Published online Sep 26, 2025. doi: 10.12998/wjcc.v13.i27.108998
Revised: May 23, 2025
Accepted: July 1, 2025
Published online: September 26, 2025
Processing time: 100 Days and 1.4 Hours
Myofascial pain syndrome (MPS) is a common musculoskeletal disease associated with myofascial trigger point (MTrP). Muscle injury is one of the common causes of MPS. Currently, there is no effective treatment for MPS.
A 24-year-old female with chronic lower back MPS secondary to quadratus lumborum (QL) injury underwent comprehensive evaluation using validated scales: Pain severity (visual analog scale, McGill Pain Questionnaire), functional disability (Oswestry Disability Index, Roland Morris Disability Questionnaire), and quality of life [short form 36 (SF-36)]. Objective assessments included sEMG of bilateral QL muscles, Myoton mechanical property analysis, and magnetic re
Ultrasound-guided PRP injections alleviated pain, restored function, and improved quality of life in post-traumatic MPS. sEMG demonstrated neuromuscular symmetry restoration in MTrP-affected muscles, supporting the therapeutic potential of PRP.
Core Tip: This case demonstrates that ultrasound-guided platelet-rich plasma (PRP) injections alleviate pain, restore neuromuscular symmetry, and improve quality of life in post-traumatic lumbar myofascial pain syndrome. Surface elec
- Citation: Ai SL, Xiang XN, Yu X, Li N, Zhang XY, Zhang KB, Jiang HY, Wang Q, He HC. Ultrasound-guided platelet-rich plasma injection improves pain, function and symmetry in lumbar myofascial pain syndrome: A case report. World J Clin Cases 2025; 13(27): 108998
- URL: https://www.wjgnet.com/2307-8960/full/v13/i27/108998.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i27.108998
Myofascial pain syndrome (MPS) is a prevalent musculoskeletal disorder characterized by myofascial trigger points (MTrPs); palpable taut bands within skeletal muscles that elicit localized or referred pain[1]. The main manifestations are palpable abnormal muscle nodules and referred pain around the trigger point. It is a common dysfunction with lifetime prevalence of up to 85% in the general population[2]. The formation of MTrPs arises from heightened energy demands in contracted muscle fibers of taut bands, resulting in localized hypoxia. Persistent MTrP activity dysregulates inflammatory mediators in surrounding tissues, including bradykinin, substance P, calcitonin gene-related peptide, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, serotonin, and norepinephrine[3]. This disruption exacerbates the contraction of muscle fibers, creating a vicious cycle. Muscle injury can cause MPS, especially the formation of some chronic pain areas that are difficult to cure[4], so the key to the treatment of MPS is the inactivation of MTrP and the regeneration and repair of muscle fibers. At present, conventional rehabilitation can alleviate the symptoms of individuals with MPS, but the effectiveness of current treatment remains a topic of controversy in clinical research, and there is no evidence-based medical evidence to prove the effective treatment of MPS[5].
Platelet-rich plasma (PRP), an autologous concentrate derived from centrifuged whole blood, contains abundant growth factors [e.g., platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-β] and cytokines that modulate inflammation and stimulate tissue regeneration[6]. Research has shown that the principle of PRP therapy is to utilize the growth factors in autologous platelets to promote tissue repair and regeneration[7]. Emerging evidence suggests that PRP disrupts the MPS cycle by suppressing inflammatory mediators (e.g., TNF-α and IL-6), enhancing muscle repair, and restoring microcirculation in MTrPs[8].
Current diagnostic modalities for MTrPs, such as palpation and thermography, lack the sensitivity to objectively quantify therapeutic outcomes[9]. In this study, surface electromyography (sEMG) was used to evaluate whether symmetry in neuromuscular activity, specifically comparing affected and unaffected sides, could be restored following PRP intervention. This secondary aim sought to validate sEMG as a quantifiable tool for assessing MTrP inactivation and muscle rebalancing. The primary objective was to assess ultrasound-guided PRP injections for pain relief (visual analog scale, VAS), functional recovery (Oswestry Disability Index, ODI), and quality-of-life improvement (short form 36; SF-36) in post-traumatic MPS. We also examined whether sEMG-derived parameters – specifically bilateral symmetry in average electromyographic amplitude (AEMG) and median frequency (MF) – could objectively quantify neuromuscular re
Clinically, post-traumatic MPS frequently manifests as persistent pain and dysfunction refractory to conventional therapies. We present a representative case of chronic post-traumatic lumbar MPS unresponsive to 2 years of multimodal conservative care. Longitudinal outcomes were systematically evaluated using a novel protocol combining sEMG, MyotonPRO mechanical analysis, magnetic resonance imaging (MRI)-based volumetric quantification, and standardized patient-reported outcomes (VAS, ODI, and SF-36) over a 6-month post-PRP period.
A 24-year-old right-handed female office worker presented with a “2-year history of chronic left lower back and thoracic pain”, exacerbated by prolonged standing, sitting, or specific movements (e.g., trunk rotation). She reported gait impairment due to progressive left lumbar pain and described the pain as a persistent dull ache with intermittent sharp episodes triggered by palpation of the left lumbar region.
