Published online Oct 26, 2025. doi: 10.12998/wjcc.v13.i30.109028
Revised: May 19, 2025
Accepted: August 11, 2025
Published online: October 26, 2025
Processing time: 166 Days and 15.2 Hours
Primary biliary cholangitis is a chronic cholestatic autoimmune liver disease that progressively damages the bile ducts, leading to cholestasis and, in advanced stages, cirrhosis. While it primarily affects middle-aged women, recent data indicate a rising incidence in men. The interplay between genetic susceptibility, environmental exposures, and gut microbiome alterations is thought to drive disease onset. Diagnosis relies on persistent cholestatic enzyme elevation, disease-specific autoantibodies, and, in select cases, liver biopsy. Ursodeoxycholic acid remains the cornerstone of treatment, but many patients show an incomplete response. The recent withdrawal of obeticholic acid from the market, due to insufficient evidence of long-term benefit, has highlighted the urgent need for effective second-line therapies. Agonists of peroxisome proliferator- activated receptors, such as elafibranor and seladelpar, have demonstrated promising biochemical improvements and may reshape the therapeutic landscape. Future research is focused on refining risk assessment, optimizing treatment combinations, and addressing symptoms such as fatigue and pruritus to enhance patient well-being. A shift toward early intervention and personalized treatment strat
Core Tip: Primary biliary cholangitis (PBC) is a progressive autoimmune liver disease driven by a complex interplay of genetic, environmental, and microbial factors. Despite advances in early diagnosis and the widespread use of ursodeoxycholic acid, a significant subset of patients experiences incomplete biochemical response and disease progression. This review highlights recent breakthroughs in understanding PBC pathogenesis, the limitations of current therapies, including the withdrawal of obeticholic acid, and the emerging role of novel agents such as selective peroxisome proliferator-activated receptors agonists. Future strategies focusing on early intervention, personalized treatment, and symptom management may reshape the clinical management and prognosis of PBC.
- Citation: Curto A, Iamello RG, Lynch EN, Galli A. Advancing the management of primary biliary cholangitis: From pathogenesis to emerging therapies. World J Clin Cases 2025; 13(30): 109028
- URL: https://www.wjgnet.com/2307-8960/full/v13/i30/109028.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i30.109028
Primary biliary cholangitis (PBC) is a progressive autoimmune liver disease characterized by chronic inflammation of the interlobular bile ducts, leading to bile acid (BA) retention in the liver and subsequent hepatocyte damage. If left untreated, PBC can progress to end-stage liver disease, ultimately requiring liver transplantation[1]. This review aims to provide a comprehensive overview of PBC, covering its epidemiology, pathogenesis, clinical features, and current treatment options, with a particular focus on therapeutic approaches and future perspectives.
The global pooled incidence and prevalence of PBC are estimated at 1.76 cases and 14.60 cases per 100000 individuals, respectively[2]. Europe and North America report the highest disease burden worldwide[2,3]. In Europe specifically, the pooled point prevalence is 22.27 cases per 100000, with an annual incidence of 1.87 new cases per 100000 inhabitants[4]. Italian epidemiological data indicate an incidence between 2.21 and 5.31 cases per 100000 and a prevalence ranging from 3.86 to 27.90 per 100000 inhabitants[5,6]. Historically, PBC predominantly affected women, with a female-to-male ratio of 9:1; however, this ratio has decreased to approximately 4:1 over the past two decades[7,8], but in Central and Eastern European countries is still almost 95% of all PBC diagnosed in women. Sex-related differences in PBC susceptibility are attributed to hormonal influences, particularly the modulatory role of oestrogens on BA metabolism, and to genetic predisposition[7,9]. Notably, male patients tend to present with more advanced disease, show poorer response rates to Ursodeoxycholic acid (UDCA), and are at higher risk of developing hepatocellular carcinoma (HCC)[7,10]. Extrahepatic autoimmune manifestations are common in PBC (Table 1), especially rheumatologic disorders. Sjogren’s syndrome exhibits the strongest association (21.4% in PBC vs 3% in controls), followed by Raynaud’s phenomenon (12.3% vs 1%), rheumatoid arthritis-like polyarthritis (5% vs 3%), systemic sclerosis (3.7% vs 0%), and systemic lupus erythematosus (2% vs 0%)[11]. Increased prevalence of thyroid disorders (11.3%), autoimmune thyroiditis (9.9%), osteoporosis (21.1%), celiac disease (1%), and chronic bronchitis (4.6%) has also been documented among PBC patients[12].
| Condition | Prevalence in PBC (%) |
| Sjogren’s syndrome | 21.4 |
| Raynaud’s phenomenon | 12.3 |
| Rheumatoid arthritis-like polyarthritis | 5 |
| Systemic sclerosis | 3.7 |
| Systemic lupus erythematosus | 2 |
| Thyroid disorders | 11.3 |
| Autoimmune thyroiditis | 9.9 |
| Osteoporosis | 21.1 |
| Celiac disease | 1 |
| Chronic bronchitis | 4.6 |
Although the precise pathogenic mechanisms underlying PBC remain incompletely defined, recent advances have emphasized the critical roles of genetic predisposition and gut-liver axis dysfunction in disease development.
Genetic studies have consistently demonstrated the strongest associations between PBC and variants within the human leukocyte antigen region, with genome-wide association studies (GWAS) playing a pivotal role in elucidating this genetic contribution[13-15]. A large international GWAS meta-analysis identified 56 genome-wide significant loci, including 20 novel susceptibility loci, across European, Asian, and combined cohorts (46, 13, and 41 Loci, respectively). Candidate genes within these regions, such as FCRL3, INAVA, PRDM1, IRF7, CCR6, CD226, and IL12RB1, are predominantly involved in immune regulation and inflammatory pathways[16]. Further, protein tyrosine phosphatase non-receptor type 2 has been identified as a novel susceptibility gene in PBC through GWAS and meta-analysis conducted in a Japanese cohort comprising 2181 cases and 2699 controls[17]. MicroRNA dysregulation has also been implicated in PBC pathogenesis. Specifically, miR-122a and miR-26a are upregulated, while miR-328 and miR-299-5p are downregulated in patients. Among these, miR-506 has emerged as a notable contributor to PBC immunopathogenesis, and its localization on the X chromosome may provide insights into the disease’s strong female predominance[18].
The differentiation and expansion of Th17 cells play a central role in biliary epithelial cell injury in PBC. Antigen-presenting cells within the liver secrete interleukin (IL)6 and IL23, which together with transforming growth factor β drive naive CD4+ T cells toward a Th17 phenotype by upregulating retinoic acid receptor-related orphan receptor gamma t and IL21 expression[19]. IL23, composed of p19 and the shared p40 subunit, signals through the IL23R/IL12RB1 receptor complex to maintain and amplify this Th17 pool[20,21]. In PBC patients and murine models, increased Th17 infiltrates localize around damaged bile ducts, where secreted IL17A induces cholangiocyte production of chemokines (e.g., C-X-C motif chemokine 1, C-C motif chemokine ligand 20) that recruit neutrophils and perpetuate local inflammation[19,22]. Moreover, IL17 synergizes with tumor necrosis factor α - produced by Th1 and natural killer cells in response to IL12 signaling - to upregulate Fas ligand on biliary epithelial cells, triggering caspase mediated apoptosis and promoting fibrogenesis[23]. This IL6/IL23/IL17-tumor necrosis factor α cytokine network thus establishes a self-sustaining loop of immune-mediated bile duct destruction and fibrosis in PBC.
