Published online Nov 16, 2025. doi: 10.12998/wjcc.v13.i32.111879
Revised: August 18, 2025
Accepted: October 10, 2025
Published online: November 16, 2025
Processing time: 124 Days and 4.2 Hours
Pleuroparenchymal fibroelastosis (PPFE) is a rare form of interstitial lung disease affecting the upper lobes. Its atypical radiological appearance frequently mimics lung malignancy, complicating early diagnosis. This case highlighted the imp
A 62-year-old male with a significant smoking history presented with progressive dyspnea and a chronic nonproductive cough. High-resolution computed tomo
Histopathology is essential for distinguishing PPFE from malignancy. Early dia
Core Tip: Pleuroparenchymal fibroelastosis (PPFE) frequently mimics lung malignancy on radiographs, leading to challenges in diagnosing PPFE early. This case illustrated that the localized fibrotic lesions with moderate fluorodeoxyglucose uptake on positron emission tomography/computed tomography initially suggested malignancy. However, after surgical biopsy and histopathological examination including elastic fiber staining, the patient was accurately diagnosed with PPFE. Early suspicion of PPFE and initiation of antifibrotic therapy, such as nintedanib, may effectively stabilize early-stage disease and prevent progression.
- Citation: Jung HS, Kim HJ, Kim KW. Early pleuroparenchymal fibroelastosis mimicking lung malignancy: A case report. World J Clin Cases 2025; 13(32): 111879
- URL: https://www.wjgnet.com/2307-8960/full/v13/i32/111879.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i32.111879
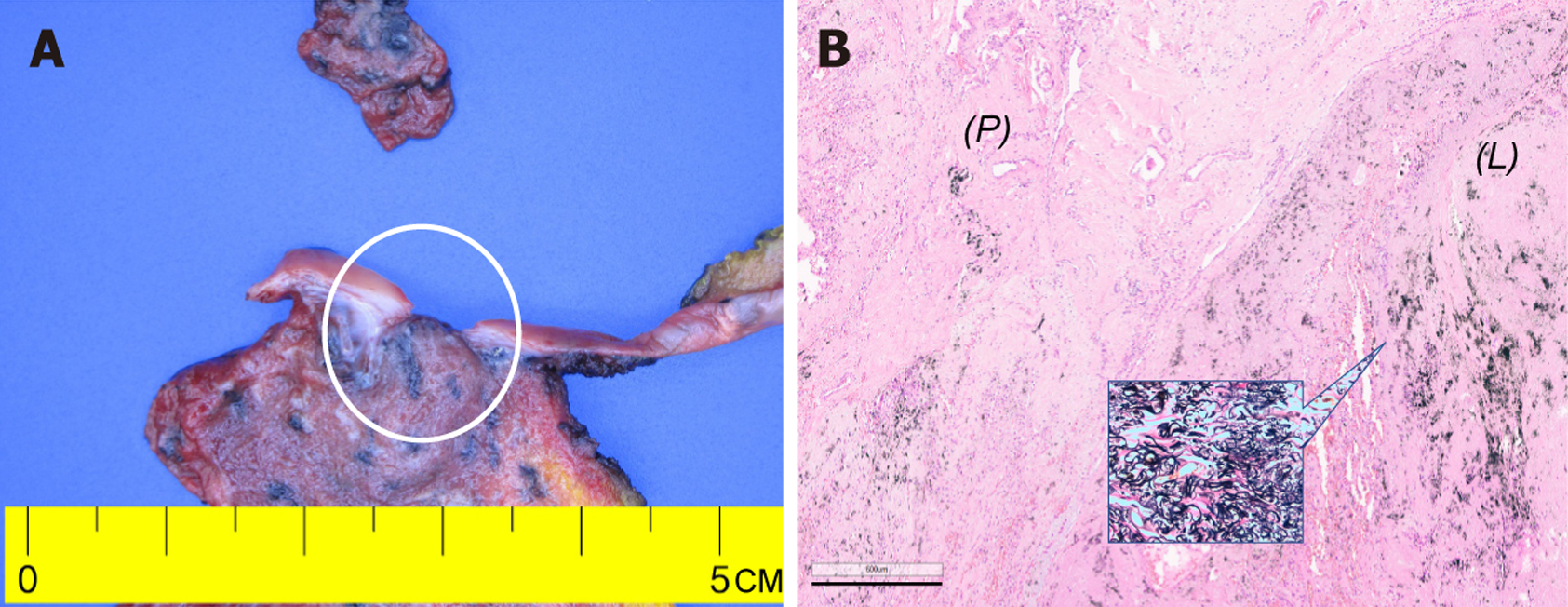
Pleuroparenchymal fibroelastosis (PPFE) is a rare interstitial lung disease with a predominance in the upper lobe. It is characterized by dense fibroelastic remodeling of the visceral pleura and adjacent subpleural lung parenchyma[1-3]. Radiologically, PPFE typically manifests as pleural thickening with subpleural consolidation/fibrosis and traction bronchiectasis[2,4,5]. Histopathological examination often includes elastic fiber stains such as Elastic Van Gieson (EVG) (also known as Verhoeff-van Gieson) and confirms dense subpleural elastosis and low levels of accompanying inflammation. Histopathology distinguishes PPFE from other fibrotic lung diseases[1-3].