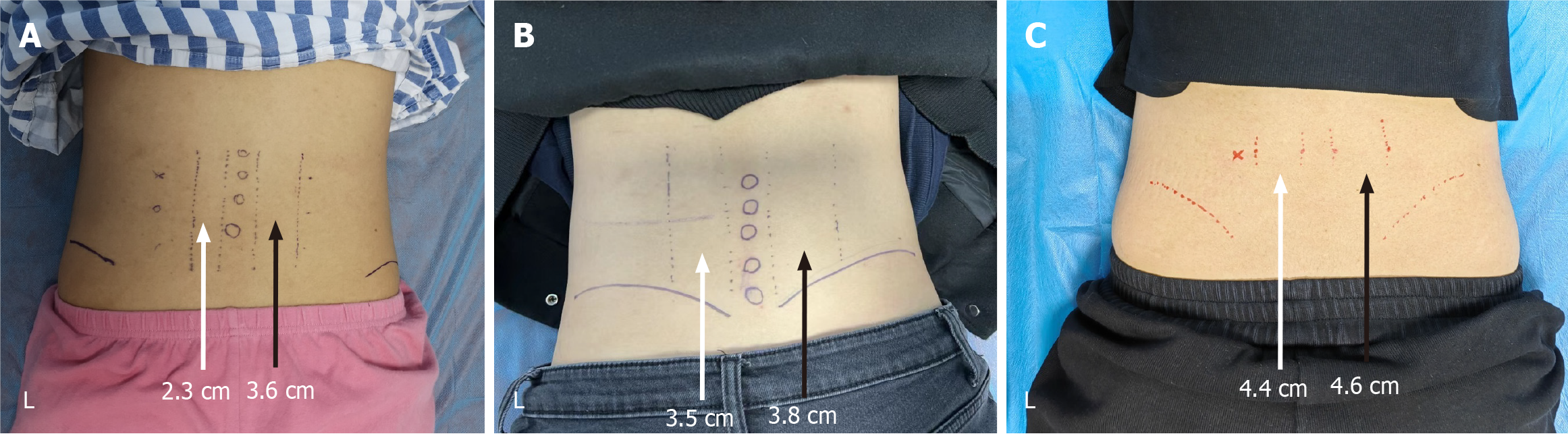
The patient’s symptoms originated from a workplace injury (falling heavy object) at age 22, resulting in immediate lower back pain without open wounds or fractures. Initial conservative management included oral nonsteroidal anti-inflammatory drugs, physical therapy (thermal modalities and stretching), and lidocaine patch application (5% topical, 12 hours daily). Despite compliance with these interventions for 6 months, pain persisted, and she developed compensatory right-sided muscle overuse and disuse atrophy of the left erector spinae (ES) muscle (confirmed by clinical palpation). Palpation of the L2–L5 spinous processes and left transverse processes elicited significant discomfort, accompanied by marked asymmetry in the bilateral ES muscle girth (left: 2.3 cm vs right: 3.6 cm).
At presentation, she rated her baseline pain as 7/10 on the VAS, with functional disability scores of 54/100 on the ODI and 15/24 on the Roland Morris Disability Questionnaire (RMDQ). Quality-of-life metrics from the Medical Outcomes Study SF-36 highlighted limitations across multiple domains: Physical Functioning (PF: 55), Role-Physical (RP: 50), Bodily Pain (BP: 57), General Health (GH: 35), Vitality (VT: 100), Social Functioning (SF: 50), Role-Emotional (RE: 66.7), and Mental Health (MH: 48).
No prior history of chronic diseases. No prior surgeries except for a minor left ankle sprain at age 18, fully resolved with conservative care.
Occupation: Sedentary office work (8 hours/day, minimal physical activity).
Social history: Non-smoker, occasional alcohol consumption (≤ 1 drink/week).
Family history: No family history of musculoskeletal or autoimmune disorders.
Palpation of the L2–L5 spinous processes and left transverse processes elicited significant discomfort, accompanied by marked asymmetry in the bilateral ES muscle girth (left: 2.3 cm vs right: 3.6 cm).
No special notes.