Antimitochondrial antibodies (AMAs) represent a hallmark diagnostic marker for PBC, although they are not universally detected in all cases[24]. The primary antigenic target of AMAs is the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2). Given the high degree of evolutionary conservation of PDC-E2 across species, the mechanism of molecular mimicry has been proposed to explain AMA generation. In this model, prior exposure to pathogens bearing structurally similar epitopes may trigger a cross-reactive autoimmune response. Among bacterial species, Escherichia coli (E. coli) has long been implicated in PBC immunopathogenesis, potentially accounting for the observed association between PBC onset and recurrent urinary tract infections, for which E. coli is a common etiological agent. More recently, exposure to Novosphingobium aromaticivorans, a Gram-negative bacterium capable of metabolizing xenobiotics, has been suggested as a potent environmental trigger, with studies reporting stronger immune reactivity to Novosphingobium aromaticivorans than to E. coli among PBC patients[25]. Despite their diagnostic utility, AMAs do not directly mediate cholangiocyte injury. The presence of AMAs in asymptomatic first-degree relatives of PBC patients further suggests that AMA production alone is insufficient for disease development, implicating additional pathogenic mechanisms. A recent comprehensive review proposed a refined model for PBC pathogenesis[26]. Serum antibodies from PBC patients demonstrate cross-reactivity with bacterial and yeast proteins, reinforcing the concept of antigenic mimicry. Nevertheless, the persistence of stable IgM AMA levels throughout disease progression challenges a purely infectious etiology. Current models suggest that immunological recognition is initiated when a modified form of PDC-E2, aberrantly externalized from cholangiocytes, becomes accessible to T and B lymphocytes[26]. Concurrently, epigenetic downregulation of the chloride/bicarbonate exchanger AE2 in both cholangiocytes and immune cells results in impaired biliary bicarbonate secretion. Loss of the protective “alkaline umbrella” exposes cholangiocytes to toxic bile salts, promoting intracellular alkalinization, activation of soluble adenylyl cyclase, and heightened susceptibility to bile salt–induced apoptosis[27]. The resultant cholangiocyte senescence, apoptosis, and dysregulated proliferation ultimately lead to progressive ductopenia. In parallel, pyruvate dehydrogenase complex involvement in mitochondrial dysfunction contributes to impaired ATP synthesis, energy depletion, and the characteristic symptom of fatigue in PBC patients[26].
The liver, as the primary recipient of blood from the gastrointestinal tract, is intimately influenced by the gut microbiota and its metabolites. Perturbations in intestinal permeability and microbial dysbiosis are recognized as key contributors to hepatic immune activation. In PBC, a significant reduction in microbial diversity has been consistently documented[28-30]. The contribution of microbial factors to PBC pathogenesis is further supported by epidemiological studies linking recurrent urinary tract infections with an increased risk of disease onset[31]. Moreover, the recurrence of PBC following liver transplantation, despite immunosuppressive therapy, implicates non-immune mechanisms, including the potential role of microbial metabolites, in disease persistence and recurrence[32]. Microbiota profiling in PBC reveals an enrichment of Haemophilus, Veillonella, Clostridium, Lactobacillus, Streptococcus, Pseudomonas, Klebsiella, and an unclassified genus within the Enterobacteriaceae family, alongside a depletion of Bacteroidetes spp., Sutterella, Oscillospira, and Faecalibacterium[33]. Of note, Faecalibacterium abundance is significantly lower in patients with an inadequate response to UDCA therapy[29].
Fatigue is one of the most prevalent and debilitating symptoms in PBC, affecting approximately 50%-80% of patients. It is typically classified into two distinct components: Peripheral and central fatigue. Peripheral fatigue is attributed to neuromuscular dysfunction and reduced exercise tolerance, potentially linked to PDC-E2 involvement and a metabolic shift toward anaerobic pathways, resulting in lactic acid accumulation and impaired muscle performance with delayed recovery[34]. In contrast, central fatigue is characterized by a diminished sense of motivation and volition, impacting both physical and cognitive domains. Although the precise pathophysiological mechanisms of central fatigue remain unclear, it is hypothesized to involve altered central nervous system processing[34]. Pruritus is another hallmark symptom of PBC, although it occurs in only a subset of patients. Cholestasis-associated pruritus often follows a circadian pattern, typically intensifying in the evening and nighttime hours. It most frequently involves the extremities, including the soles, palms, and forearms, but may present as generalized pruritus[35]. Given the profound impact of fatigue and pruritus on health-related quality of life, thorough evaluation is essential (Table 2 summarize effect of current therapies on these symptoms). The PBC-40 questionnaire is a validated tool specifically designed to assess the spectrum of symptoms associated with PBC, encompassing fatigue, pruritus, cognitive dysfunction, sleep disturbances, physical functioning, and emotional well-being[36]. For the targeted assessment of pruritus severity, a simple Numerical Rating Scale may be employed. As the disease progresses toward cirrhosis, patients become susceptible to complications typical of advanced liver disease, including HCC. The incidence of HCC is approximately 13 per 1000 person-years among patients with PBC-related cirrhosis, compared to 2.7 per 1000 person-years in those without cirrhosis[37]. Cirrhosis and male sex have been identified as the principal risk factors for HCC development in this population[38].
| Therapy | Effect on fatigue | Effect on pruritus |
| UDCA[43,45] | No significant improvement2 | Often insufficient2 |
| OCA[70,92] | No improvement2 | Worsening or new-onset, dose-dependent3 |
| Fibrates[84] | Mild to moderate improvement1 | Clinically meaningful reduction1 |
| Elafibranor[95] | Mild to moderate improvement1 | Clinically meaningful reduction1 |
| Seladelpar[98] | Mild to moderate improvement1 | Significant reduction1 |
| Budesonide[108] | Little or no documented effect2 | Marginal or no specific effect2 |
| IBAT inhibitors (e.g., linerixibat, maralixibat)[107] | Data limited | Marked improvement1 |
PBC is frequently associated with a broad spectrum of extrahepatic autoimmune conditions[12]. Consequently, clinical evaluation should systematically explore symptoms indicative of rheumatologic diseases, with particular attention to sicca symptoms given the strong association with Sjogren’s syndrome. In addition, thyroid function, calcium and vitamin D metabolism, bone mineral density, and lipid profiles should be routinely assessed[39]. Hyperlipidaemia in PBC differs mechanistically from that observed in other metabolic disorders. In PBC, hypercholesterolemia predominates over hypertriglyceridemia, with elevated low-density lipoprotein cholesterol levels that could, in theory, suggest an increased risk for cardiovascular events. However, these patients typically also exhibit elevated high-density lipoprotein cholesterol concentrations. Moreover, the concurrent elevation of adiponectin and the presence of lipoprotein-X, both of which exert anti-atherogenic effects, may mitigate cardiovascular risk in this setting[40-43]. Thus, despite the frequent finding of hypercholesterolemia, patients with PBC appear to have a lower-than-expected risk for clinically significant cardiovascular disease[44].
In clinical practice, PBC is established when at least two of the following are present: Chronic cholestatic biochemistry, seropositivity for AMA or PBC-specific antinuclear antibodies (anti-gp210/anti-sp100), and liver histology consistent with chronic nonsuppurative destructive cholangitis and ductopenia[43,45,46]. Any patient with sustained or fluctuating alkaline phosphatase (ALP)/gamma glutamyl transferase (GGT) elevations should undergo a thorough evaluation, detailed history (including drugs, supplements, alcohol intake and symptoms of fatigue or pruritus), physical examination, and abdominal ultrasound to exclude extrahepatic obstruction[43,45,46]. Biochemically, a sustained rise in ALP, confirmed by concomitant GGT elevation, often represents the earliest clue, whereas modest transaminase increases and hyperbilirubinemia reflect periportal inflammation and advancing ductopenia, however serial liver biopsies are the only option to assess progression of ductopenia in the ductopenic form of PBC; marked alanine aminotransferase (ALT) or IgG elevations should prompt consideration of an overlap with autoimmune hepatitis and thus liver biopsy[47,48]. Measurement of serum IgM and assessment of lipid profiles (notably hypercholesterolaemia) provide additional supportive evidence. Serological confirmation relies on AMA detection, by indirect immunofluorescence in over 90% of cases or by high-sensitivity enzyme-linked immunosorbent assay against PDC-E2 and related antigens, while anti-gp210 and anti-sp100, despite sensitivities of only about 23%-25%, are highly specific (> 95%) and carry independent prognostic value in AMA-negative patients[49-51]. Cross-sectional imaging, principally ultrasonography, serves to rule out biliary dilatation or mass lesions; in contrast, hepatic imaging in early PBC is typically unremarkable. Transient elastography performed at diagnosis affords non-invasive staging of fibrosis and may obviate the need for immediate biopsy[43,45,52]. Liver biopsy, adequate when sampling ≥ 10-15 portal tracts, is reserved for patients lacking serological markers, those with normal ALP despite positive AMA, or when concomitant liver diseases (e.g., metabolic dysfunction-associated steatotic liver disease, small-duct primary sclerosing cholangitis, or autoimmune hepatitis overlap) are suspected. Histologically, identification of florid duct lesions, characterized by epithelioid granulomas adjacent to damaged bile ducts, and the progressive loss of interlobular ducts confirm the diagnosis and guide prognostication[45,48].
