Published online Oct 6, 2025. doi: 10.12998/wjcc.v13.i28.109077
Revised: May 26, 2025
Accepted: July 22, 2025
Published online: October 6, 2025
Processing time: 100 Days and 19.1 Hours
Splenic histiocytic sarcoma (SHS) is a rare, aggressive hematological malignancy with unclear progression and management. Our case illustrates the progression and pathophysiological processes of SHS and provides key data for the diagnosis, treatment and management of SHS. A 60-year-old female with incidentally detected splenic mass (6.0 cm × 5.7 cm) underwent splenectomy, confirmed as SHS in 2020. Post-op imatinib therapy was given. In 2022, hepatic metastases (2.4 cm × 2.9 cm) with pancytopenia led to supportive care. Lesions enlarged to 4.3 cm × 2.7 cm, leading to multi-organ failure and death at 33 months. The case was categorized into three distinct stages based on the pathophysiology of SHS: Early-stage splenic tumor growth, mid-stage liver metastasis with hematological abnormalities, and late-stage tumor infiltration leading to multiorgan failure. For SHS, this case highlights the pivotal role of early intervention and the value of personalized treatment strategies.
Core Tip: Splenic histiocytic sarcoma (SHS) is an exceedingly rare malignant tumor of the spleen. Owing to its low incidence rate and a relatively limited number of clinical cases, the medical community's comprehension of SHS remains in a phase of continuous exploration and refinement. Currently, the pathophysiological process of SHS has not been fully elucidated. A comprehensive clarification of the pathophysiological process of SHS and an in-depth understanding of its pathogenesis are fundamental for the development of effective treatment strategies.
- Citation: Yao MT, Wang T, Luo H, Yao MY, Chen K, Zhu YQ. Splenic histiocytic sarcoma: Disease progression from the perspective of pathophysiology. World J Clin Cases 2025; 13(28): 109077
- URL: https://www.wjgnet.com/2307-8960/full/v13/i28/109077.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i28.109077
A 60-year-old female patient had a 10-year history of hypertension and was not on regular antihypertensive medication. She underwent surgery for a comminuted elbow fracture 10 years ago. Thirty years ago, she received a blood transfusion during a gynecological procedure (details unspecified).
The patient was initially admitted on April 10, 2020, due to a splenic lesion detected during a routine physical exami
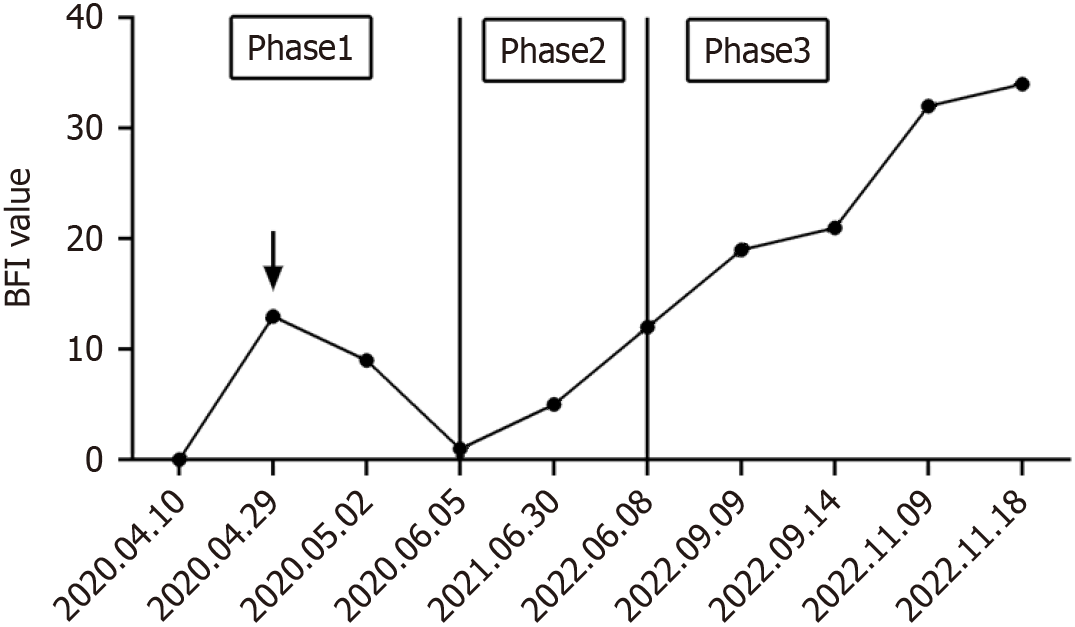
The patient was readmitted on June 8, 2022, presenting symptoms of cough, dry mouth, joint pain, fatigue, and weight loss. The brief fatigue inventory (BFI) was utilized to assess the patient’s fatigue level, with scores ranging from 0 to 40, where 40 signifies severe fatigue (Figure 1). The patient reported a slight reduction in activity, occasionally requiring rest, with a self-assessed BFI total score of 12.