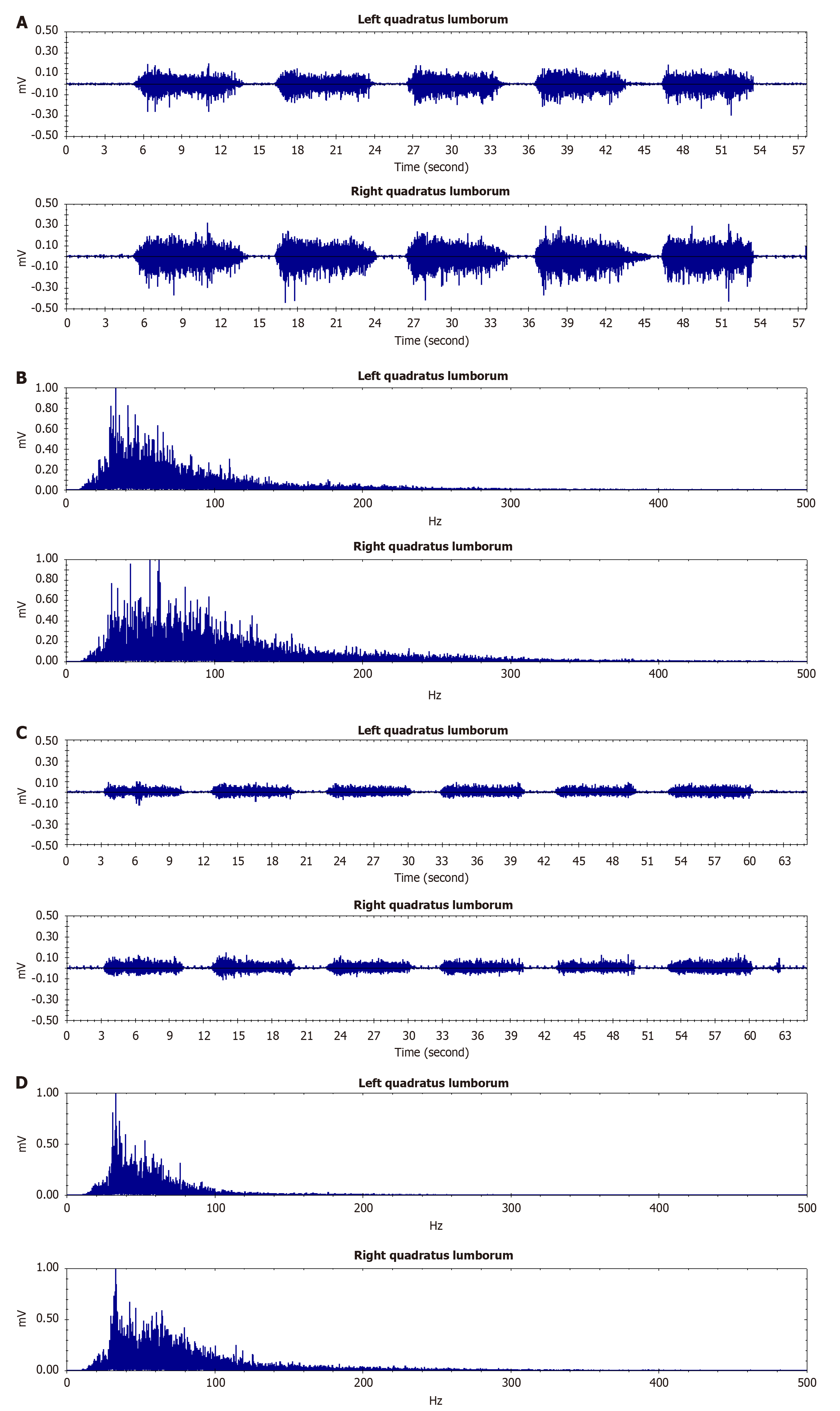
Single channel sEMG (BTS Inc. FreeEMG1000, Milan, Italy): The electrodes need to be placed on the line from the posterior superior iliac spine to the lowest point of the lower rib at the L3 level. The testing movement is lifting the trunk from a prone position to ensure that the bilateral QL muscles contract simultaneously. Left side AEMG was 28.735 µV, EMG median frequency (MPF) 63.601 Hz, and right side AEMG 40.763 µV, MPF 88.201 Hz. The mechanical properties of the QL and ES muscles were assessed using the Myoton PRO (Myoton AS, Tallinn, Estonia). Those results were left side QL: Natural oscillation frequency (F) 12.8 Hz, dynamic stiffness (S) 206 N/m, logarithmic decrement (D) 0.9 (relative unit), mechanical stress relaxation time (R) 24.9 ms; ratio of relaxation and deformation time (C) 1.46 (relative unit); right side QL: F 13.3 Hz, S 221 N/m, D 1.27, R 25.9 ms; C 1.56; left side ES: F 17 Hz, S 394 N/m, D 1.6, R 14.3 ms; C 0.91; right side ES: F 16.6 Hz, S 342 N/m, D 1.18, R 17.1 ms; C 1.07.
The patient was diagnosed with left QL injury and MPS.