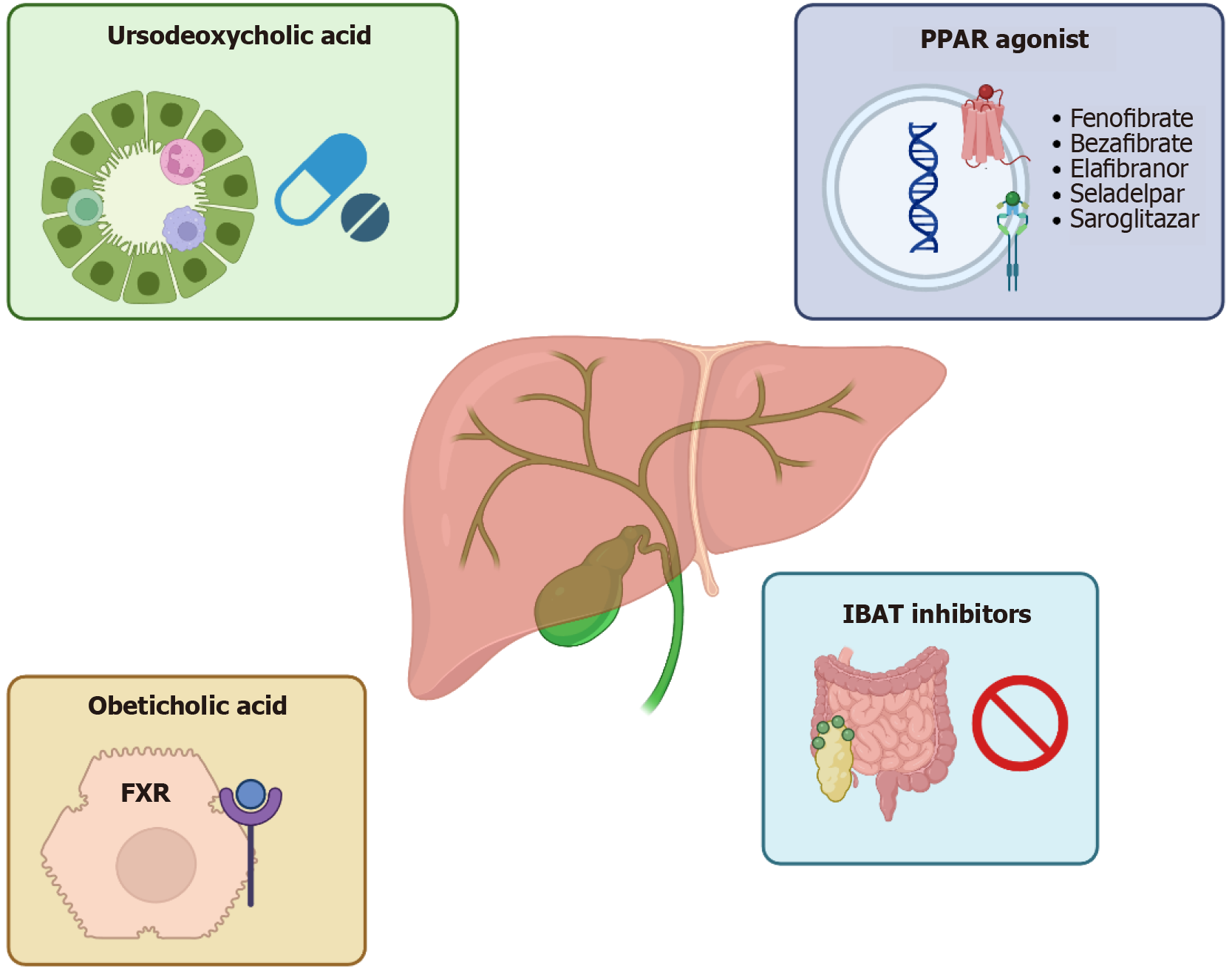
The cornerstone of therapy for PBC (Figure 1) is UDCA[43], which has been shown to significantly alter the natural course of the disease[53,54]. Approved for clinical use in 1997, UDCA acts through a cytoprotective mechanism, shielding cholangiocytes from bile toxicity. Treatment with UDCA, administered at 13-15 mg/kg/day for at least one year, has been proven to improve transplant-free survival regardless of initial disease severity[49]. It effectively reduces biochemical markers such as ALP, total bilirubin, cholesterol, and IgM[50,51]. Therapeutic response is typically assessed after 12 months of therapy, although improvements in serum parameters may be observed as early as six months[55]. Several models have been developed to define treatment response, including the Paris, Toronto, and Global Assessment of Liver Outcomes (GLOBE) criteria[56-59]. Although there are several models that evaluate the therapeutic response to UDCA in PBC patients, normalisation of ALP and total bilirubin should be main outcome of first-line PBC treatment. PBC patients with achieved deep response have a better prognosis compared to patients without achieve it[60]. The main histological goal of PBC treatment is fibrosis regression; this is achieved by only a small proportion of UDCA responders, while it is not achieved by non-responders[59]. Nonetheless, approximately 40% of patients exhibit an inadequate response to UDCA, a condition that significantly increases the risk of progression to cirrhosis and overall mortality[61]. Additionally, UDCA has limited impact on extrahepatic symptoms such as fatigue and pruritus, and a minority of patients may experience intolerance due to gastrointestinal side effects or cosmetic concerns[62]. Obeticholic acid (OCA), a potent and selective farnesoid X receptor (FXR) agonist, was approved in 2016 by the United States Food and Drug Administration (FDA) as a second-line treatment for PBC in patients who exhibit an inadequate response to UDCA or who are intolerant to it[45]. Farnesoid X receptor is a nuclear receptor highly expressed in the liver and gastrointestinal tract that plays a central role in regulating BA homeostasis through transcriptional control of genes involved in BA synthesis, transport, and efflux[63-66].
OCA exerts hepatoprotective effects by enhancing BA excretion, suppressing hepatic BA synthesis, and modulating inflammatory and fibrogenic pathways. These effects are mediated in part by the induction of fibroblast growth factor 19 and suppression of nuclear factor-kappa B-mediated inflammation[67-69]. Clinical efficacy was demonstrated in the phase 3 PBC OCA international study of efficacy (POISE) trial, where OCA significantly reduced serum ALP, surrogate marker of disease progression, with 46%-47% of patients in the 5 mg and 10 mg treatment arms, respectively, achieving the composite biochemical endpoint, compared with 10% in the placebo group[70]. The biochemical improvements observed in POISE were sustained in a long-term extension study[71]. In two real world cohorts of UDCA refractory PBC patients, obeticholic acid demonstrated robust biochemical and prognostic benefits over 12 months of follow up. Both studies reported significant median reductions in ALP (by approximately 32-80 U/L), ALT (22%-31%), and bilirubin (0.12 mg/dL to 11.2%), accompanied by meaningful declines in composite risk scores such as the Globe PBC score (to 0.17) and a non significant trend in the United Kingdom PBC score[72,73]. Using POISE criteria, response rates ranged from 29.5% to 42.9%, while only about 11% achieved full normalization of liver tests. Although patients with cirrhosis, present in roughly one third of subjects, experienced similar biochemical improvements, they discontinued OCA more often (up to 30% vs 12% in non cirrhotic patients, primarily due to pruritus) and had lower response rates[72,73]. However, the most commonly reported adverse effect was pruritus, which was dose-dependent and led to treatment discontinuation in up to 25% of patients[70]. Other side effects included fatigue, dyslipidemia, characterized by decreased high-density lipoprotein and increased low-density lipoprotein[74], as well as nasopharyngitis and headache[70]. Despite early regulatory approval under the FDA’s accelerated approval program, confirmatory evidence from the COBALT trial, a phase 4 randomized controlled study, failed to demonstrate a statistically significant benefit of OCA on hard clinical outcomes such as mortality or liver transplantation, although functional unblinding and treatment crossover, particularly in the placebo arm, confounded the intenttotreat estimate of OCA’s effects in the randomized clinical trial randomized clinical trial[75]. The trial was terminated early due to statistical futility, and its results, along with post-marketing safety data indicating an increased risk of hepatic decompensation and failure in patients with advanced cirrhosis, prompted the addition of a black box warning for this subgroup[74,76]. In June 2024, based on these findings and the insufficient demonstration of clinical efficacy, the European Medicines Agency recommended revoking the conditional marketing authorization for OCA. This decision was upheld by the General Court of the European Union, leading to the drug’s withdrawal from the European market in November 2024. Nevertheless, the real world data suggest that OCA could still hold value within carefully monitored treatment algorithms.