On September 9, 2022, the patient was readmitted due to abdominal distension and pain, nausea, vomiting, and lower limb edema. After consuming small amounts of food, the patient experienced upper abdominal pain, which could resolve spontaneously, accompanied by a decreased appetite. The ability to walk decreased significantly, and activity levels were reduced, resulting in a BFI self-assessment total score of 19. Mild edema appeared in both ankles after prolonged standing or walking, with finger pressure causing indentations that resolved quickly.
By November 9, 2022, the patient showed signs of malnutrition, edema in both lower limbs, electrolyte imbalance, and hypokalemia, with blood potassium levels as low as 2.7 mmol/L. At this stage, the patient probably required rest most of the time, with noticeably decreased activity, often feeling heavy, weak, and dizzy, with a BFI self-assessed total score of 32. Noticeable swelling was present in the calves, becoming more pronounced over time, with a prolonged recovery time for indentations caused by finger pressure. Additionally, pleural effusion led to symptoms of shortness of breath, chest tightness, as well as difficulty breathing.
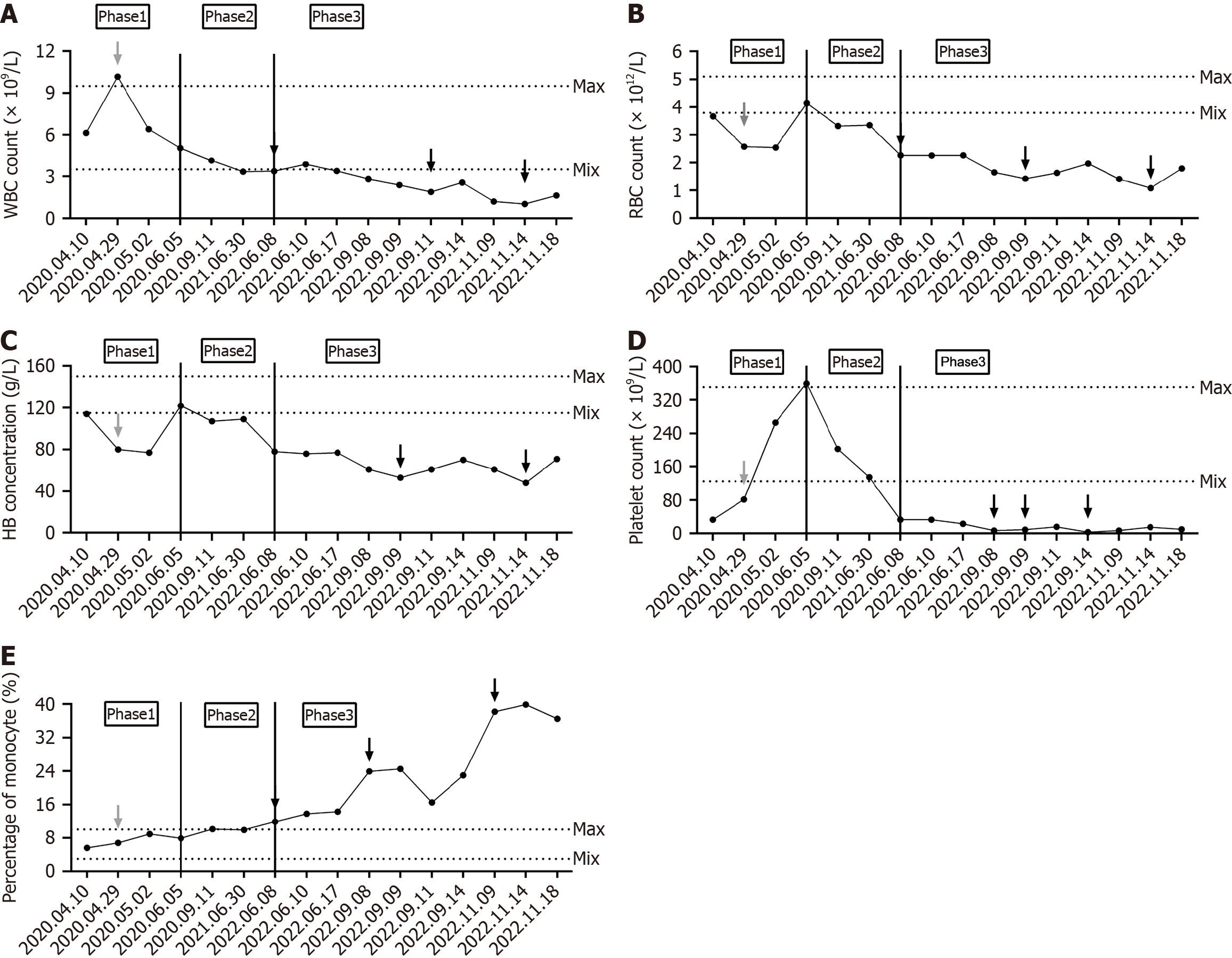
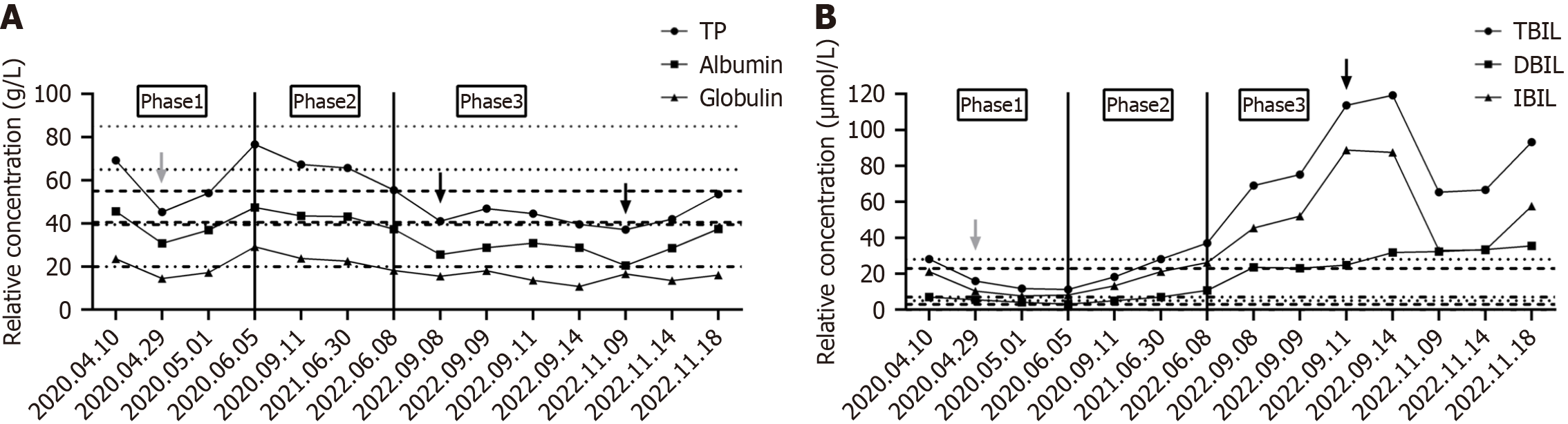
In June 2022, the patient’s complete blood count revealed reductions in white blood cells, red blood cells, hemoglobin, and platelets, with the platelet count dropping to 33 × 109/L. Concurrently, ferritin levels increased and total iron binding capacity decreased, indicating iron deficiency anemia. Bone marrow biopsy indicated hyperplastic anemia with a predominance of middle to late erythroblast proliferation and decreased granulocyte proliferation. Liver function tests showed decreased levels of total protein, albumin, and globulin, with increased total bilirubin, direct bilirubin, and indirect bilirubin, suggesting possible liver inflammation or damage. In September 2022, upon admission, the patient showed progressive decreases in white blood cells, hemoglobin, and platelets, with the platelet count dropping to 7 ×
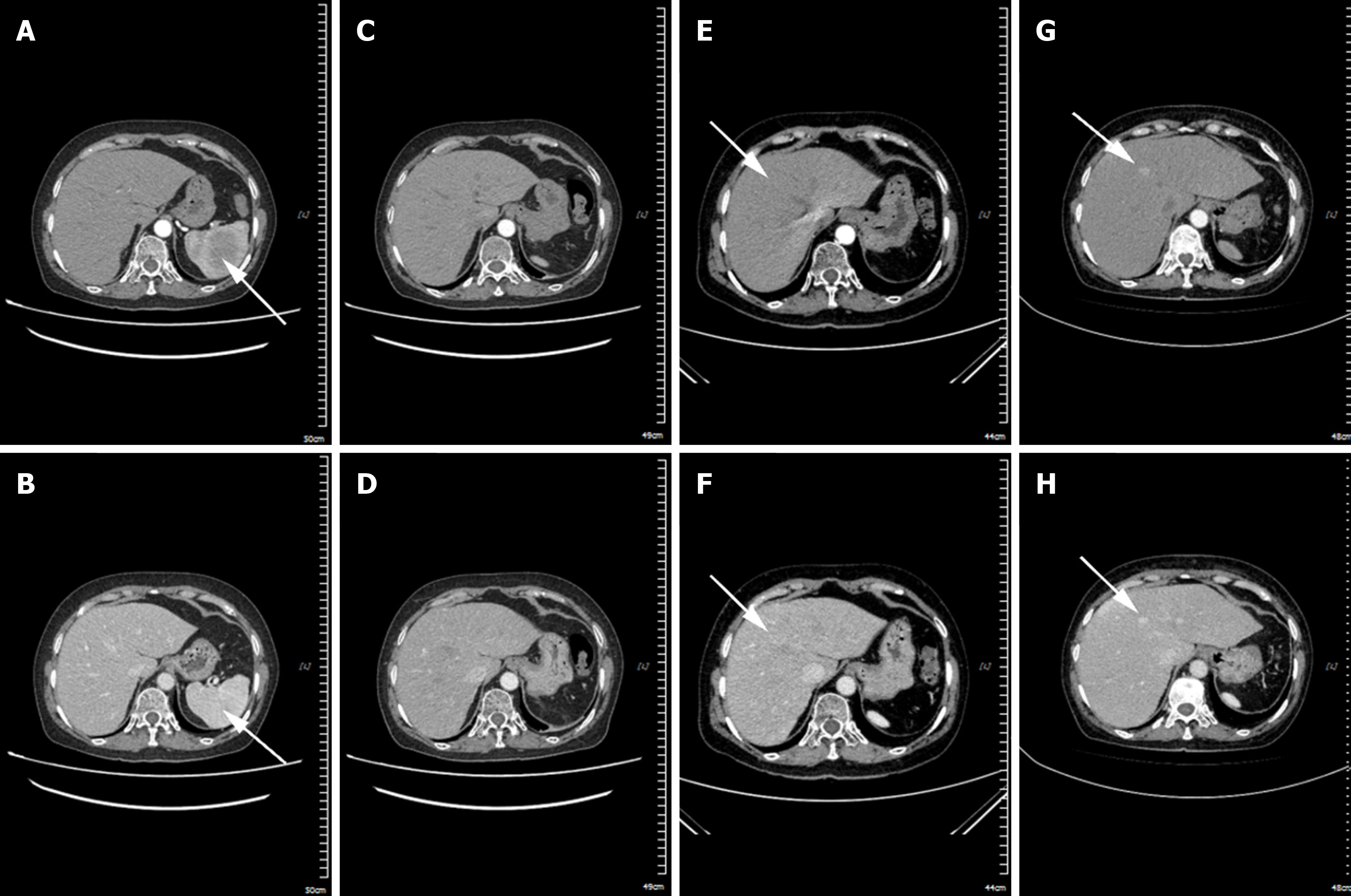
In April 2020, abdominal computed tomography (CT) revealed a slightly hypodense mass shadow within the splenic parenchyma of the patient. The mass had ill - defined margins, measured approximately 6.0 cm × 5.7 cm in size, with a CT value of around 48 HU and demonstrated progressive enhancement[1]. Subsequently, the patient underwent splenectomy. A post-operation CT examination in June 2020 indicated the absence of the spleen in the splenic region. There was a slight disorganization of the fat space surrounding the operative area and the presence of a striped hyperdense shadow (Phase 1). A CT scan in June 2022 showed the emergence of multiple new nodular enhancement shadows in the liver. The larger one was located in the left medial lobe of the liver, with an area of approximately 2.4 cm × 2.9 cm (Phase 2). Meanwhile, the negative results of tumor markers (alpha-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 199, 125, and 153) excluded the possibility of certain cancers. In Phase 3, a CT re-examination in September 2022 demonstrated that the extent of the nodular enhancement shadow in the left medial lobe of the liver had increased to 4.3 cm × 2.7 cm (Figure 4).