Approximately 40 mL of venous blood was drawn from the elbow vein using a specialized large-bore needle. The blood was then thoroughly mixed with an anticoagulant (sodium citrate) in a 10:1 ratio to prevent microbubble formation. Subsequently, the mixture was transferred into anticoagulant vacuum tubes. PRP was prepared utilizing the WEGO (II-
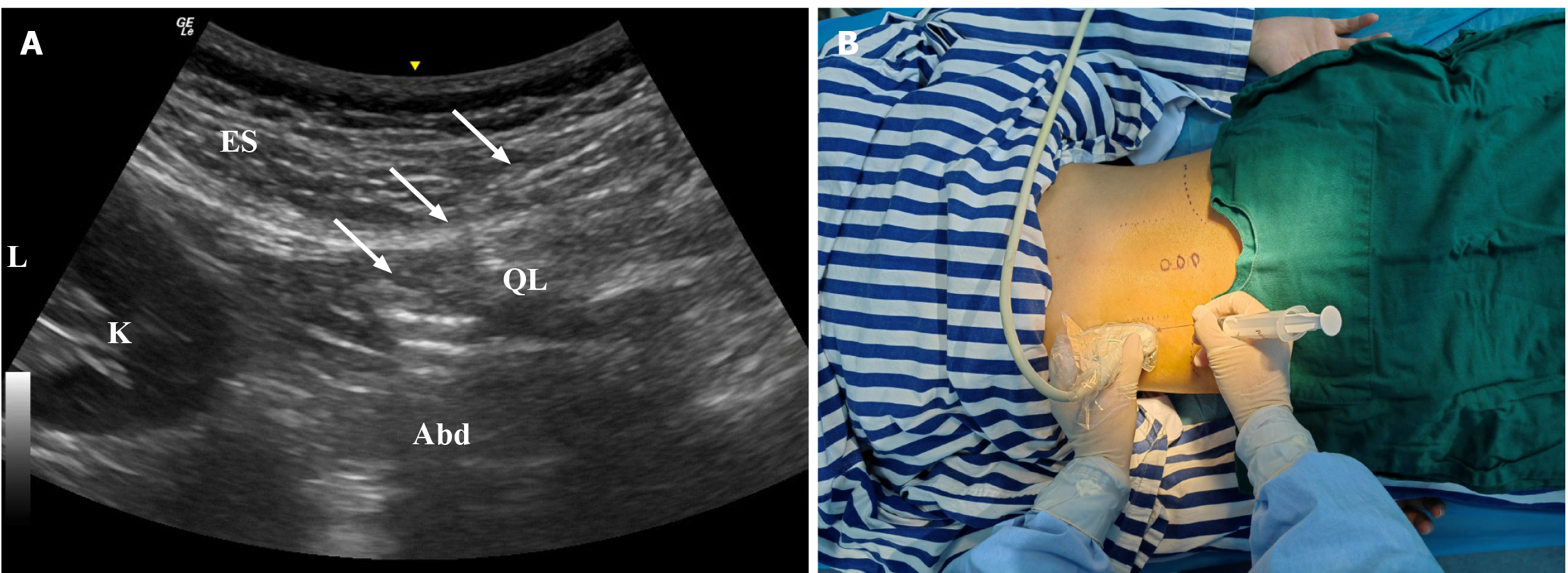
MTrP was determined according to clinical symptoms, palpation pain points and sEMG examination results. Under the guidance of ultrasound, an appropriate amount of PRP was injected into MTrP and the surrounding myofascial system. About 3 mL: PRP was injected into the space between MTrP and adjacent tissues, and the remaining 3 mL was used to release the surrounding myofascia layer by layer (Figure 1).
At baseline, the individual exhibited severe pain and functional impairment, with a VAS score of 7, McGill score of 15, RMDQ score of 15, and ODI score of 54. Immediate pain relief was observed 24 hours after the first PRP treatment, with VAS decreasing to 6. By 1 month post-treatment, further improvement was noted: VAS and McGill scores both dropped to 2, indicating substantial pain reduction. Functional recovery progressed steadily, with RMDQ improving to 8 and ODI decreasing to 30 at 1 month. These improvements were sustained throughout the 6-month follow-up period, with VAS, McGill, and RMDQ remaining stable at 2, 2, and 8, respectively, and ODI further improving to 15 by 6 month, demonstrating long-term efficacy of the intervention (Table 1). She noticed that her quality of life improved significantly and thought her response was satisfactory (Table 2). Three months after treatment, she was able to return to normal work and enjoy her social life again. During follow-up, the pain score, functional assessment, and life therapy assessment were significantly improved. During the follow-up of 6 month, there were no adverse reactions. After PRP treatment, there was no significant change in the muscle hardness or elasticity index test data of the bilateral psoas muscles of the individual.
| Baseline | 24 hours | 1 month | 3 months | 6 months | |
| VAS | 7 | 6 | 2 | 2 | 2 |
| McGill | 15 | 4 | 2 | 2 | 2 |
| RMDQ | 15 | 8 | 8 | 8 | 8 |
| ODI | 54 | 54 | 30 | 30 | 15 |
| Baseline | 1 month | 3 months | 6 months | |
| PF | 55 | 70 | 70 | 85 |
| RP | 50 | 50 | 75 | 75 |
| BP | 57 | 69 | 84 | 84 |
| GH | 35 | 30 | 30 | 50 |
| VT | 100 | 110 | 110 | 110 |
| SF | 50 | 75 | 75 | 75 |
| RE | 66.7 | 33.3 | 66.7 | 66.7 |
| MH | 48 | 48 | 68 | 68 |
The muscle circumference of the ES muscle of the left and right sides of the individual was significantly improved. In the dimension measurement of the left ES at the last follow-up, both sides became symmetrical (left 4.4 cm, right 4.6 cm). The measurement and comparison of lumbar ES muscle width were conducted before, at 3 month, and at 6 month after treatment. The initial difference between the left and right sides was 1.3 cm, which decreased to 0.3 cm after 3 month and further reduced to 0.2 cm after 6 month (Figure 2).