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors, first cloned in 1990, that transcriptionally regulate genes involved in BA synthesis, transport, inflammation, and fibrogenesis[77]. Fibrates are synthetic PPAR agonists with varying affinities for PPAR-α, -γ, and -δ: PPAR-α activation upregulates genes critical for bile and lipid metabolism and downregulates immune-related pathways[78], while PPAR-γ/δ induction confers anti-inflammatory and antifibrotic effects[79,80]. Bezafibrate (BZF), a weak pan-PPAR activator, also enhances biliary phospholipid excretion via upregulation of multidrug resistance protein 3[81,82]. On this mechanistic basis, the American Association for the Study of Liver Diseases recommends off-label use of fibrates for PBC patients with an inadequate response to UDCA[45]. Early Japanese pilot studies administering BZF 400 mg/day to pre-cirrhotic PBC patients demonstrated complete normalization of ALP and significant reductions in IgM levels[83]. These findings were confirmed in the phase 3 BEZURSO trial, in which 100 UDCA-refractory, non-cirrhotic PBC patients were randomized to receive BZF 400 mg/day or placebo in addition to UDCA. At 24 months, 31% of the BZF cohort achieved complete biochemical response, defined as normalization of ALP, aminotransferases, total bilirubin, albumin, and prothrombin index, vs 0% in the placebo arm; secondary endpoints included ALP normalization in 67% vs 2%, a 15% reduction vs 22% increase in liver stiffness, and significant improvements in pruritus and fatigue[84].
Real-world evidence from a large Japanese observational cohort (n = 3908) further substantiated these results, revealing that UDCA + BZF was associated with significantly improved transplant-free survival compared to UDCA alone (adjusted hazard ratios 0.33 all-cause, 0.27 Liver-related) and a marked GLOBE score reduction from 0.504 ± 0.090 pre-treatment to 0.115 ± 0.085 at one year (P < 0.0001). Retrospective analyses of dual (UDCA + OCA or fibrate) and triple (UDCA + OCA + fibrate) regimens have also reported superior reductions in ALP, GGT, transaminases, and bilirubin compared to dual therapy alone[85-87]. In 255 UDCA-nonresponsive PBC patients, adding fibrates to UDCA + obeticholic acid markedly improved biochemical response and long-term prognostic scores vs dual therapy. Adverse events affected 41.2%, with 18.8% discontinuing OCA; hepatic decompensation occurred only in cirrhotic patients with significant portal hypertension[88].
Fenofibrate, a selective PPAR-α agonist, has likewise demonstrated efficacy. In small cohorts, 55% of UDCA non-responders met the Barcelona criteria (≥ 40% ALP reduction or normalization) after 48 weeks of fenofibrate monotherapy[89], and a 12-month combination of UDCA + fenofibrate produced an 81.4% vs 64.3% response rate compared to UDCA alone[90]. A retrospective study of 120 PBC patients treated with fenofibrate showed improved decompensation-free and transplant-free survival (hazard ratio = 0.09) and a 41% biochemical response rate per Toronto criteria[91]. Nationwide United Kingdom data (n = 457) indicated that biochemical response and discontinuation rates were comparable between fibrates and OCA[92]. While generally well tolerated, fibrates can transiently elevate transaminases and creatinine, reflecting altered production rather than true renal injury, and may induce myalgia, especially when co-administered with statins. Regular monitoring of liver enzymes and renal function is therefore recommended during therapy[93]. Selective PPAR agonists have emerged as promising second-line therapies for PBC. Elafibranor (GFT505), a dual PPAR-α/δ agonist, received United States FDA approval in June 2024, for use in combination with UDCA or as monotherapy in UDCA-intolerant patients[94]. In the multinational, double-blind, placebo-controlled phase 3 ELATIVE trial involving 161 UDCA-refractory or -intolerant patients randomized 2:1 to 80 mg elafibranor daily or placebo for 52 weeks, the primary endpoint, ALP < 1.67 × upper limit of normal with ≥ 15% reduction from baseline and normal total bilirubin, was met by 51% of the elafibranor group vs 4% of placebo; 15% of treated patients achieved ALP normalization compared to none on placebo[95]. Elafibranor’s safety profile was favorable, with gastrointestinal adverse events, nausea, vomiting, diarrhea, in approximately 11%, pruritus in 20%, and rare aminotransferase elevations leading to discontinuation in a small subset[96]. Seladelpar, a highly selective PPAR-δ agonist that decreases BA synthesis and suppresses inflammatory cytokines[97], was evaluated in the phase 3 RESPONSE trial, wherein 193 PBC patients with inadequate UDCA response or intolerance were randomized 2:1 to 10 mg seladelpar daily or placebo (UDCA continued if tolerated). The primary endpoint, ALP < 1.67 × upper limit of normal and normalized bilirubin at 12 months, was achieved by 62% of seladelpar-treated patients vs 20% of placebo; 25% of seladelpar recipients normalized ALP vs none in the placebo arm[98]. Importantly, seladelpar significantly reduced worst-itch Numerical Rating Scale scores (-3.2 vs -1.7 points at six months) and improved 5-D itch scores (least squares mean difference: -3.5 points at 12 months). Gastrointestinal symptoms (abdominal pain, nausea, distension) were more frequent with seladelpar, but earlier safety concerns regarding drug-induced liver injury raised in NASH trials were not confirmed, as an independent histological review found no increase in drug-induced liver injury risk[99,100]. Based on these data, the FDA granted accelerated approval for seladelpar in August 2024.
Saroglitazar, a dual PPAR-α/γ agonist with both anti-inflammatory and antifibrotic effects, was assessed in the phase 2 EPICS trial. In that study 37 UDCA-refractory or -intolerant PBC patients were randomized to saroglitazar 2 mg, 4 mg, or placebo for 4 weeks. The composite biochemical endpoint, which included a ≥ 15% reduction in ALP or ALP normalization, was achieved by 71% of all saroglitazar-treated patients, with the 4 mg cohort exhibiting a mean 50% decrease in ALP levels at study end[101]. Although generally well tolerated, four patients experienced transient elevations in alanine and aspartate aminotransferases, necessitating close monitoring of transaminases in future studies[101]. These promising early results support further evaluation of saroglitazar’s long-term efficacy and safety in larger, controlled trials. PPAR agonists appeared more effective than OCA in improving ALP biochemical levels, with elafibranor ranking highest; however, this finding should be interpreted with caution given the limited number and early-phase nature of the supporting studies[102].
The ileal BA transporter in the distal ileum is essential for reclaiming BA s into the enterohepatic cycle, and its blockade shifts BA elimination into the feces, lowering particularly toxic, hydrophobic species while sparing protective bicarbonate and phospholipid secretion[103]. Oral, intestine-restricted ileal BA transporter inhibitors have been shown to reduce serum–conjugated BA levels and meaningfully relieve itch in PBC patients, although early phase I studies noted dose-related nausea and diarrhea[104-106]. In the 2024 GLISTEN trial, 24 weeks of linerixibat produced a significant decrease in pruritus, suggesting it may become the first approved antipruritic specifically for PBC[107]. Meta analyses in PBC-autoimmune hepatitis overlap demonstrate that UDCA + budesonide yields superior biochemical normalization [odds ratio (OR) = 0.25; P < 0.0001], attenuates histological progression (OR = 2.57; P = 0.02) and lowers death/transplant risk (OR = 0.26; P = 0.02) vs UDCA monotherapy, with no excess adverse events[108]. In a 36 month randomized clinical trial of PBC patients with an incomplete biochemical response to UDCA, addition of budesonide did not significantly improve histology (42% vs 29%, P = 0.225) but achieved greater ALP normalization (35% vs 9%, P = 0.023) and more pronounced ALT/aspartate aminotransferase reductions. However, the histological endpoint was underpowered, relied on invasive paired biopsies without non invasive elastography, and was accompanied by corticosteroid related effects (cortisol suppression, osteopenia), highlighting the need for non invasive monitoring and long-term safety surveillance[109].
