Published online Jan 26, 2026. doi: 10.12998/wjcc.v14.i3.113275
Revised: September 30, 2025
Accepted: January 9, 2026
Published online: January 26, 2026
Processing time: 155 Days and 0.1 Hours
The management of stage IV pressure ulcers (PUs) in elderly, high-risk surgical patients remains a formidable clinical challenge, often limited by the invasiveness and risks of standard surgical options. Although Aloe vera has known wound-healing properties, clinical evidence of its efficacy in complex, severe PUs is scarce. This case report details a novel, non-invasive combination therapy cen
A 90-year-old, severely malnourished female (body mass index 13.3 kg/m2) pre
AVBER-based combination therapy represents a promising non-invasive option for managing severe PUs in surgically ineligible patients.
Core Tip: This case report describes the successful management of severe (stage IV) pressure ulcers in a high-risk, non-surgical 90-year-old patient using a novel aloe-derived micellar emulsion dressing combined with red light therapy and nutritional support. The aloe-derived micellar emulsion dressing, containing the bioactive component acemannan, was associated with significant wound healing, including complete closure of one ulcer and a marked reduction in another. This multimodal, non-invasive approach is a promising and cost-effective palliative strategy for managing complex wounds in frail, elderly patients for whom surgical options are contraindicated.
- Citation: Mei ST, Li L, Li JJ. Aloe-derived micellar emulsion dressing combination therapy for pressure ulcers: A case report and review of literature. World J Clin Cases 2026; 14(3): 113275
- URL: https://www.wjgnet.com/2307-8960/full/v14/i3/113275.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v14.i3.113275
Pressure ulcers (PUs) represent a global health challenge, particularly among elderly, immobilized, and debilitated patients. They are associated with high mortality, patient suffering, and substantial socioeconomic burdens. PUs typically develop when prolonged pressure exceeds capillary filling thresholds (approximately 32 mmHg arterial and 8-12 mmHg venous)[1]. They are exacerbated by risk factors such as malnutrition, neurological impairment, mental disorders, immobility, venous thrombosis, long-term use of drugs (such as anesthetics and muscle relaxants), and metabolic diseases such as diabetes[2,3]. Sustained pressure leads to tissue ischemia, injury, and necrosis, especially in the tissue over a bony prominence, leading to PUs. Although PUs can occur at any site of prolonged pressure, approximately 70% occur over the sacrum, ischial tuberosity and greater trochanter[4,5]. The National Pressure Ulcer Advisory Panel (NPUAP) staging system (revised 2016 revision) classifies PUs into six categories: Stage I (non-blanchable erythema), stage II (partial-thickness skin loss), stage III (full-thickness skin loss), stage IV (full-thickness skin and tissue loss), unstageable pressure injury (full-thickness loss obscured by slough or eschar), and deep tissue pressure injury (persistent non-blanchable deep discoloration)[6]. Healing progress is objectively evaluated using the Pressure Ulcer Scale for Healing (PUSH) tool, which yields a total score from 0 to 17. This score integrates three clinical parameters: Evaluates wound size (0-10 points), exudate amount (0-3 points), and tissue type observed in the wound bed (0-4 points), with decreasing score indicating improved healing trajectories[7]. Despite many advances in medical knowledge, dressings and therapies, PU care remains a challenging problem.
Aloe vera has been used medicinally since 1500 before common era, with demonstrated anti-inflammatory, antimicrobial, and wound-healing properties[8,9]. Its mechanisms include immunomodulation, promotion of cytokine synthesis, thromboxane inhibition, and collagen activation[10-12]. Our previous work characterized Aloe vera barbadensis extract R (AVBER), an acemannan-based microemulsion with anti-inflammatory, immunomodulatory, and pro-healing effects in cellular and animal models[13,14]. Previous literature has mainly focused on the benefits of Aloe vera in mild to moderate ulcers[15], with limited clinical evidence on complex wounds such as stage III/IV PUs and diabetic foot ulcers. Here, we present a case of severe (stage IV) PU treated with AVBER. Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images.
The patient was a 90-year-old woman with bilateral ischial tuberosity and heel PUs (duration: > 2 months), who sought nonsurgical interventions aimed at symptom relief and improved quality of life.