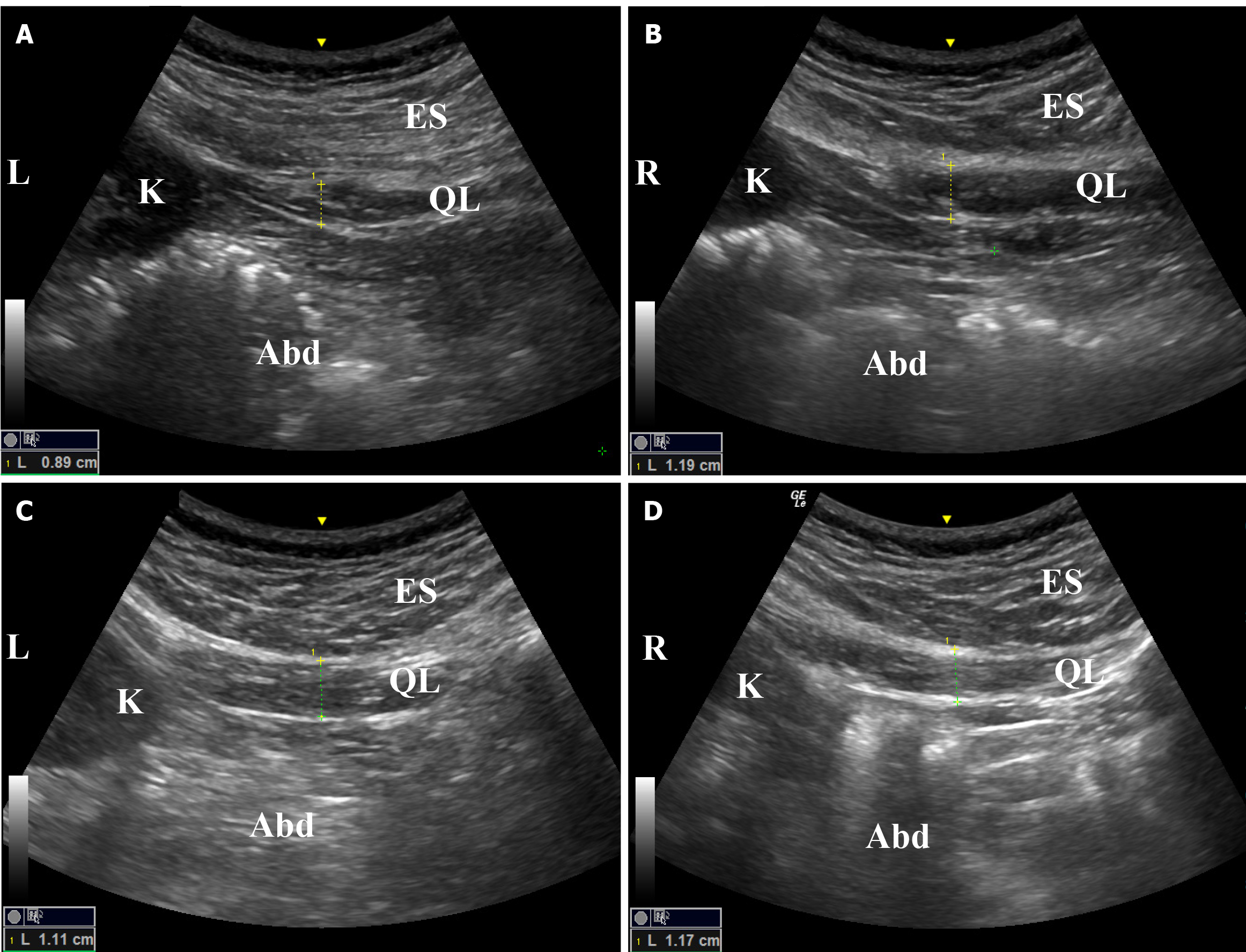
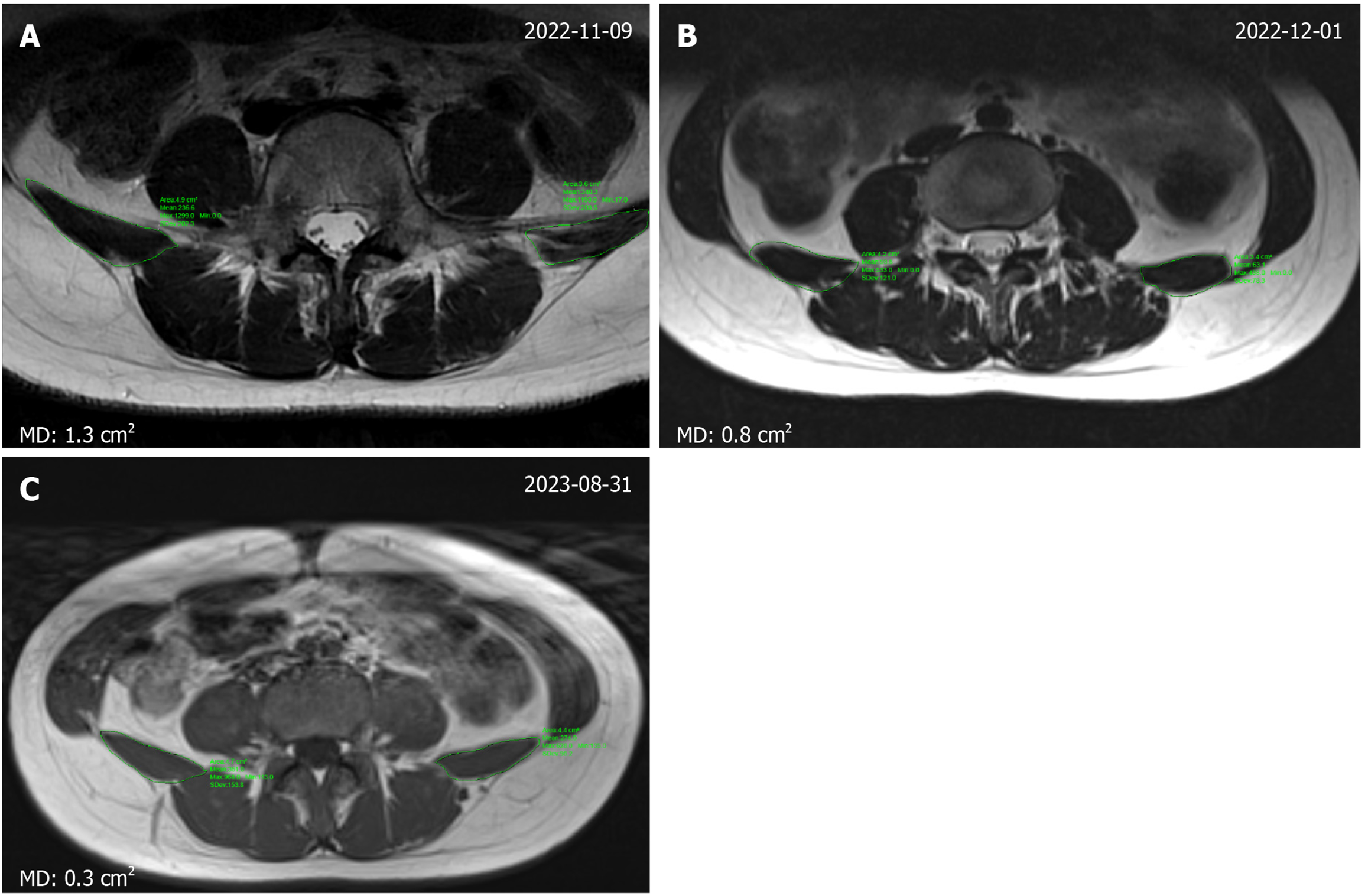
Ultrasound imaging proved to be a convenient method for measuring the thickness of the lumbar paraspinal muscles. Following treatment, the muscle thickness on both sides exhibited significant improvement in symmetry. Prior to treatment, the left side measured 0.89 cm, while the right side measured 1.19 cm, resulting in a difference of 0.3 cm between the two sides. However, after treatment, the left side increased to 1.11 cm, the right side to 1.17 cm, and the difference between the two sides reduced to 0.06 cm. These findings demonstrate a notable enhancement in bilateral symmetry (Figure 3). During the previous post-treatment follow-up, the lumbar MRI of individuals revealed a reduction in the average difference in cross-sectional area of the bilateral psoas quadratus muscle from 1.3 to 0.8 cm2. This gap was further narrowed to 0.3 cm2 during the 6-month follow-up assessment (Figure 4).
From the single channel sEMG, we found that the AEMG and MPF on both sides of the individual became symmetrical. At the last follow-up, the left side AEMG was 25.707 µV, MPF was 66.413 Hz, and the right side AEMG was 26.910 µV, MPF was 70.745 Hz. Following treatment with PRP, there was a notable improvement in the symmetry of electromyographic measurements of the individual's bilateral QL muscles (Figure 5 and Table 3).
