | 78.3 ± 10.1 | 78.9 ± 8.8 | 0.728 |
| Walking speed (milliseconds) | 1.1 ± 0.4 | 1.2 ± 0.5 | 1.1 ± 0.3 | 0.020 |
| 5-CS (second) | 10.9 ± 4.2 | 11.5 ± 3.5 | 10.7 ± 4.4 | 0.310 |
| SPPB balance score | 3.8 ± 0.7 | 3.6 ± 0.8 | 3.8 ± 0.6 | 0.121 |
| SPPB standing score | 3.1 ± 1.2 | 3.3 ± 1.1 | 3.0 ± 1.3 | 0.342 |
| SPPB walking score | 3.6 ± 0.7 | 3.6 ± 0.8 | 3.6 ± 0.7 | 0.844 |
| Grip strength (kg) | 25.2 ± 8.4 | 23.1 ± 7.3 | 26.0 ± 8.7 | 0.058 |
The purpose of this study was to develop a model to predict HAFD in patients who have undergone cardiovascular surgery and to reveal factors associated with HAFD.
Factors influencing the model in our study included sex and preoperative walking speed. Postoperative complications and in-hospital mortality tend to be higher in women than in men[13]. This may be related to a slower recovery during postoperative rehabilitation, which could contribute to a decline in physical function. Walking speed has long been considered a prognostic factor in postoperative cardiovascular surgery[4,14]. Morisawa et al[15] reported that the risk of poor prognosis was 12 times higher in patients with HAFD associated with preoperative gait slowing than in those without gait slowing. This suggests that preoperative gait speed may modify HAFD, highlighting the importance of monitoring walking speed during preoperative assessments.
Machine learning has become an increasingly valuable tool in the medical field, assisting in diagnosis and prognosis by analyzing, training, and modeling medical data[16,17]. In our study, we utilized the XGBoost model, which is a gradient-boosting decision tree machine learning algorithm. This approach creates new trees by learning from the residuals of previous trees, enhancing generalization performance and robustness compared to those achieved by conventional SVMs and Random Forests. The XGBoost model is considered to have superior generalization performance and robustness[18,19]. In this study, overfitting was assumed to be prevented, and model performance was maximized by training with XGBoost.
We successfully developed a model to predict HAFD with high accuracy, using preoperative personal and physical function data as well as the XGBoost machine-learning model. The accuracy and reliability of the model were validated using test data, and the variables contributing to the model were analyzed using SHAP[20,21], which explains the influence of each variable. Our model may be implemented in actual clinical practice, potentially contributing to improvements in accuracy and clinical quality, with updates to the model data daily.
However, this study has some limitations. First, the data used to construct this model were collected retrospectively. Second, the sample size was small. Although the sample size of this study was insufficient to construct a machine learning model, an AUC of 0.87 was achieved, demonstrating the potential of the model. Further accumulation of data should improve accuracy of the model and broaden the scope of its clinical applications.
In summary, we constructed multiple machine learning models to predict HAFD in older patients who underwent postoperative cardiovascular surgery. We found that the XGBoost model provided the best predictive performance, with gender and preoperative walking speed identified as key relevant factors.
We thank the staff of the Oita Oka Hospital for their cooperation in data collection.
| 1. | Makita S, Yasu T, Akashi YJ, Adachi H, Izawa H, Ishihara S, Iso Y, Ohuchi H, Omiya K, Ohya Y, Okita K, Kimura Y, Koike A, Kohzuki M, Koba S, Sata M, Shimada K, Shimokawa T, Shiraishi H, Sumitomo N, Takahashi T, Takura T, Tsutsui H, Nagayama M, Hasegawa E, Fukumoto Y, Furukawa Y, Miura SI, Yasuda S, Yamada S, Yamada Y, Yumino D, Yoshida T, Adachi T, Ikegame T, Izawa KP, Ishida T, Ozasa N, Osada N, Obata H, Kakutani N, Kasahara Y, Kato M, Kamiya K, Kinugawa S, Kono Y, Kobayashi Y, Koyama T, Sase K, Sato S, Shibata T, Suzuki N, Tamaki D, Yamaoka-Tojo M, Nakanishi M, Nakane E, Nishizaki M, Higo T, Fujimi K, Honda T, Matsumoto Y, Matsumoto N, Miyawaki I, Murata M, Yagi S, Yanase M, Yamada M, Yokoyama M, Watanabe N, Itoh H, Kimura T, Kyo S, Goto Y, Nohara R, Hirata KI; Japanese Circulation Society/the Japanese Association of Cardiac Rehabilitation Joint Working Group. JCS/JACR 2021 Guideline on Rehabilitation in Patients With Cardiovascular Disease. Circ J. 2022;87:155-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 2. | Blokzijl F, Dieperink W, Keus F, Reneman MF, Mariani MA, van der Horst IC. Cardiac rehabilitation for patients having cardiac surgery: a systematic review. J Cardiovasc Surg (Torino). 2018;59:817-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Ministry of Health, Labour and Welfare. Overview of the 2023 medical facilities (static/dynamic) survey and hospital report. [cited December 24, 2024]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/iryosd/23/dl/04toukei05.pdf. |