On March 10, 2020, the patient was hospitalized following a left intertrochanteric fracture sustained in an accidental fall, and presented with left hip swelling, pain, and restricted mobility that persisted for 1 week. The patient underwent open reduction and internal fixation surgery on March 13, 2020, and was discharged on March 27, 2020. On April 14, the patient was readmitted due to redness, swelling, and pain in the buttocks, and presented with bilateral ischial tuberosity PUs showing skin loss, measuring 2 cm × 3 cm and 5 cm × 6 cm, respectively. The patient was in poor physical condition, exhibiting weakness, severe malnutrition, and high surgical risk, which limited surgical intervention. Conservative treatment with medication was recommended. After symptomatic management, including anti-infective therapy, pain relief, and nutritional intravenous fluids, the patient declined further treatment and was discharged. The patient adopted conservative measures, including oral Qi-regulating traditional Chinese medicine, herbal wound dressings, and foam pressure-relief cushions. However, the PUs continued to worsen in both size and depth, leading to the expansion of bilateral ischial tuberosity ulcers, as well as the development of smaller heel ulcers.
The patient underwent open reduction and internal fixation surgery on March 13, 2020. She had developed PU infection, with complications including malnutrition, anemia, emphysema, and bilateral deep vein thrombosis.
The patient reported no significant family or other personal history.
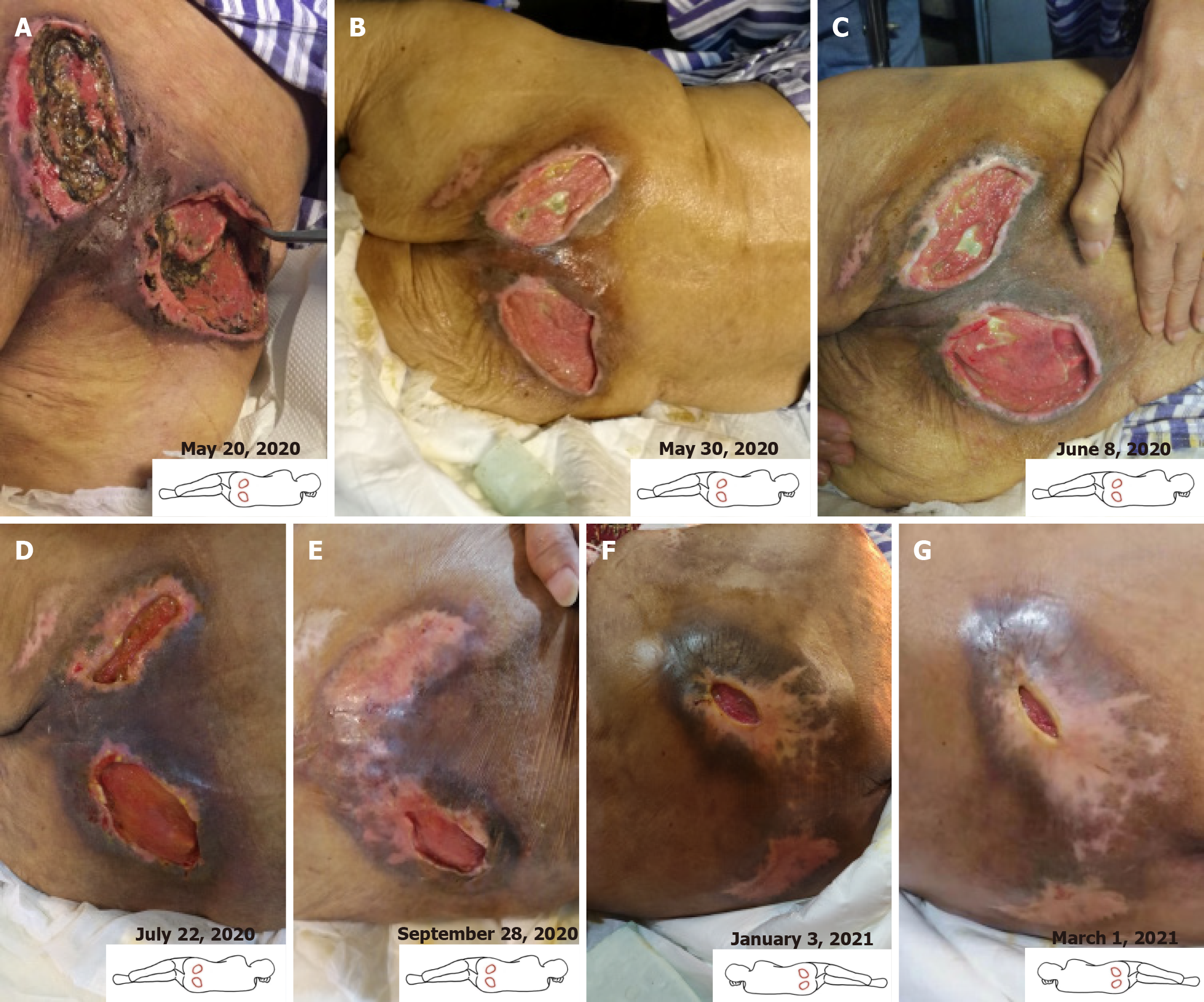
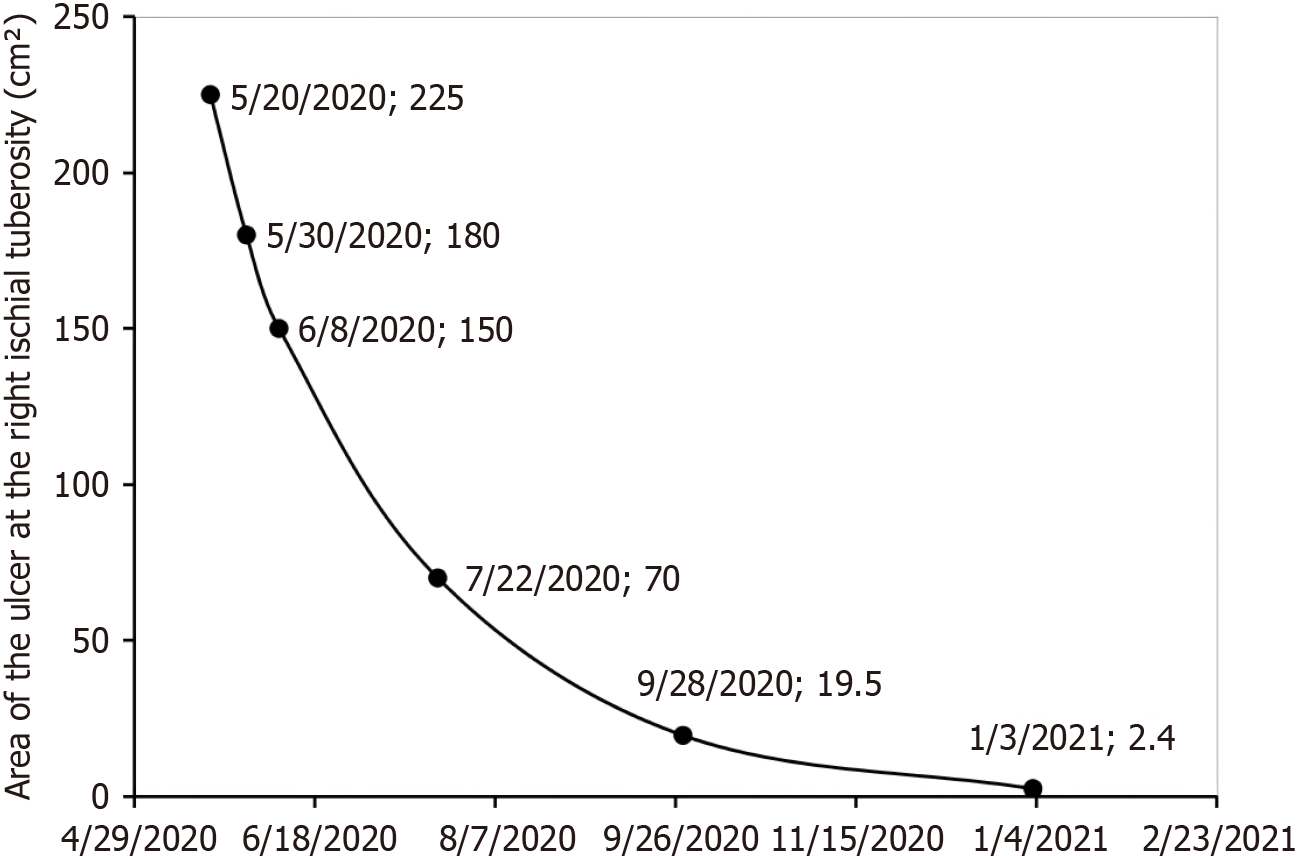
By May 20, 2020, the patient had a body mass index of 13.3 kg/m2 and a weight of 32 kg, exhibiting the following: Left ischial ulcer: 180 cm2 (12 cm × 15 cm), with full-thickness tissue loss and exposed muscle, along with a small amount of active bleeding at the lower edge; right ischial ulcer: 225 cm2 (15 cm × 15 cm), with exposed bone and undermined edges (3 cm in depth); heel ulcers: Right: 2.25 cm2 (1.5 cm × 1.5 cm) with adipose tissue exposure; left: 1 cm2 (1 cm × 1 cm) with erythema. Notably, the extensive ulcers were not accompanied by pain, which is consistent with underlying tissue neuropathy.