Due to its rarity and radiological similarities to lung malignancy, there are significant diagnostic challenges for PPFE, resulting in misdiagnosis and inappropriate clinical management[2,5,6]. This report illustrated the complexity of diagnosing PPFE and emphasized the necessity of histological confirmation to distinguish PPFE from neoplastic lesions[1-3,7]. We described a patient whose clinical and imaging features initially indicated lung cancer, but surgical biopsy confirmed PPFE, leading to the appropriate management.
A 62-year-old Asian man presented with progressive dyspnea occurring for 1 week and a chronic nonproductive cough lasting for 1 year.
The patient reported worsening exertional dyspnea that had developed acutely over the prior week. A chronic dry cough had persisted without fever, weight loss, hemoptysis, nor night sweats. He had a significant smoking history (50 packyears) but had quit smoking 2 months prior to presentation.
The patient had no significant prior medical history, including previous illnesses, surgeries, allergies, or adverse drug reactions.
The patient was a former office worker with no known occupational exposure to asbestos nor silica and denied any history of alcohol or illicit drug use. His family medical history was unremarkable.
Upon admission physical examination revealed decreased breath sounds over the left hemithorax with an oxygen saturation of 92% on room air. There was no evidence of cyanosis, digital clubbing, or peripheral edema.
Findings from laboratory evaluations including complete blood count, coagulation profile, renal and hepatic function tests (alanine aminotransferase, aspartate aminotransferase, bilirubin), autoimmune serology, infection markers, and routine urine and fecal analyses were all within normal limits.
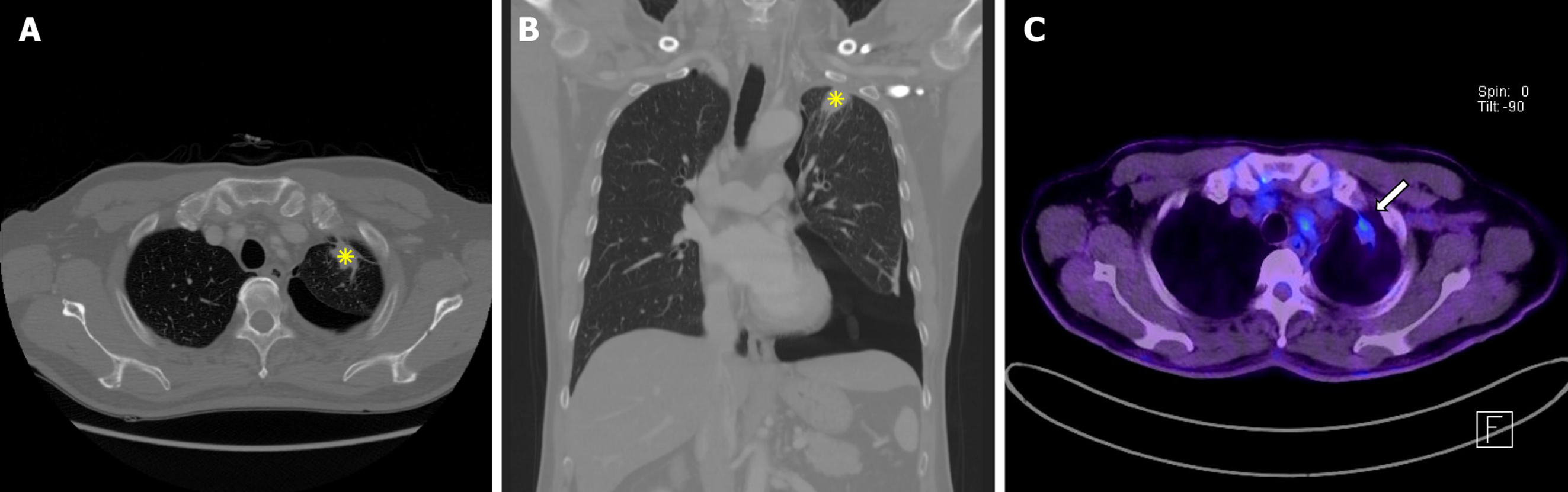
Chest radiography revealed a left pneumothorax. High-resolution computed tomography (CT) of the chest showed a localized, lobulated subpleural consolidation in the apicoposterior segment of the left upper lobe with apical pleural thickening, pleural retraction, and contiguous traction bronchiectasis (Figure 1A and B). 18F-fluorodeoxyglucose positron emission tomography (PET)/CT demonstrated moderate fluorodeoxyglucose uptake (maximum standardized uptake value = 3.2) within the lesion (Figure 1C). No abdominopelvic nor cranial imaging was performed.