The clinical manifestations and pathologic features of SHS overlap with those of various hematologic diseases, solid tumors, and benign lesions, thus necessitating careful differentiation through multidimensional examinations. Malignant lymphoma can be identified via bone marrow biopsy showing lymphoma cell infiltration. Additionally, lymphoma cells express B-cell markers (e.g., CD20, CD79a) or T-cell markers (e.g., CD3, CD4) but do not express histiocytic markers (e.g., CD68, lysozyme). Splenic angiosarcoma is prone to spontaneous splenic rupture, and its tumor cells express vascular endothelial markers (CD31, CD34, Factor VIII) rather than CD68 or lysozyme. Splenic metastatic tumors (e.g., splenic metastases from hepatocellular carcinoma or gastric carcinoma) often have a history of primary tumors (e.g., hepatic lesions, gastrointestinal symptoms) and elevated tumor markers (e.g., alpha fetoprotein, carcino-embryonic antigen). Langerhans cell histiocytosis can involve multiple systems (bone, skin, lung), with proliferating cells being Langerhans cells that express CD1a, S-100, CD207 (langerin). In conclusion, the differential diagnosis of SHS requires close integration of clinical features, imaging manifestations, and pathologic immunohistochemistry. For patients with unexplained splenomegaly accompanied by hematopenia and liver injury, the possibility of SHS should be highly suspected, and pathologic specimens should be obtained promptly through MDT to avoid missed or incorrect diagnoses.
After the resection of SHS, the patient has been tumor-free for over 26 months. During this period, the patient consistently took imatinib mesylate at a daily dose of 400 mg. Subsequently, from June to September 2022, due to a progressive decrease in the patient’s white blood cells, hemoglobin, and platelets, we administered transfusions of type A frozen plasma, suspended red blood cells, platelets, type A cryoprecipitate, and 20% human albumin. The patient’s symptoms of dizziness, fatigue, and shortness of breath improved, with hemoglobin and platelet levels increasing, though they remained below normal. In November 2022, symptomatic treatment was administered, including liver protection, correction of electrolyte imbalances, diuretics, and transfusions of suspended red blood cells. The patient’s symptoms of ascites, hypoproteinemia, hypokalemia, and liver failure showed improvement.
The primary complications in this SHS patient were pancytopenia, hepatic failure, and electrolyte imbalance. In January 2023, the patient succumbed to multiple organ failure, with a total survival duration exceeding 33 months.
The disease progression in this case can be categorized into three distinct stages based on pathophysiology. During the early phase of SHS (pre- and post-surgery), the spleen, being the primary site for histiocytes and macrophages, exhibits tumor-like growth on imaging, and splenomegaly is evident upon physical examination. Laboratory tests may demonstrate anemia or thrombocytopenia, with reductions in one or two blood cell lineages. In the mid-stage of the disease (post-splenectomy), liver-enhanced CT scans reveal nodular enhancement, primarily due to abnormal proliferation of histiocytes and macrophages in the liver. Laboratory tests indicate pancytopenia, monocytosis, and liver dysfunction. In the late stage of SHS, CT scans show increased nodular enhancement in the liver, primarily due to the further uncontrolled proliferation of histiocytes and macrophages. Laboratory tests reveal decreased albumin, pancytopenia, increased monocytes, and deteriorating liver function.