Laboratory tests showed the following: Albumin 26.6 g/L, hemoglobin 100 g/L, lymphocyte percentage 11.8%, and monocyte percentage 0.72%.
Imaging examinations were not performed.
The primary diagnosis was severe PUs affecting the bilateral ischial tuberosities (NPUAP grade: IV, PUSH score: 17), right heel (NPUAP grade: III, PUSH score: 8), and left heel (NPUAP grade: I, PUSH score: 3), along with severe malnutrition and mild anemia.
The PU sites were cleansed daily with sterile normal saline and gently dried using aseptic gauze to remove surface debris and exudate. The micellar emulsion dressing (AVBER; Crescel®, Guangzhou Oriplamacy Technology Co., Ltd., Gu
Considering the patient’s compromised nutritional status, standardized intravenous nutritional supplementation was initiated. The regimen consisted of a total daily volume of 1500 mL, providing approximately 1500 kcal and 60 g of protein. It included a glucose-electrolyte solution (10% glucose), a compound amino acid injection (8.5%), and a 20% fat emulsion. Essential vitamins, trace elements, and electrolytes were supplemented daily according to standard guidelines to meet the patient’s nutritional requirements. Empirical antibiotic therapy (ceftriaxone, 0.5 g/day) was also ad
On May 20, 2020, the patient initiated the treatment regimen (Figure 1). By May 30 (day 10 of treatment), clinical ob
At the follow-up on June 8, spontaneous detachment of the crust on the right heel was observed, indicating complete epithelialization. The ulcer at the left ischial tuberosity had further reduced to 104 cm2 (8 cm × 13 cm), and the right-sided lesion to 150 cm2 (10 cm × 15 cm), with corresponding PUSH scores declining to 13 (Figure 1). The patient reported new-onset mild periwound pruritus in previously insensate areas, which was documented as an AE. Concurrently, the patient’s nutritional status had improved significantly, allowing for adequate oral intake. Following clinical reassessment by the multidisciplinary team, intravenous nutritional support and antibiotic therapy were discontinued. Given the overall clinical improvement and to address the pruritus, the treatment protocol was modified: AVBER application was reduced to twice daily, with each session preceded by RLT. This adjustment aimed to alleviate discomfort while sustaining the anti-inflammatory and wound-healing effects.
As of July 22 (2 months after initiation of treatment), further substantial reductions in the wound areas were observed. The left ulcer had decreased to 12 cm2 (1.5 cm × 8 cm), and the right ulcer to 70 cm2 (7 cm × 10 cm) (Figure 2). The un
On September 28, the left ulcer had completely healed. The right ulcer continued to decrease in size, measuring 19.25 cm2 (3.5 cm × 5.5 cm) (PUSH score: 11) (Figure 1). The patient and her family members were satisfied with the wound healing outcome. The patient reported recurrence of pruritus symptoms despite initial alleviation, and this was again documented as an AE. Notably, two days prior to this assessment, the patient independently initiated treatment with a traditional Chinese medicine decoction for regulating Qi and simultaneously reduced the frequency of AVBER app
On January 3, 2021 (nearly 8.5 months after treatment initiation), the right ulcer area further decreased to 2.4 cm2 (0.8 cm × 3.0 cm) (Figure 2), with complete epithelialization, a PUSH score of 6, and significantly reduced periwound pigmentation (Figure 1). A subsequent follow-up on March 1, 2021, showed that the wound condition remained stable (Figure 1). The PUSH score thereafter remained between 5 and 6 until the patient died of pneumonia on December 14, 2021. Throughout the entire follow-up period, all AEs, primarily transient pruritus, were mild and systematically monitored using a standardized AE record form. Importantly, no ulcer exacerbation or new PU development occurred. A summary of PUSH score changes is provided in Table 1.