PBC is a chronic, immune-mediated cholestatic liver disorder driven by a multifactorial interaction between genetic predisposition, environmental influences, and gut-liver axis dysfunction. Although early diagnosis and UDCA as first-line therapy have significantly improved prognosis, a considerable proportion of patients experience inadequate biochemical response, predisposing them to progressive fibrosis and cirrhosis. The recent market withdrawal of OCA, due to concerns over its long-term efficacy, has underscored the urgent need for novel and reliable second-line treatment options. Emerging therapies targeting PPARs, such as elafibranor and seladelpar, have shown encouraging biochemical and clinical results, offering potential to redefine current management approaches. Future directions in PBC research aim to enhance individualized risk stratification, develop combination therapies, and address burdensome symptoms like fatigue and pruritus, with the ultimate goal of improving both survival and quality of life through earlier and more personalized intervention strategies.
| 1. | Tanaka A, Ma X, Takahashi A, Vierling JM. Primary biliary cholangitis. Lancet. 2024;404:1053-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 2. | Lv T, Chen S, Li M, Zhang D, Kong Y, Jia J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2021;36:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Galoosian A, Hanlon C, Zhang J, Holt EW, Yimam KK. Clinical Updates in Primary Biliary Cholangitis: Trends, Epidemiology, Diagnostics, and New Therapeutic Approaches. J Clin Transl Hepatol. 2020;8:49-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Gazda J, Drazilova S, Janicko M, Jarcuska P. The Epidemiology of Primary Biliary Cholangitis in European Countries: A Systematic Review and Meta-Analysis. Can J Gastroenterol Hepatol. 2021;2021:9151525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Alvaro D, Carpino G, Craxi A, Floreani A, Moschetta A, Invernizzi P. Primary biliary cholangitis management: controversies, perspectives and daily practice implications from an expert panel. Liver Int. 2020;40:2590-2601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Marzioni M, Bassanelli C, Ripellino C, Urbinati D, Alvaro D. Epidemiology of primary biliary cholangitis in Italy: Evidence from a real-world database. Dig Liver Dis. 2019;51:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Floreani A, Gabbia D, De Martin S. Are Gender Differences Important for Autoimmune Liver Diseases? Life (Basel). 2024;14:500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Colapietro F, Bertazzoni A, Lleo A. Contemporary Epidemiology of Primary Biliary Cholangitis. Clin Liver Dis. 2022;26:555-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Ismail A, Kennedy L, Francis H. Sex-Dependent Differences in Cholestasis: Why Estrogen Signaling May Be a Key Pathophysiological Driver. Am J Pathol. 2023;193:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Invernizzi F, Cilla M, Trapani S, Guarino M, Cossiga V, Gambato M, Morelli MC, Morisco F, Burra P, Floreani A; Special Interest Group Gender in Hepatology of the Italian Association for the Study of the Liver (AISF). Gender and Autoimmune Liver Diseases: Relevant Aspects in Clinical Practice. J Pers Med. 2022;12:925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Liu Z, Shao Y, Duan X. Genetic link between primary biliary cholangitis and connective tissue diseases in European populations: A two-sample Mendelian randomization study. PLoS One. 2024;19:e0298225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Liang Y, Li J, Zhang Z, Jiang T, Yang Z. Extrahepatic conditions of primary biliary cholangitis: A systematic review and meta-analysis of prevalence and risk. Clin Res Hepatol Gastroenterol. 2024;48:102321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 13. | Mulinacci G, Palermo A, Gerussi A, Asselta R, Gershwin ME, Invernizzi P. New insights on the role of human leukocyte antigen complex in primary biliary cholangitis. Front Immunol. 2022;13:975115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 14. | Ellinghaus D. How genetic risk contributes to autoimmune liver disease. Semin Immunopathol. 2022;44:397-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Tanaka A. Current understanding of primary biliary cholangitis. Clin Mol Hepatol. 2021;27:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Cordell HJ, Fryett JJ, Ueno K, Darlay R, Aiba Y, Hitomi Y, Kawashima M, Nishida N, Khor SS, Gervais O, Kawai Y, Nagasaki M, Tokunaga K, Tang R, Shi Y, Li Z, Juran BD, Atkinson EJ, Gerussi A, Carbone M, Asselta R, Cheung A, de Andrade M, Baras A, Horowitz J, Ferreira MAR, Sun D, Jones DE, Flack S, Spicer A, Mulcahy VL, Byan J, Han Y, Sandford RN, Lazaridis KN, Amos CI, Hirschfield GM, Seldin MF, Invernizzi P, Siminovitch KA, Ma X, Nakamura M, Mells GF; PBC Consortia; Canadian PBC Consortium; Chinese PBC Consortium; Italian PBC Study Group; Japan-PBC-GWAS Consortium; US PBC Consortium; UK-PBC Consortium. An international genome-wide meta-analysis of primary biliary cholangitis: Novel risk loci and candidate drugs. J Hepatol. 2021;75:572-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 17. | Hitomi Y, Ueno K, Aiba Y, Nishida N, Kono M, Sugihara M, Kawai Y, Kawashima M, Khor SS, Sugi K, Kouno H, Kohno H, Naganuma A, Iwamoto S, Katsushima S, Furuta K, Nikami T, Mannami T, Yamashita T, Ario K, Komatsu T, Makita F, Shimada M, Hirashima N, Yokohama S, Nishimura H, Sugimoto R, Komura T, Ota H, Kojima M, Nakamuta M, Fujimori N, Yoshizawa K, Mano Y, Takahashi H, Hirooka K, Tsuruta S, Sato T, Yamasaki K, Kugiyama Y, Motoyoshi Y, Suehiro T, Saeki A, Matsumoto K, Nagaoka S, Abiru S, Yatsuhashi H, Ito M, Kawata K, Takaki A, Arai K, Arinaga-Hino T, Abe M, Harada M, Taniai M, Zeniya M, Ohira H, Shimoda S, Komori A, Tanaka A, Ishigaki K, Nagasaki M, Tokunaga K, Nakamura M. A genome-wide association study identified PTPN2 as a population-specific susceptibility gene locus for primary biliary cholangitis. Hepatology. 2024;80:776-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Qiu ZX, Huang LX, Wang XX, Wang ZL, Li XH, Feng B. Exploring the Pathogenesis of Autoimmune Liver Diseases from the Heterogeneity of Target Cells. J Clin Transl Hepatol. 2024;12:659-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5543] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 20. | Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2046] [Cited by in RCA: 2135] [Article Influence: 82.1] [Reference Citation Analysis (4)] |
| 21. | Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O'Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699-5708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 1037] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 22. | Lan RY, Salunga TL, Tsuneyama K, Lian ZX, Yang GX, Hsu W, Moritoki Y, Ansari AA, Kemper C, Price J, Atkinson JP, Coppel RL, Gershwin ME. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun. 2009;32:43-51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 657] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 24. | Trivedi PJ, Hirschfield GM, Adams DH, Vierling JM. Immunopathogenesis of Primary Biliary Cholangitis, Primary Sclerosing Cholangitis and Autoimmune Hepatitis: Themes and Concepts. Gastroenterology. 2024;166:995-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 25. | Lenci I, Carnì P, Milana M, Bicaj A, Signorello A, Baiocchi L. Sequence of events leading to primary biliary cholangitis. World J Gastroenterol. 2023;29:5305-5312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Reshetnyak VI, Maev IV. New insights into the pathogenesis of primary biliary cholangitis asymptomatic stage. World J Gastroenterol. 2023;29:5292-5304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Prieto J, Banales JM, Medina JF. Primary biliary cholangitis: pathogenic mechanisms. Curr Opin Gastroenterol. 2021;37:91-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Wang L, Cao ZM, Zhang LL, Li JM, Lv WL. The Role of Gut Microbiota in Some Liver Diseases: From an Immunological Perspective. Front Immunol. 2022;13:923599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 29. | Liwinski T, Heinemann M, Schramm C. The intestinal and biliary microbiome in autoimmune liver disease-current evidence and concepts. Semin Immunopathol. 