| 4. | Afilalo J, Sharma A, Zhang S, Brennan JM, Edwards FH, Mack MJ, McClurken JB, Cleveland JC Jr, Smith PK, Shahian DM, Peterson ED, Alexander KP. Gait Speed and 1-Year Mortality Following Cardiac Surgery: A Landmark Analysis From the Society of Thoracic Surgeons Adult Cardiac Surgery Database. J Am Heart Assoc. 2018;7:e010139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Yuguchi S, Saitoh M, Oura K, Tahara M, Kamisaka K, Kawamura T, Kato M, Morisawa T, Takahashi T. Impact of preoperative frailty on regaining walking ability in patients after cardiac surgery: Multicenter cohort study in Japan. Arch Gerontol Geriatr. 2019;83:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Zisberg A, Shadmi E, Gur-Yaish N, Tonkikh O, Sinoff G. Hospital-associated functional decline: the role of hospitalization processes beyond individual risk factors. J Am Geriatr Soc. 2015;63:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 324] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 7. | Tasheva P, Vollenweider P, Kraege V, Roulet G, Lamy O, Marques-Vidal P, Méan M. Association Between Physical Activity Levels in the Hospital Setting and Hospital-Acquired Functional Decline in Elderly Patients. JAMA Netw Open. 2020;3:e1920185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Morisawa T, Saitoh M, Otsuka S, Takamura G, Tahara M, Ochi Y, Takahashi Y, Iwata K, Oura K, Sakurada K, Takahashi T. Association between hospital-acquired functional decline and 2-year readmission or mortality after cardiac surgery in older patients: a multicenter, prospective cohort study. Aging Clin Exp Res. 2023;35:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 9. | Saitoh M, Saji M, Kozono-Ikeya A, Arimitsu T, Sakuyama A, Ueki H, Nagayama M, Isobe M. Hospital-Acquired Functional Decline and Clinical Outcomes in Older Patients Undergoing Transcatheter Aortic Valve Implantation. Circ J. 2020;84:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Kojima G, Taniguchi Y, Kitamura A, Shinkai S. Are the Kihon Checklist and the Kaigo-Yobo Checklist Compatible With the Frailty Index? J Am Med Dir Assoc. 2018;19:797-800.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Satake S, Shimokata H, Senda K, Kondo I, Arai H, Toba K. Predictive Ability of Seven Domains of the Kihon Checklist for Incident Dependency and Mortality. J Frailty Aging. 2019;8:85-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 7110] [Article Influence: 222.2] [Reference Citation Analysis (0)] |
| 13. | Maraschini A, Seccareccia F, D'Errigo P, Rosato S, Badoni G, Casali G, Musumeci F. Role of gender and age on early mortality after coronary artery bypass graft in different hospitals: data from a national administrative database. Interact Cardiovasc Thorac Surg. 2010;11:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56:1668-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 557] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 15. | Morisawa T, Saitoh M, Otsuka S, Takamura G, Tahara M, Ochi Y, Takahashi Y, Iwata K, Oura K, Sakurada K, Takahashi T. Hospital-Acquired Functional Decline and Clinical Outcomes in Older Cardiac Surgical Patients: A Multicenter Prospective Cohort Study. J Clin Med. 2022;11:640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Mo X, Chen X, Ieong C, Zhang S, Li H, Li J, Lin G, Sun G, He F, He Y, Xie Y, Zeng P, Chen Y, Liang H, Zeng H. Early Prediction of Clinical Response to Etanercept Treatment in Juvenile Idiopathic Arthritis Using Machine Learning. Front Pharmacol. 2020;11:1164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Waljee AK, Higgins PD. Machine learning in medicine: a primer for physicians. Am J Gastroenterol. 2010;105:1224-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Deng J, Fu Y, Liu Q, Chang L, Li H, Liu S. Automatic Cardiopulmonary Endurance Assessment: A Machine Learning Approach Based on GA-XGBOOST. Diagnostics (Basel). 2022;12:2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Hung TNK, Le NQK, Le NH, Van Tuan L, Nguyen TP, Thi C, Kang JH. An AI-based Prediction Model for Drug-drug Interactions in Osteoporosis and Paget's Diseases from SMILES. Mol Inform. 2022;41:e2100264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Li X, Tian Y, Li S, Wu H, Wang T. Interpretable prediction of 30-day mortality in patients with acute pancreatitis based on machine learning and SHAP. BMC Med Inform Decis Mak. 2024;24:328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 21. | Wang K, Tian J, Zheng C, Yang H, Ren J, Liu Y, Han Q, Zhang Y. Interpretable prediction of 3-year all-cause mortality in patients with heart failure caused by coronary heart disease based on machine learning and SHAP. Comput Biol Med. 2021;137:104813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/