| Date ulcer site | May 20, 2020 | May 30, 2020 | Jun 08, 2020 | Jul 22, 2020 | Sep 28, 2020 | Jan 03, 2021 |
| Left heel | 3 | Healed | Healed | Healed | Healed | Healed |
| Right heel | 8 | 5 | Healed | Healed | Healed | Healed |
| Left ischial tuberosity | 17 | 14 | 13 | 10 | Healed | Healed |
| Right ischial tuberosity | 17 | 14 | 13 | 12 | 11 | 6 |
The clinical management of PUs remains a significant challenge, particularly in elderly bedridden patients with multiple comorbidities such as malnutrition and diabetes[2]. For advanced PUs (stage III-IV), surgical interventions such as flap reconstruction are considered the primary treatment modality. However, their clinical application is often constrained by the risk of complications and recurrence in patients with high-risk profiles[16]. This case report describes a 90-year-old female patient with a prolonged bedridden status and severe malnutrition, presenting with bilateral stage IV PUs over the ischial tuberosities. Given the patient’s high surgical risk and overall frailty, the family declined surgical intervention and opted for palliative care instead. To enhance the patient’s quality of life and alleviate clinical symptoms, our research team implemented a comprehensive treatment strategy incorporating AVBER, RLT, and nutritional support. This comprehensive strategy led to substantial wound healing and improved patient outcomes, suggesting that AVBER may serve as a valuable component within an integrated treatment plan for high-risk, surgically ineligible patients.
Notably, the healing rate during the first 4 months of treatment surpassed that reported in the literature for similar severe PUs managed with standard care[17]. Previous studies have indicated that standard care for advanced PUs often results in persistent inflammation and impaired tissue remodeling[18]. The rapid formation of granulation tissue and reduction in wound size observed in this case are consistent with the known complementary mechanisms of the interventions employed. Specifically, acemannan - a primary bioactive polysaccharide in Aloe vera - has demonstrated anti-inflammatory and immunomodulatory properties in preclinical models, such as by promoting macrophage polarization toward a pro-regenerative phenotype[19]. Similarly, RLT has been established in prior clinical studies to enhance microcirculation and accelerate tissue repair. Although the concurrent use of these modalities precludes definitive attribution of outcomes to any single component, the temporal association between AVBER application and marked reduction in inflammation - evidenced by the discontinuation of antibiotics after one week - hints at its potential role in modulating the wound microenvironment.
In this case, the outcomes observed with AVBER contrast with the limitations of conventional dressings, suggesting potential advantages. Unlike hydrogels and foam dressings, which often fail to adequately recalibrate the pathological wound milieu[20], or silver-based dressings that lack regenerative capacity[21], AVBER combines barrier protection with potential bioactivity. Although direct evidence from human chronic wound models remains limited, the unique amphiphilic structure of AVBER is theorized to enhance the delivery of bioactive substances[22]. Li et al[14] further demonstrated that acemannan-based microemulsions can activate macrophage immune responses and promote tail fin regeneration in zebrafish. AVBER also differs from more advanced options such as extracellular matrix scaffolds or growth factor dressings, which can be limited by high cost, potential immune rejection, and prolonged inflammation[23,24]. In terms of cost, both AVBER and RLT are generally considered more affordable than advanced biological therapies or surgery, though further health-economic evaluations are needed. Similarly, although negative pressure wound therapy is effective in reducing wound volume, its invasiveness and potential for tissue damage render it less suitable for highly frail patients[25,26]. In contrast, the non-invasive nature of AVBER makes it more suitable for vulnerable populations, offering lower operational demands and greater patient comfort. Studies by Hekmatpou et al[27] and Malek Hosseini et al[28] have confirmed that Aloe vera gel can alleviate periwound erythema, edema, heat, and pain, as well as significantly reduce postoperative discomfort.