2022;44:485-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Furukawa M, Moriya K, Nakayama J, Inoue T, Momoda R, Kawaratani H, Namisaki T, Sato S, Douhara A, Kaji K, Kitade M, Shimozato N, Sawada Y, Saikawa S, Takaya H, Kitagawa K, Akahane T, Mitoro A, Yamao J, Tanaka Y, Yoshiji H. Gut dysbiosis associated with clinical prognosis of patients with primary biliary cholangitis. Hepatol Res. 2020;50:840-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 31. | Smyk DS, Bogdanos DP, Kriese S, Billinis C, Burroughs AK, Rigopoulou EI. Urinary tract infection as a risk factor for autoimmune liver disease: from bench to bedside. Clin Res Hepatol Gastroenterol. 2012;36:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Schneider KM, Kummen M, Trivedi PJ, Hov JR. Role of microbiome in autoimmune liver diseases. Hepatology. 2024;80:965-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Zheng Y, Ran Y, Zhang H, Wang B, Zhou L. The Microbiome in Autoimmune Liver Diseases: Metagenomic and Metabolomic Changes. Front Physiol. 2021;12:715852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Lynch EN, Campani C, Innocenti T, Dragoni G, Biagini MR, Forte P, Galli A. Understanding fatigue in primary biliary cholangitis: From pathophysiology to treatment perspectives. World J Hepatol. 2022;14:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 35. | Langedijk JAGM, Beuers UH, Oude Elferink RPJ. Cholestasis-Associated Pruritus and Its Pruritogens. Front Med (Lausanne). 2021;8:639674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Jacoby A, Rannard A, Buck D, Bhala N, Newton JL, James OF, Jones DE. Development, validation, and evaluation of the PBC-40, a disease specific health related quality of life measure for primary biliary cirrhosis. Gut. 2005;54:1622-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 198] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Sy AM, Ferreira RD, John BV. Hepatocellular Carcinoma in Primary Biliary Cholangitis. Clin Liver Dis. 2022;26:691-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 38. | Natarajan Y, Tansel A, Patel P, Emologu K, Shukla R, Qureshi Z, El-Serag HB, Thrift AP, Kanwal F. Incidence of Hepatocellular Carcinoma in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2021;66:2439-2451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | AISF Expert Panel. Position paper of the Italian Association for the Study of the Liver (AISF): Management and treatment of primary biliary cholangitis. Dig Liver Dis. 2024;56:1461-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Reshetnyak VI, Maev IV. Features of Lipid Metabolism Disorders in Primary Biliary Cholangitis. Biomedicines. 2022;10:3046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 41. | Floreani A, Variola A, Niro G, Premoli A, Baldo V, Gambino R, Musso G, Cassader M, Bo S, Ferrara F, Caroli D, Rizzotto ER, Durazzo M. Plasma adiponectin levels in primary biliary cirrhosis: a novel perspective for link between hypercholesterolemia and protection against atherosclerosis. Am J Gastroenterol. 2008;103:1959-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Chang PY, Lu SC, Su TC, Chou SF, Huang WH, Morrisett JD, Chen CH, Liau CS, Lee YT. Lipoprotein-X reduces LDL atherogenicity in primary biliary cirrhosis by preventing LDL oxidation. J Lipid Res. 2004;45:2116-2122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 1006] [Article Influence: 111.8] [Reference Citation Analysis (1)] |
| 44. | Greco S, Campigotto M, D'Amuri A, Fabbri N, Passaro A. Dyslipidemia, Cholangitis and Fatty Liver Disease: The Close Underexplored Relationship: A Narrative Review. J Clin Med. 2024;13:2714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary Biliary Cholangitis: 2018 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology. 2019;69:394-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 492] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 46. | You H, Ma X, Efe C, Wang G, Jeong SH, Abe K, Duan W, Chen S, Kong Y, Zhang D, Wei L, Wang FS, Lin HC, Yang JM, Tanwandee T, Gani RA, Payawal DA, Sharma BC, Hou J, Yokosuka O, Dokmeci AK, Crawford D, Kao JH, Piratvisuth T, Suh DJ, Lesmana LA, Sollano J, Lau G, Sarin SK, Omata M, Tanaka A, Jia J. APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis. Hepatol Int. 2022;16:1-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 47. | Terziroli Beretta-Piccoli B, Stirnimann G, Mertens J, Semela D, Zen Y, Mazzucchelli L, Voreck A, Kolbus N, Merlo E, Di Bartolomeo C, Messina P, Cerny A, Costantini S, Vergani D, Mieli-Vergani G; Swiss PBC Cohort Study Group. Primary biliary cholangitis with normal alkaline phosphatase: A neglected clinical entity challenging current guidelines. J Autoimmun. 2021;116:102578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 643] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 49. | Harms MH, van Buuren HR, Corpechot C, Thorburn D, Janssen HLA, Lindor KD, Hirschfield GM, Parés A, Floreani A, Mayo MJ, Invernizzi P, Battezzati PM, Nevens F, Ponsioen CY, Mason AL, Kowdley KV, Lammers WJ, Hansen BE, van der Meer AJ. Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2019;71:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 50. | Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. N Engl J Med. 1994;330:1342-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 348] [Article Influence: 10.9] [Reference Citation Analysis (1)] |
| 51. | Parés A, Caballería L, Rodés J, Bruguera M, Rodrigo L, García-Plaza A, Berenguer J, Rodríguez-Martínez D, Mercader J, Velicia R. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 52. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 1286] [Article Influence: 257.2] [Reference Citation Analysis (1)] |
| 53. | Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 560] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 54. | Lindor KD, Dickson ER, Baldus WP, Jorgensen RA, Ludwig J, Murtaugh PA, Harrison JM, Wiesner RH, Anderson ML, Lange SM. Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology. 1994;106:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 325] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 55. | Lammers WJ, van Buuren HR, Hirschfield GM, Janssen HL, Invernizzi P, Mason AL, Ponsioen CY, Floreani A, Corpechot C, Mayo MJ, Battezzati PM, Parés A, Nevens F, Burroughs AK, Kowdley KV, Trivedi PJ, Kumagi T, Cheung A, Lleo A, Imam MH, Boonstra K, Cazzagon N, Franceschet I, Poupon R, Caballeria L, Pieri G, Kanwar PS, Lindor KD, Hansen BE; Global PBC Study Group. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147:1338-49.e5; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 362] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 56. | Parés A, Caballería L, Rodés J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology. 2006;130:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 559] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 57. | Kuiper EM, Hansen BE, de Vries RA, den Ouden-Muller JW, van Ditzhuijsen TJ, Haagsma EB, Houben MH, Witteman BJ, van Erpecum KJ, van Buuren HR; Dutch PBC Study Group. Improved prognosis of patients with primary biliary cirrhosis that have a biochemical response to ursodeoxycholic acid. Gastroenterology. 2009;136:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 366] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 58. | Corpechot C, Chazouillères O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 348] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 59. | Kumagi T, Guindi M, Fischer SE, Arenovich T, Abdalian R, Coltescu C, Heathcote EJ, Hirschfield GM. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:2186-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 60. | Corpechot C, Lemoinne S, Soret PA, Hansen B, Hirschfield G, Gulamhusein A, Montano-Loza AJ, Lytvyak E, Pares A, Olivas I, Eaton JE, Osman KT, Schramm C, Sebode M, Lohse AW, Dalekos G, Gatselis N, Nevens F, Cazzagon N, Zago A, Russo FP, Floreani A, Abbas N, Trivedi P, Thorburn D, Saffioti F, Barkai L, Roccarina D, Calvaruso V, Fichera A, Delamarre A, Sobenko N, Villamil AM, Medina-Morales E, Bonder A, Patwardhan V, Rigamonti C, Carbone M, Invernizzi P, Cristoferi L, van der Meer A, de Veer R, Zigmond E, Yehezkel E, Kremer AE, Deibel A, Bruns T, Große K, Wetten A, Dyson JK, Jones D, Dumortier J, Pageaux GP, de Lédinghen V, Chazouillères O, Carrat F; Global & ERN Rare-Liver PBC Study Groups. Adequate versus deep response to ursodeoxycholic acid in primary biliary cholangitis: To what extent and under what conditions is normal alkaline phosphatase level associated with complication-free survival gain? Hepatology. 