Because the left pneumothorax necessitated immediate chest tube drainage, baseline pulmonary function testing was deferred for the patient’s safety, precluding a full functional assessment. Malignancy could not be definitively ruled out due to the results from the high-resolution CT and 18F-fluorodeoxyglucose PET/CT (lobulated subpleural consolidation with moderate metabolic activity). After case presentation at the interstitial lung disease multidisciplinary discussion (MDD), video assisted thoracoscopic surgery was recommended to secure a tissue diagnosis. Segmentectomy with talc pleurodesis was performed, and histopathology revealed dense fibroelastosis with prominent EVG positive elastic fibers, scant inflammation, and no malignant cells (Figure 2). Ancillary molecular testing including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase, receptor tyrosine kinase, and RET immunohistochemistry plus EGFR polymerase chain reaction on the resected specimen was negative for driver mutations. Differential diagnoses including chronic hypersensitivity pneumonitis, granulomatous infection, apical cap, and asbestos-related fibrosis were systematically excluded by clinicoradiological correlation and histological features.
Idiopathic PPFE of the left upper lobe.
Antifibrotic therapy (nintedanib, 150 mg orally twice daily) was initiated postoperatively due to risk of disease pro
At the 26-month postoperative visit, the patient had remained clinically stable with no recurrence of pneumothorax. Pulmonary function testing demonstrated a forced vital capacity (FVC) of 2.85 L and a diffusing capacity for carbon monoxide (DLCO) of 78% of the predicted value, confirming preserved lung function. Regular outpatient monitoring will continue at 6-month intervals.
PPFE is a rare subtype of idiopathic interstitial pneumonia. It is characterized by predominant fibroelastic changes affecting the upper lobes and adjacent pleura[1,2]. Although PPFE classically presents with diffuse fibrosis, it can occ
Unlike idiopathic pulmonary fibrosis, which typically exhibits diffuse honeycombing and prominent inflammatory infiltrates, PPFE demonstrates dense fibroelastic remodeling with minimal inflammation and predominantly involves subpleural regions[1,2,4,5]. Differential diagnoses including apical cap, chronic hypersensitivity pneumonitis, granulomatous infection, and asbestos-related fibrosis can be rigorously excluded through clinicoradiological and pathological examinations[5,7]. The absence of relevant exposure and the characteristic histopathology effectively ruled out these other diagnoses in our patient. Consultation with the interstitial-lung-disease MDD minimized diagnostic error. The MDD recommended video-assisted thoracoscopic biopsy[7,8], which is consistent with the 2022 American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society guidelines. Histology confirmed PPFE. Furthermore, immunohistochemistry and polymerase chain reaction detected no EGFR, anaplastic lymphoma kinase, receptor tyrosine kinase, or RET alterations, thereby excluding a diagnosis of scar-associated adenocarcinoma and averting inappropriate oncological therapy[1-3].
Proposed poor prognostic markers for PPFE include a baseline DLCO < 55% of the predicted value, an annual FVC decline ≥ 10%, progressive platythorax, and serum Krebs von den Lungen-6 concentration > 600 U/mL[4,9,10]. Our patient presented with a Krebs von den Lungen-6 concentration of 230 U/mL, which was well below the poor prognostic threshold. Additionally, no other poor prognostic factors were present, supporting the preservation of lung function observed 26 months after resection (FVC: 2.85 L; DLCO: 78% of the predicted value). Observational data suggested that nintedanib attenuated FVC decline in secondary PPFE, whereas another cohort reported limited efficacy of nintedanib in idiopathic PPFE with usual interstitial pneumonia[11,12]. An individualized, early-intervention strategy in patients at risk of progression is supported by these data. The radiographic and physiologic stability observed in the present case after 2 years of nintedanib treatment also supports these preliminary data. However, controlled trials are needed to confirm the therapeutic efficacy of nintedanib and to identify the patients who will derive the greatest benefit from this treatment. Several limitations of this case must be acknowledged. The baseline pulmonary function testing was absent due to the initial pneumothorax, and it limited our assessment of early functional impairment and the therapeutic response of nintedanib. Furthermore, inherent limitations associated with single-case observations restrict generalizability. Nonetheless, this report provided meaningful insights into diagnostic and management strategies for atypical presentations of PPFE.