Dysregulation of the immune system can result in cytokine-mediated inappropriate activation and proliferation of macrophages, which subsequently phagocytize the body's blood cells, a condition known as hemophagocytic syndrome[2]. The pathogenesis primarily involves various triggers (e.g., infections, tumors, and autoimmune diseases), leading to abnormal activation of T lymphocytes, natural killer cells, and other immune-active cells, which continuously release large amounts of inflammatory cytokines such as interferon-gamma, further stimulating macrophages. Activated macrophages phagocytose a variety of blood cells, including erythrocytes, leukocytes, platelets, and their hematopoietic precursors. Concurrently, activated macrophages secrete cytokines like tumour necrosis factor alpha and interleukin-6, further intensifying the inflammatory response and perpetuating a vicious cycle[3]. In this case, hemophagocytic syndrome primarily manifests as tumor-associated macrophages phagocytosing albumin, immunoglobulins, and blood cells. The clinical symptoms of SHS range from splenomegaly in the early stage to anemia (78 g/L), leukopenia (3.37 ×
Upon reviewing the patient’s disease progression, the infiltration of tumor cells into the liver and bone marrow, along with the onset of hemophagocytic syndrome, appears to have contributed to the patient’s demise. The infiltration of tumor cells into the bone marrow further suppresses normal hematopoiesis, resulting in a severe deficiency in the production of all three blood cell lineages. This disruption prevents the maintenance of normal blood cell counts, leading to severe anemia, serious infections (due to critically low white blood cell counts), and uncontrollable bleeding tendencies (due to severe thrombocytopenia), ultimately resulting in multiple organ failure. Metastasis of tumor cells to the liver leads to progressive liver failure, impairing the liver's capacity to metabolize, detoxify, and synthesize essential substances, which in turn results in severe hypoalbuminemia. Consequently, this results in systemic edema, pleural effusion, and ascites, adversely affecting cardiopulmonary function and gastrointestinal absorption, further causing systemic organ dysfunction and ultimately culminating in death.
Previous studies have shown that ring-enhancing nodules on liver CT or magnetic resonance imaging scans in patients with SHS often indicate liver involvement[6]. In the late stage of SHS in this case, a nodular enhancement measuring approximately 2.4 cm × 2.9 cm was observed in June 2022, which increased to 4.3 cm × 2.7 cm within three months. Previous case reports similar to this one have noted normal liver texture and appearance during abdominal exploration in patients with primary SHS, with no evidence of metastatic lesions in the liver. However, after splenectomy, the tumor continued to progress to the liver, resulting in a rapid deterioration of the patient’s condition[7]. Consequently, the rapid development of a liver mass raises suspicion of metastasis from SHS. Although splenectomy may temporarily alleviate symptoms, the invasive nature of SHS can result in liver or bone marrow infiltration[4]. Therefore, early diagnosis and intervention in SHS, prior to tumor cell dissemination, may improve patient survival and is crucial for enhancing prognosis.
SHS is an exceptionally rare and highly aggressive tumor of the lymphohematopoietic system, with average survival influenced by factors such as the stage at diagnosis, treatment modalities, and individual patient variability. Data from various studies indicate that some patients succumb within months of diagnosis[8], with a median overall survival of 6 months[9], reflecting a short survival period and poor prognosis. However, a minority of patients achieve extended survival for several years post-active treatment, such as surviving 3.5 years following chemotherapy selected via a collagen gel droplet-embedded culture drug sensitivity test[10]. In this case, early surgical intervention and pathological diagnosis, mid-term maintenance therapy with imatinib, and late-stage transfusion and albumin support therapy resulted in a total survival time exceeding 33 months, which is relatively long for SHS patients. These findings suggest that current treatment strategies may be effective for some SHS patients and could inform the optimization of future treatment plans.