2024;79:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 61. | Goel A, Kim WR. Natural History of Primary Biliary Cholangitis in the Ursodeoxycholic Acid Era: Role of Scoring Systems. Clin Liver Dis. 2018;22:563-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 62. | Cortez-Pinto H, Liberal R, Lopes S, Machado MV, Carvalho J, Dias T, Santos A, Agostinho C, Figueiredo P, Loureiro R, Martins A, Alexandrino G, Cotrim I, Leal C, Presa J, Mesquita M, Nunes J, Gouveia C, Vale AHE, Alves AL, Coelho M, Maia L, Pedroto I, Banhudo A, Pinto JS, Gomes MV, Oliveira J, Andreozzi V, Calinas F. Predictors for incomplete response to ursodeoxycholic acid in primary biliary cholangitis. Data from a national registry of liver disease. United European Gastroenterol J. 2021;9:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Smith SM, Pegram AH. Obeticholic Acid: A Farnesoid X Receptor Agonist for Primary Biliary Cholangitis. J Pharm Technol. 2017;33:66-71. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Floreani A, Gabbia D, De Martin S. Obeticholic Acid for Primary Biliary Cholangitis. Biomedicines. 2022;10:2464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 65. | Pellicciari R, Costantino G, Camaioni E, Sadeghpour BM, Entrena A, Willson TM, Fiorucci S, Clerici C, Gioiello A. Bile acid derivatives as ligands of the farnesoid X receptor. Synthesis, evaluation, and structure-activity relationship of a series of body and side chain modified analogues of chenodeoxycholic acid. J Med Chem. 2004;47:4559-4569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 66. | Halilbasic E, Baghdasaryan A, Trauner M. Nuclear receptors as drug targets in cholestatic liver diseases. Clin Liver Dis. 2013;17:161-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Chapman RW, Lynch KD. Obeticholic acid-a new therapy in PBC and NASH. Br Med Bull. 2020;133:95-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 68. | Pablo Arab J, Cabrera D, Arrese M. Bile Acids in Cholestasis and its Treatment. Ann Hepatol. 2017;16 Suppl 1:S53-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 69. | Verbeke L, Farre R, Verbinnen B, Covens K, Vanuytsel T, Verhaegen J, Komuta M, Roskams T, Chatterjee S, Annaert P, Vander Elst I, Windmolders P, Trebicka J, Nevens F, Laleman W. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats. Am J Pathol. 2015;185:409-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 70. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D; POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 851] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 71. | Murillo Perez CF, Fisher H, Hiu S, Kareithi D, Adekunle F, Mayne T, Malecha E, Ness E, van der Meer AJ, Lammers WJ, Trivedi PJ, Battezzati PM, Nevens F, Kowdley KV, Bruns T, Cazzagon N, Floreani A, Mason AL, Parés A, Londoño MC, Invernizzi P, Carbone M, Lleo A, Mayo MJ, Dalekos GN, Gatselis NK, Thorburn D, Verhelst X, Gulamhusein A, Janssen HLA, Smith R, Flack S, Mulcahy V, Trauner M, Bowlus CL, Lindor KD, Corpechot C, Jones D, Mells G, Hirschfield GM, Wason J, Hansen BE; GLOBAL PBC Study Group and the members of the UK-PBC Consortium. Greater Transplant-Free Survival in Patients Receiving Obeticholic Acid for Primary Biliary Cholangitis in a Clinical Trial Setting Compared to Real-World External Controls. Gastroenterology. 2022;163:1630-1642.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 72. | D'Amato D, De Vincentis A, Malinverno F, Viganò M, Alvaro D, Pompili M, Picciotto A, Palitti VP, Russello M, Storato S, Pigozzi MG, Calvaruso V, De Gasperi E, Lleo A, Castellaneta A, Pellicelli A, Cazzagon N, Floreani A, Muratori L, Fagiuoli S, Niro GA, Feletti V, Cozzolongo R, Terreni N, Marzioni M, Pellicano R, Pozzoni P, Baiocchi L, Chessa L, Rosina F, Bertino G, Vinci M, Morgando A, Vanni E, Scifo G, Sacco R, D'Antò M, Bellia V, Boldizzoni R, Casella S, Omazzi B, Poggi G, Cristoferi L, Gerussi A, Ronca V, Venere R, Ponziani F, Cannavò M, Mussetto A, Fontana R, Losito F, Frazzetto E, Distefano M, Colapietro F, Labanca S, Marconi G, Grassi G, Galati G, O'Donnell SE, Mancuso C, Mulinacci G, Palermo A, Claar E, Izzi A, Picardi A, Invernizzi P, Carbone M, Vespasiani-Gentilucci U; Italian PBC Registry and the Club Epatologi Ospedalieri (CLEO)/Associazione Italiana Gastroenterologi ed Endoscopisti Digestivi Ospedalieri (AIGO) PBC Study Group. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. 2021;3:100248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 73. | Gomez E, Garcia Buey L, Molina E, Casado M, Conde I, Berenguer M, Jorquera F, Simón MA, Olveira A, Hernández-Guerra M, Mesquita M, Presa J, Costa-Moreira P, Macedo G, Arenas JI, Manuel Sousa J, Ampuero J, Morillas RM, Santos A, De Carvalho A, Uriz J, Carrión JA, Luisa Gutiérrez M, Pérez-Fernández E, Fernández-Rodríguez CM; IBER-PBC leading Cooperative Group. Effectiveness and safety of obeticholic acid in a Southern European multicentre cohort of patients with primary biliary cholangitis and suboptimal response to ursodeoxycholic acid. Aliment Pharmacol Ther. 2021;53:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 74. | Siddiqui MS, Van Natta ML, Connelly MA, Vuppalanchi R, Neuschwander-Tetri BA, Tonascia J, Guy C, Loomba R, Dasarathy S, Wattacheril J, Chalasani N, Sanyal AJ; NASH CRN. Impact of obeticholic acid on the lipoprotein profile in patients with non-alcoholic steatohepatitis. J Hepatol. 2020;72:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 75. | Kowdley KV, Hirschfield GM, Coombs C, Malecha ES, Bessonova L, Li J, Rathnayaka N, Mells G, Jones DE, Trivedi PJ, Hansen BE, Smith R, Wason J, Hiu S, Kareithi DN, Mason AL, Bowlus CL, Muller K, Carbone M, Berenguer M, Milkiewicz P, Adekunle F, Villamil A. Correction to COBALT: A Confirmatory Trial of Obeticholic Acid in Primary Biliary Cholangitis With Placebo and External Controls. Am J Gastroenterol. 2025;120:263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 76. | Eaton JE, Vuppalanchi R, Reddy R, Sathapathy S, Ali B, Kamath PS. Liver Injury in Patients With Cholestatic Liver Disease Treated With Obeticholic Acid. Hepatology. 2020;71:1511-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 77. | Ghonem NS, Assis DN, Boyer JL. Fibrates and cholestasis. Hepatology. 2015;62:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 78. | Kersten S, Stienstra R. The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie. 2017;136:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 315] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 79. | Nozaki Y, Harada K, Sanzen T, Nakanuma Y. PPARγ ligand attenuates portal inflammation in the MRL-lpr mouse: a new strategy to restrain cholangiopathy in primary biliary cirrhosis. Med Mol Morphol. 2013;46:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Harada K, Isse K, Kamihira T, Shimoda S, Nakanuma Y. Th1 cytokine-induced downregulation of PPARgamma in human biliary cells relates to cholangitis in primary biliary cirrhosis. Hepatology. 2005;41:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1402] [Cited by in RCA: 1419] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 82. | Corpechot C, Poupon R, Chazouillères O. New treatments/targets for primary biliary cholangitis. JHEP Rep. 2019;1:203-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Iwasaki S, Tsuda K, Ueta H, Aono R, Ono M, Saibara T, Maeda T, Onishi S. Bezafibrate may have a beneficial effect in pre-cirrhotic primary biliary cirrhosis. Hepatol Res. 1999;16:12-18. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Corpechot C, Chazouillères O, Rousseau A, Le Gruyer A, Habersetzer F, Mathurin P, Goria O, Potier P, Minello A, Silvain C, Abergel A, Debette-Gratien M, Larrey D, Roux O, Bronowicki JP, Boursier J, de Ledinghen V, Heurgue-Berlot A, Nguyen-Khac E, Zoulim F, Ollivier-Hourmand I, Zarski JP, Nkontchou G, Lemoinne S, Humbert L, Rainteau D, Lefèvre G, de Chaisemartin L, Chollet-Martin S, Gaouar F, Admane FH, Simon T, Poupon R. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N Engl J Med. 2018;378:2171-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 431] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 85. | Reig A, Álvarez-Navascués C, Vergara M, Gómez-Domínguez E, Gallego-Moya A, Pérez-Medrano IM, Fábrega E, Hernández-Guerra M, Berenguer M, Estevez P, Arencibia A, Morillas RM, Horta D, Albillos A, Casado M, De la Cruz G, Fernandez-Bonilla E, Molina E, Hijona L, Diago M, Fernández-Rodriguez CM, González-Santiago JM, Sala M, Gómez-Camarero J, Romero-Gomez M, Suárez F, Vargas V, Ferre-Aracil C, Andrade RJ, Chahri N, Parés A. Obeticholic Acid and Fibrates in Primary Biliary Cholangitis: Comparative Effects in a Multicentric Observational Study. Am J Gastroenterol. 