| Baseline | 1 month | 3 months | 6 months | |
| Left Aemg | 28.74 | 23.17 | 24.59 | 25.71 |
| Left iemg | 83.64 ± 7.51 | 59.49 ± 4.63 | 61.12 ± 8.12 | 73.73 ± 11.41 |
| Left RMS | 35.77 ± 1.88 | 28.96 ± 2.50 | 30.79 ± 0.97 | 32.01 ± 1.30 |
| Left MF | 63.60 | 87.29 | 80.06 | 68.52 |
| Right Aemg | 40.76 | 27.82 | 26.95 | 26.91 |
| Right iemg | 112.47 ± 8.39 | 68.87 ± 6.27 | 69.37 ± 6.69 | 75.48 ± 9.79 |
| Right RMS | 51.36 ± 2.94 | 34.86 ± 1.63 | 33.84 ± 1.16 | 33.53 ± 1.03 |
| Right MF | 88.20 | 85.17 | 83.20 | 70.48 |
This case demonstrates that ultrasound-guided PRP injections effectively alleviate pain, restore motor function, and improve quality of life in post-traumatic MPS. During a 6-month follow-up period, no adverse events were observed, indicating that this treatment approach is both safe and effective. Pain relief may stem from MTrP inactivation via PRP anti-inflammatory properties and tissue regeneration mediated by growth factors (e.g., PDGF and TGF-β)[10-12]. Functional recovery likely reflects muscle repair and biomechanical rebalancing, as demonstrated by restored ES symmetry (ultrasound/MRI) and sEMG-documented neuromuscular equilibrium. Quality-of-life improvements correlate with these multidimensional gains.