This case underscored the necessity for clinicians to recognize atypical, localized manifestations of PPFE because it can mimic lung malignancy. Histopathological confirmation is needed to guide accurate diagnosis and management. The positive clinical outcome observed following antifibrotic therapy highlights its potential utility in treating early-stage PPFE. Further prospective investigations are essential to substantiate therapeutic recommendations and define prognostic biomarkers.
| 1. | Cottin V, Si-Mohamed S, Diesler R, Bonniaud P, Valenzuela C. Pleuroparenchymal fibroelastosis. Curr Opin Pulm Med. 2022;28:432-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Gamble JL, Müller NL, Churg A, Bilawich AM. Pleuroparenchymal Fibroelastosis: Update on CT and Histologic Findings. Radiol Cardiothorac Imaging. 2025;7:e240382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Yamakawa H, Oda T, Sugino K, Hirama T, Komatsu M, Katano T, Fukuda T, Takemura T, Kubota Y, Kishaba T, Norisue Y, Araya J, Ogura T. Proposed Clinical Algorithm for Pleuroparenchymal Fibroelastosis (PPFE). J Clin Med. 2024;13:3675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Ricoy J, Suárez-Antelo J, Antúnez J, Martínez de Alegría A, Ferreiro L, Toubes ME, Casal A, Valdés L. Pleuroparenchymal fibroelastosis: Clinical, radiological and histopathological features. Respir Med. 2022;191:106437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Franquet T, Giménez Palleiro A. Idiopathic pleuroparenchymal fibroelastosis. Radiologia (Engl Ed). 2022;64 Suppl 3:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Archer JM, Mendoza DP, Hung YP, Lanuti M, Digumarthy SR. Surgical Resection of Benign Nodules in Lung Cancer Screening: Incidence and Features. JTO Clin Res Rep. 2023;4:100605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Glenn LM, Troy LK, Corte TJ. Diagnosing interstitial lung disease by multidisciplinary discussion: A review. Front Med (Lausanne). 2022;9:1017501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 8. | Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM, Martinez FJ, Molina-Molina M, Myers JL, Nicholson AG, Ryerson CJ, Strek ME, Troy LK, Wijsenbeek M, Mammen MJ, Hossain T, Bissell BD, Herman DD, Hon SM, Kheir F, Khor YH, Macrea M, Antoniou KM, Bouros D, Buendia-Roldan I, Caro F, Crestani B, Ho L, Morisset J, Olson AL, Podolanczuk A, Poletti V, Selman M, Ewing T, Jones S, Knight SL, Ghazipura M, Wilson KC. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2022;205:e18-e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 1804] [Article Influence: 451.0] [Reference Citation Analysis (0)] |
| 9. | Kinoshita Y, Ikeda T, Miyamura T, Ueda Y, Yoshida Y, Kushima H, Fujita M, Ogura T, Watanabe K, Ishii H. A proposed prognostic prediction score for pleuroparenchymal fibroelastosis. Respir Res. 2021;22:215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Kono M, Tsunoda T, Ikeda S, Yagi S, Hirama R, Watanuki M, Oshima Y, Tsutsumi A, Miwa H, Miki Y, Hashimoto D, Suda T, Nakamura H. Clinical features of idiopathic pleuroparenchymal fibroelastosis with progressive phenotype showing a decline in forced vital capacity. Respir Investig. 2023;61:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 11. | Nasser M, Si-Mohamed S, Turquier S, Traclet J, Ahmad K, Philit F, Bonniaud P, Chalabreysse L, Thivolet-Béjui F, Cottin V. Nintedanib in idiopathic and secondary pleuroparenchymal fibroelastosis. Orphanet J Rare Dis. 2021;16:419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Sugino K, Ono H, Shimizu H, Kurosawa T, Matsumoto K, Ando M, Mori K, Tsuboi E, Homma S, Kishi K. Treatment with antifibrotic agents in idiopathic pleuroparenchymal fibroelastosis with usual interstitial pneumonia. ERJ Open Res. 2021;7:00196-02020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/