It is important to acknowledge, however, that the promising outcomes noted herein must be interpreted within the inherent constraints of a single-case design. The most significant limitation lies in the inability to disentangle the individual contributions of AVBER, RLT, and nutritional support due to their simultaneous administration. Additional confounding factors - including the patient’s self-initiated use of traditional Chinese medicine and variations in dressing frequency - further complicate interpretation of the healing trajectory. Moreover, the advanced age and unique co
An interesting secondary observation was the transient pruritus reported during healing, consistent with a previous case report involving AVBER in severe diabetic foot ulcers[29]. This may indicate a role for aloe-derived components in modulating cutaneous sensory nerves during repair - a hypothesis worthy of further investigation. From a translational perspective, the unique microcellular structure of AVBER may not only improve transdermal delivery of active compounds but also support its potential as a versatile vehicle for targeted therapy in chronic wound management[30]. This case highlights the potential utility of AVBER within a multimodal, non-surgical approach for managing severe PUs in high-risk elderly patients. Although the single-case nature and concomitant interventions preclude definitive conclusions regarding AVBER’s independent efficacy, the encouraging clinical outcomes warrant further investigation through well-designed controlled trials. Future studies should seek to validate these findings, clarify underlying mechanisms, and explore the applicability of AVBER in other complex wound scenarios.
In this study, AVBER, used as part of a comprehensive regimen including RLT and nutritional support, was associated with improved healing in a patient with severe PUs and high surgical risks. Its observed benefits warrant further investigation. The noninvasive nature and favorable safety profile of AVBER suggest it is a promising adjunct for severe chronic wound conditions in vulnerable populations. However, the specific contribution of AVBER independent of other interventions requires elucidation in future controlled studies.
| 1. | Berecek KH. Etiology of decubitus ulcers. Nurs Clin North Am. 1975;10:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Mervis JS, Phillips TJ. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 324] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 3. | Ortac Ersoy E, Ocal S, Oz A, Yilmaz P, Arsava B, Topeli A. Evaluation of Risk Factors for Decubitus Ulcers in Intensive Care Unit Patients. J Med Surg Intensive Care Med. 2014;4:9-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Leblebici B, Turhan N, Adam M, Akman MN. Clinical and epidemiologic evaluation of pressure ulcers in patients at a university hospital in Turkey. J Wound Ostomy Continence Nurs. 2007;34:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Vangilder C, Macfarlane GD, Meyer S. Results of nine international pressure ulcer prevalence surveys: 1989 to 2005. Ostomy Wound Manage. 2008;54:40-54. [PubMed] |
| 6. | Edsberg LE, Black JM, Goldberg M, McNichol L, Moore L, Sieggreen M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Revised Pressure Injury Staging System. J Wound Ostomy Continence Nurs. 2016;43:585-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 725] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 7. | Stotts NA, Rodeheaver GT, Thomas DR, Frantz RA, Bartolucci AA, Sussman C, Ferrell BA, Cuddigan J, Maklebust J. An instrument to measure healing in pressure ulcers: development and validation of the pressure ulcer scale for healing (PUSH). J Gerontol A Biol Sci Med Sci. 2001;56:M795-M799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Shelton RM. Aloe vera. Its chemical and therapeutic properties. Int J Dermatol. 1991;30:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 142] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Li D, Wang HW, Dong AD, Zhang Q, Wang C, Wang J, Sun J, Zhao C. Extraction and purification, structural characteristics, biological activities, applications, and quality control of Aloe vera polysaccharides: A review. Int J Biol Macromol. 2025;329:147927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Heck E, Head M, Nowak D, Helm P, Baxter C. Aloe vera (gel) cream as a topical treatment for outpatient burns. Burns. 1981;7:291-294. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Mirshafiey A, Aghily B, Namaki S, Razavi A, Ghazavi A, Ekhtiari P, Mosayebi G. Therapeutic approach by Aloe vera in experimental model of multiple sclerosis. Immunopharmacol Immunotoxicol. 2010;32:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:103-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 250] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Tong X, Li M, Li D, Lao C, Chen J, Xu W, Du J, Zhang M, Yang X, Li J. Aloe vera gel extract: Safety evaluation for acute and chronic oral administration in Sprague-Dawley rats and anticancer activity in breast and lung cancer cells. J Ethnopharmacol. 2021;280:114434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Li D, Deng J, Wang M, Yin H, Chen J, Xu W, Guo X, Tong X, Ye D, Li J. Aloe barbadensis polymeric acetylated mannan modified by self-assembly through a pervaporation aloe polysaccharide membrane: Safety evaluation and tail fin regeneration in zebrafish. Carbohydr Polym Technol Appl. 2025;10:100709. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Hekmatpou D, Mehrabi F, Rahzani K, Aminiyan A. The Effect of Aloe Vera Clinical Trials on Prevention and Healing of Skin Wound: A Systematic Review. Iran J Med Sci. 2019;44:1-9. [PubMed] [DOI] [Full Text] |
| 16. | Cushing CA, Phillips LG. Evidence-based medicine: pressure sores. Plast Reconstr Surg. 2013;132:1720-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Zuloff-Shani A, Adunsky A, Even-Zahav A, Semo H, Orenstein A, Tamir J, Regev E, Shinar E, Danon D. Hard to heal pressure ulcers (stage III-IV): efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Arch Gerontol Geriatr. 2010;51:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Peña LT, Escolar-Peña A, Solera RA, Martínez LSZ, Castro OG, Cerezo CT, Escribese MM, López JB, Pérez TC, Heredero XS, López-Rodríguez JC, Martínez PF. Systemic immune response alteration in patients with severe pressure ulcers. Sci Rep. 2025;15:19579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Li L, Xu W, Luo Y, Lao C, Tong X, Du J, Huang B, Li D, Chen J, Ye H, Cong F, Guo X, Li J. Aloe polymeric acemannan inhibits the cytokine storm in mouse pneumonia models by modulating macrophage metabolism. Carbohydr Polym. 2022;297:120032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 20. | Rashdan HR, El-Naggar ME. Traditional and modern wound dressings—characteristics of ideal wound dressings. In: Khan R, Gowri S, editors. Antimicrobial Dressings. Academic Press, 2023: 21-42. [DOI] [Full Text] |
| 21. | Shrestha S, Wang B, Dutta PK. Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds. Antibiotics (Basel). 2024;13:910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 22. | Kumar M, Jha A, Bharti K, Mishra B. Extraction, structural properties, and applications of aloe mucilage. In: Ahmed S, Ali A, editors. Natural Gums. Elsevier, 2023: 319-337. [DOI] [Full Text] |
| 23. | Zhang T, Liu F, Tian W. [Advance of new dressings for promoting skin wound healing]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2019;36:1055-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Onida S, Tan M, Balan V, Heatley F, Peerbux S, Bolton-Saghdaoui L, Lane T, Epstein D, Gohel M, Norrie J, Lee R, Lomas R, Davies AH. Decellularized dermis allograft for the treatment of venous leg ulceration: the DAVE RCT. Br J Surg. 2025;112:znae330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Upton D, Andrews A. Pain and trauma in negative pressure wound therapy: a review. Int Wound J. 2015;12:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Huang Y, Mao B, Hu J, Xu B, Ni P, Hou L, Xie T. Consensus on the health education of home-based negative pressure wound therapy for patients with chronic wounds: a modified Delphi study. Burns Trauma. 2021;9:tkab046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Hekmatpou D, Mehrabi F, Rahzani K, Aminiyan A. The effect of Aloe Vera gel on prevention of pressure ulcers in patients hospitalized in the orthopedic wards: a randomized triple-blind clinical trial. BMC Complement Altern Med. 2018;18:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Malek Hosseini A, Rostam Khani M, Abdi S, Abdi S, Sharifi N. Comparison of aloe vera gel dressing with conventional dressing on pressure ulcer pain reduction: a clinical trial. BMC Res Notes. 2024;17:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Mei ST, Li JJ. Effective application of an innovative acemannan-enriched glycolipid sphere dressing in diabetic foot ulcer wound healing: A case report and review of literature. World J Clin Cases. 2025;13:109358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/