2021;116:2250-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Smets L, Verbeek J, Korf H, van der Merwe S, Nevens F. Improved Markers of Cholestatic Liver Injury in Patients With Primary Biliary Cholangitis Treated With Obeticholic Acid and Bezafibrate. Hepatology. 2021;73:2598-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Soret PA, Lam L, Carrat F, Smets L, Berg T, Carbone M, Invernizzi P, Leroy V, Trivedi P, Cazzagon N, Weiler-Normann C, Alric L, Rosa-Hezode I, Heurgué A, Cervoni JP, Dumortier J, Potier P, Roux O, Silvain C, Bureau C, Anty R, Larrey D, Levy C, Pares A, Schramm C, Nevens F, Chazouillères O, Corpechot C. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis. Aliment Pharmacol Ther. 2021;53:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 88. | Gómez E, Montero JL, Molina E, García-Buey L, Casado M, Fuentes J, Simón MA, Díaz-González A, Jorquera F, Morillas RM, Presa J, Berenguer M, Conde MI, Olveira A, Macedo G, Garrido I, Hernández-Guerra M, Olivas I, Rodríguez-Tajes S, Londoño M, Sousa JM, Ampuero J, Romero-González E, González-Padilla S, Escudero-García D, Carvalho A, Santos A, Gutiérrez ML, Pérez-Fernández E, Aburruza L, Uriz J, Gomes D, Santos L, Martínez-González J, Albillos A, Fernández-Rodríguez CM. Longitudinal outcomes of obeticholic acid therapy in ursodiol-nonresponsive primary biliary cholangitis: Stratifying the impact of add-on fibrates in real-world practice. Aliment Pharmacol Ther. 2024;59:1604-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 89. | Grigorian AY, Mardini HE, Corpechot C, Poupon R, Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39:296-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 90. | Liu Y, Guo G, Zheng L, Sun R, Wang X, Deng J, Jia G, Yang C, Cui L, Guo C, Shang Y, Han Y. Effectiveness of Fenofibrate in Treatment-Naive Patients With Primary Biliary Cholangitis: A Randomized Clinical Trial. Am J Gastroenterol. 2023;118:1973-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 91. | Cheung AC, Lapointe-Shaw L, Kowgier M, Meza-Cardona J, Hirschfield GM, Janssen HL, Feld JJ. Combined ursodeoxycholic acid (UDCA) and fenofibrate in primary biliary cholangitis patients with incomplete UDCA response may improve outcomes. Aliment Pharmacol Ther. 2016;43:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 92. | Abbas N, Culver EL, Thorburn D, Halliday N, Crothers H, Dyson JK, Phaw A, Aspinall R, Khakoo SI, Kallis Y, Smith B, Patanwala I, McCune A, Chimakurthi CR, Hegade V, Orrell M, Jones R, Mells G, Thain C, Thain RM, Jones D, Hirschfield G, Trivedi PJ. UK-Wide Multicenter Evaluation of Second-line Therapies in Primary Biliary Cholangitis. Clin Gastroenterol Hepatol. 2023;21:1561-1570.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Carrion AF, Lindor KD, Levy C. Safety of fibrates in cholestatic liver diseases. Liver Int. 2021;41:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 94. | Westerouen Van Meeteren MJ, Drenth JPH, Tjwa ETTL. Elafibranor: a potential drug for the treatment of nonalcoholic steatohepatitis (NASH). Expert Opin Investig Drugs. 2020;29:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 95. | Kowdley KV, Bowlus CL, Levy C, Akarca US, Alvares-da-Silva MR, Andreone P, Arrese M, Corpechot C, Francque SM, Heneghan MA, Invernizzi P, Jones D, Kruger FC, Lawitz E, Mayo MJ, Shiffman ML, Swain MG, Valera JM, Vargas V, Vierling JM, Villamil A, Addy C, Dietrich J, Germain JM, Mazain S, Rafailovic D, Taddé B, Miller B, Shu J, Zein CO, Schattenberg JM; ELATIVE Study Investigators’ Group; ELATIVE Study Investigators' Group. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N Engl J Med. 2024;390:795-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 147] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 96. | Schattenberg JM, Pares A, Kowdley KV, Heneghan MA, Caldwell S, Pratt D, Bonder A, Hirschfield GM, Levy C, Vierling J, Jones D, Tailleux A, Staels B, Megnien S, Hanf R, Magrez D, Birman P, Luketic V. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J Hepatol. 2021;74:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 97. | Kremer AE, Mayo MJ, Hirschfield G, Levy C, Bowlus CL, Jones DE, Steinberg A, McWherter CA, Choi YJ. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022;42:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 98. | Hirschfield GM, Bowlus CL, Mayo MJ, Kremer AE, Vierling JM, Kowdley KV, Levy C, Villamil A, Ladrón de Guevara Cetina AL, Janczewska E, Zigmond E, Jeong SH, Yilmaz Y, Kallis Y, Corpechot C, Buggisch P, Invernizzi P, Londoño Hurtado MC, Bergheanu S, Yang K, Choi YJ, Crittenden DB, McWherter CA; RESPONSE Study Group. A Phase 3 Trial of Seladelpar in Primary Biliary Cholangitis. N Engl J Med. 2024;390:783-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 142] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 99. | Jones D, Boudes PF, Swain MG, Bowlus CL, Galambos MR, Bacon BR, Doerffel Y, Gitlin N, Gordon SC, Odin JA, Sheridan D, Wörns MA, Clark V, Corless L, Hartmann H, Jonas ME, Kremer AE, Mells GF, Buggisch P, Freilich BL, Levy C, Vierling JM, Bernstein DE, Hartleb M, Janczewska E, Rochling F, Shah H, Shiffman ML, Smith JH, Choi YJ, Steinberg A, Varga M, Chera H, Martin R, McWherter CA, Hirschfield GM. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: a double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol Hepatol. 2017;2:716-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 100. | Cumpian NA, Choi G, Saab S. Review of Current and Upcoming Second-Line Treatments for Primary Biliary Cholangitis. Dig Dis Sci. 2025;70:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 101. | Vuppalanchi R, González-Huezo MS, Payan-Olivas R, Muñoz-Espinosa LE, Shaikh F, Pio Cruz-Lopez JL, Parmar D. A Multicenter, Open-Label, Single-Arm Study to Evaluate the Efficacy and Safety of Saroglitazar in Patients With Primary Biliary Cholangitis. Clin Transl Gastroenterol. 2021;12:e00327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 102. | Lin W, Wang JX, Liu YJ. Optimal drug regimens for improving ALP biochemical levels in patients with primary biliary cholangitis refractory to UDCA: a systematic review and Bayesian network meta-analysis. Syst Rev. 2024;13:46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 103. | Baghdasaryan A, Fuchs CD, Österreicher CH, Lemberger UJ, Halilbasic E, Påhlman I, Graffner H, Krones E, Fickert P, Wahlström A, Ståhlman M, Paumgartner G, Marschall HU, Trauner M. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 104. | Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Richards D, Storey J, Dukes G, Gilchrist K, Vallow S, Alexander GJ, Corrigan M, Hirschfield GM, Jones DE. BAT117213: Ileal bile acid transporter (IBAT) inhibition as a treatment for pruritus in primary biliary cirrhosis: study protocol for a randomised controlled trial. BMC Gastroenterol. 2016;16:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Hegade VS, Kendrick SF, Dobbins RL, Miller SR, Thompson D, Richards D, Storey J, Dukes GE, Corrigan M, Oude Elferink RP, Beuers U, Hirschfield GM, Jones DE. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 106. | Graffner H, Gillberg PG, Rikner L, Marschall HU. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther. 2016;43:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Karatza E, Swift B, Carreño F, Mukherjee S, Casillas L, Lennie J, Fettiplace J, McLaughlin MM, Kremer AE. Serum bile acid change correlates with improvement in pruritus in patients with primary biliary cholangitis receiving linerixibat. Liver Int. 2024;44:2293-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 108. | Zhang H, Yang J, Zhu R, Zheng Y, Zhou Y, Dai W, Wang F, Chen K, Li J, Wang C, Li S, Liu T, Abudumijiti H, Zhou Z, Wang J, Lu W, Wang J, Xia Y, Zhou Y, Lu J, Guo C. Combination therapy of ursodeoxycholic acid and budesonide for PBC-AIH overlap syndrome: a meta-analysis. Drug Des Devel Ther. 2015;9:567-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 109. | Hirschfield GM, Beuers U, Kupcinskas L, Ott P, Bergquist A, Färkkilä M, Manns MP, Parés A, Spengler U, Stiess M, Greinwald R, Pröls M, Wendum D, Drebber U, Poupon R. A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA. J Hepatol. 2021;74:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/