In the context of this case, SHS represents a relatively rare and intricate disease. Its pathogenesis, diagnostic modalities, and treatment strategies are still under continuous exploration within the medical research domain. Notably, early diagnosis and treatment play a crucial role in enhancing patient survival. In the early phase of SHS, aggressive interventions like splenectomy might contribute to preventing tumor dissemination. However, the majority of patients are diagnosed at advanced stages, frequently accompanied by liver and bone marrow infiltration, and thus have a dismal prognosis. Despite undergoing treatments such as splenectomy, some patients ultimately succumb to disease progression[4]. Additionally, further research efforts are required to comprehensively understand the mechanisms underlying the hematological abnormalities and multi-organ functional impairments induced by this disease. Regarding treatment, monotherapy is frequently inadequate for significantly enhancing long-term patient survival. Transfusion and albumin therapy are frequently employed as symptomatic supportive measures for anemia and thrombocytopenia in these patients. Most patients exhibit a poor response to chemotherapy; nevertheless, under certain circumstances, for example, when appropriate chemotherapeutic agents are selected via drug sensitivity testing, the treatment outcomes might be enhanced. Furthermore, targeted therapies may offer viable treatment options for malignant histiocytic sarcoma harboring specific gene mutations[11]. For more precise treatment modalities like targeted therapy, additional means such as genetic testing might be necessary to guide the treatment process. However, in practical applications, it is also constrained by factors related to the patients’ physical condition (e.g., high risk of puncture due to low platelet count). Future research may focus on identifying safer and more effective treatment options to improve the quality of survival and prognosis of patients. The genetic mutation status of the tumor and the immune microenvironment could potentially serve as avenues for future research on SHS. Moreover, the discovery of biomarkers or potential therapeutic targets associated with prolonged survival will furnish a theoretical foundation for the development of novel therapeutic methods and drugs, thereby promoting the advancement of the SHS treatment field.
The pathophysiology of SHS is intricate, and its pathogenesis, diagnosis, and treatment remain significant challenges within the medical community. Additional research efforts are required to explore more effective treatment strategies. Thus, early diagnosis and treatment of SHS are of utmost importance. Moreover, future treatment plans should be more personalized and precise to improve the survival rate and quality of life of patients.
| 1. | Luo H, Wang T, Xiao L, Wang C, Yi H. Multiple disciplinary team management of rare primary splenic malignancy: Two case reports. World J Clin Cases. 2022;10:10535-10542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 2. | Dhawale MS, Joshi AR, Bhatkule MA, Kumbhakarna NR, Bindu RS. Hemophagocytic syndrome. Indian J Hematol Blood Transfus. 2014;30:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Larroche C, Mouthon L. Pathogenesis of hemophagocytic syndrome (HPS). Autoimmun Rev. 2004;3:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Yamada S, Tasaki T, Satoh N, Nabeshima A, Kitada S, Noguchi H, Yamada K, Takeshita M, Sasaguri Y. Primary splenic histiocytic sarcoma complicated with prolonged idiopathic thrombocytopenia and secondary bone marrow involvement: a unique surgical case presenting with splenomegaly but non-nodular lesions. Diagn Pathol. 2012;7:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Montalvo N, Lara-Endara J, Redrobán L, Leiva M, Armijos C, Russo L. Primary splenic histiocytic sarcoma associated with hemophagocytic lymphohistiocytosis: A case report and review of literature of next-generation sequencing involving FLT3, NOTCH2, and KMT2A mutations. Cancer Rep (Hoboken). 2022;5:e1496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Zhou Y, Zhang K, Yu C. A case of primary splenic histiocytic sarcoma with liver metastasis. Asian J Surg. 2024;47:4623-4624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Oka K, Nakamine H, Maeda K, Yamakawa M, Imai H, Tada K, Ito M, Watanabe Y, Suzuki H, Iwasa M, Tanaka I. Primary histiocytic sarcoma of the spleen associated with hemophagocytosis. Int J Hematol. 2008;87:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Kobayashi S, Kimura F, Hama Y, Ogura K, Torikai H, Kobayashi A, Ikeda T, Sato K, Aida S, Kosuda S, Motoyoshi K. Histiocytic sarcoma of the spleen: case report of asymptomatic onset of thrombocytopenia and complex imaging features. Int J Hematol. 2008;87:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Kommalapati A, Tella SH, Durkin M, Go RS, Goyal G. Histiocytic sarcoma: a population-based analysis of incidence, demographic disparities, and long-term outcomes. Blood. 2018;131:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Yamamoto Y, Hiasa Y, Hirooka M, Koizumi Y, Takeji S, Tokumoto Y, Tsubouchi E, Ikeda Y, Abe M, Matsuura B, Onji M. Complete response of a patient with advanced primary splenic histiocytic sarcoma by treatment with chemotherapeutic drugs selected using the collagen gel droplet-embedded culture drug sensitivity test. Intern Med. 2012;51:2893-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Gounder MM, Solit DB, Tap WD. Trametinib in Histiocytic Sarcoma with an Activating MAP2K1 (MEK1) Mutation. N Engl J Med. 2018;378:1945-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/