MPS represents a prevalent etiology of musculoskeletal pain, yet its diagnosis remains challenging due to the absence of high-quality evidence-based guidelines[13]. Current clinical consensus relies on a combination of criteria, including regional pain, palpable taut bands, localized tenderness, restricted range of motion, and either sensory abnormalities or a local twitch response upon palpation[14]. The patient in this study met all primary diagnostic criteria; specifically, persistent lower back pain with trigger point-associated referred pain, taut band identification, and motion limitation, alongside secondary features of reproducible tenderness and twitch response, aligning with established specialist recommendations. The mechanism underlying this phenomenon likely involves PRP dual anti-inflammatory and regenerative effects[15]. PRP suppresses proinflammatory cytokines (e.g., TNF-α and IL-1β) via nuclear factor-κB pathway inhibition while promoting tissue repair through growth factors such as TGF-β and PDGF15. In MPS, chronic inflammation perpetuates MTrP activity[16], leading to muscle fiber taut bands and nociceptor sensitization.
The restoration of neuromuscular symmetry observed via sEMG in this case provides robust evidence for the efficacy of ultrasound-guided PRP therapy in treating MPS. Post-treatment sEMG analysis revealed a significant convergence in AEMG and MF between the affected and unaffected sides of the QL muscles (AEMG asymmetry: 28.74 vs 40.76 µV at baseline to 25.71 vs 26.91 µV at 6 month; MF asymmetry: 63.60 vs 88.20 Hz to 68.52 vs 70.48 Hz). These electrophysiological improvements paralleled clinical outcomes, including pain relief (VAS: 7 to 2), functional recovery (ODI: 54 to 15), and enhanced quality of life (SF-36 PF: 55 to 85), suggesting that sEMG symmetry restoration is not merely a biomarker but a direct reflection of neuromuscular rebalancing post-PRP intervention.
Some studies have reported that muscle injury can lead to chronic MPs, and they are difficult to cure[17,18]. The treatment of MPS includes physical therapy, oral medication, and trigger point injection. Some of the most commonly used oral medications are: Nonsteroidal anti-inflammatory drugs, tricyclic antidepressants, muscle spasms, opioid analgesics, neuropathic analgesics, and antidepressants[19]. In noninvasive treatment, mechanical pressure or stretching of affected muscles at the trigger point is also used[20]. Invasive treatment includes injection of local anesthetics, corticosteroids, neurotransmitters, and botulinum toxin into MTrP[12]. Other reported treatments can relieve the pain of MPS individuals to some extent, but the direct intervention of MTrP is lacking. Our innovation lies in the application of sEMG and other objective examinations for accurate evaluation and ultrasound-guided precise treatment, which fully play the role of PRP regeneration. This paper introduces the diagnostic difficulties of MPs from the perspective of objective evaluation. From the perspective of structure to function, the application of regenerative therapy will bring better curative effect to individuals.
The advantage of PRP treatment is that autologous blood PRP has no rejection reaction in theory and is a safe treatment. PRP could alleviate inflammation and inactivate MTrP locally[21]. At the same time, the regeneration of growth factors released by PRP could repair local myofascial tissue and restore muscle function[10,11]. PRP has unique properties in promoting tissue repair and modulating the local environment around the trigger point. The combination of PRP and exercise likely had a synergistic effect, but the specific mechanism by which each component acted requires further investigation. PRP significantly improved motor function and quality of daily life. This treatment scheme has a good effect on MPS in individuals with muscle injury. There is evidence suggesting that dry needling can provide short-term local symptom relief[22]. Nevertheless, there is a lack of sufficient evidence for its long-term effect on muscle function recovery.
Our report had some limitations. As a case report, the single individual restricts the generalization of our findings due to individual variations. The absence of a control group complicates the determination of the specific effect of PRP, as other factors might have influenced the results. Future randomized controlled trials with larger cohorts are needed to validate PRP efficacy.
This case underscores the transformative potential of ultrasound-guided PRP therapy in MPS, combining targeted anti-inflammatory action, tissue regeneration, and objective monitoring via sEMG. By addressing both symptom relief and pathophysiological repair, PRP outperforms conventional therapies constrained by their transient or mechanistic limitations. Future research should focus on standardizing sEMG applications and expanding the role of PRP in regenerative rehabilitation, ultimately improving outcomes for patients with refractory MPS.
Thank you to our colleagues. We thank the Rehabilitation Medicine Department and Geriatrics Center of West China Hospital of Sichuan University for their strong support.
| 1. | Diep D, Chen KJQ, Kumbhare D. Ultrasound-guided interventional procedures for myofascial trigger points: a systematic review. Reg Anesth Pain Med. 2021;46:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Fleckenstein J, Zaps D, Rüger LJ, Lehmeyer L, Freiberg F, Lang PM, Irnich D. Discrepancy between prevalence and perceived effectiveness of treatment methods in myofascial pain syndrome: results of a cross-sectional, nationwide survey. BMC Musculoskelet Disord. 2010;11:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Duarte FCK, West DWD, Linde LD, Hassan S, Kumbhare DA. Re-Examining Myofascial Pain Syndrome: Toward Biomarker Development and Mechanism-Based Diagnostic Criteria. Curr Rheumatol Rep. 2021;23:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 4. | Zhuang X, Tan S, Huang Q. Understanding of myofascial trigger points. Chin Med J (Engl). 2014;127:4271-4277. [PubMed] |
| 5. | Urits I, Charipova K, Gress K, Schaaf AL, Gupta S, Kiernan HC, Choi PE, Jung JW, Cornett E, Kaye AD, Viswanath O. Treatment and management of myofascial pain syndrome. Best Pract Res Clin Anaesthesiol. 2020;34:427-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Cecerska-Heryć E, Goszka M, Serwin N, Roszak M, Grygorcewicz B, Heryć R, Dołęgowska B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 7. | Gao H, Zhao Z, Li J, Guo Z, Zhang F, Wang K, Bai X, Wang Q, Guan Y, Wang Y, Zhang P, Lv N, Zhu H, Li Z. Platelet-rich plasma promotes skeletal muscle regeneration and neuromuscular functional reconstitution in a concentration-dependent manner in a rat laceration model. Biochem Biophys Res Commun. 2023;672:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Solakoglu Ö, Heydecke G, Amiri N, Anitua E. The use of plasma rich in growth factors (PRGF) in guided tissue regeneration and guided bone regeneration. A review of histological, immunohistochemical, histomorphometrical, radiological and clinical results in humans. Ann Anat. 2020;231:151528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Xu J, Chen K, Ding B, Zhu M, Yao S, Ren M, Shen Y. Effectiveness of self-myofascial release combined with biofeedback and electrical stimulation for the management of myofascial pelvic pain: A randomized controlled trial. Eur J Pain. 2022;26:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Le ADK, Enweze L, DeBaun MR, Dragoo JL. Current Clinical Recommendations for Use of Platelet-Rich Plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (1)] |
| 11. | Sha K, Chen M, Liu F, Xu S, Wang B, Peng Q, Zhang Y, Xie H, Li J, Deng Z. Platelet factor 4 inhibits human hair follicle growth and promotes androgen receptor expression in human dermal papilla cells. PeerJ. 2020;8:e9867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | De Andrés J, Adsuara VM, Palmisani S, Villanueva V, López-Alarcón MD. A double-blind, controlled, randomized trial to evaluate the efficacy of botulinum toxin for the treatment of lumbar myofascial pain in humans. Reg Anesth Pain Med. 2010;35:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 13. | Yang S, Chang MC. Effect of Repetitive Transcranial Magnetic Stimulation on Pain Management: A Systematic Narrative Review. Front Neurol. 2020;11:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Grosman-Rimon L, Clarke H, Chan AK, Mills PB, Rathbone ATL, Kumbhare D. Clinicians' perspective of the current diagnostic criteria for myofascial pain syndrome. J Back Musculoskelet Rehabil. 2017;30:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Nakajima R, Saita Y, Kobayashi Y, Wakayama T, Uchino S, Momoi Y, Yamamoto N, Ishijima M. Comparison of bioactive substances in novel-developed freeze-dried platelet-rich plasma (PRP) and activated normal PRP, and investigation of bioactive substance levels after long-term storage. Regen Ther. 2024;27:200-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Tsai PF, Edison JL, Wang CH, Gramlich MW, Manning KQ, Deshpande G, Bashir A, Sefton J. Characteristics of patients with myofascial pain syndrome of the low back. Sci Rep. 2024;14:11912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Monclús P, Bosque M, Margalef R, Colomina MT, Valderrama-Canales FJ, Just L, Santafé MM. Shock waves as treatment of mouse myofascial trigger points. Pain Pract. 2023;23:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Zhou X, Li X, Wang Z, Huang D. Preliminary evidence of safety and effectiveness of Loxoprofen Sodium Cataplasm combined with physiotherapy for myofascial pain syndrome treatment: A randomized controlled pilot clinical trial. Front Neurol. 2022;13:998327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Liu C, Wang Y, Yu W, Xiang J, Ding G, Liu W. Comparative effectiveness of noninvasive therapeutic interventions for myofascial pain syndrome: a network meta-analysis of randomized controlled trials. Int J Surg. 2024;110:1099-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Gül K, Onal SA. [Comparison of non-invasive and invasive techniques in the treatment of patients with myofascial pain syndrome]. Agri. 2009;21:104-112. [PubMed] |
| 21. | Chen YL, Chao TT, Wu YN, Chen MC, Lin YH, Liao CH, Wu CC, Chen KC, Chou SP, Chiang HS. nNOS-positive minor-branches of the dorsal penile nerves is associated with erectile function in the bilateral cavernous injury model of rats. Sci Rep. 2018;8:929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Sánchez Romero EA, Lim T, Villafañe JH, Boutin G, Riquelme Aguado V, Martin Pintado-Zugasti A, Alonso Pérez JL, Fernández Carnero J. The Influence of Verbal Suggestion on Post-Needling Soreness and Pain Processing after Dry Needling Treatment: An Experimental Study. Int J Environ Res Public Health. 2021;18:4206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/