Published online Sep 26, 2025. doi: 10.12998/wjcc.v13.i27.107805
Revised: April 22, 2025
Accepted: June 19, 2025
Published online: September 26, 2025
Processing time: 129 Days and 19.1 Hours
Acute pancreatitis (AP) is commonly encountered in gastroenterology, with bi
We describe the case of a 50-year-old male recently diagnosed with small-cell lung cancer (SCLC). The patient was admitted to the emergency department with acute abdominal pain and subsequently diagnosed with AP. He was hospitalized under the care of the gastroenterology service. During the etiological workup, metastatic pancreatic lesions were identified on imaging, which had not been observed on the initial cancer staging. Following resolution of the initial episode, oral intake was introduced, but the patient experienced recurrent abdominal pain and labo
Oncologic treatment can be considered as part of the therapeutic approach in AP secondary to SCLC metastasis, especially chemotherapy.
Core Tip: Acute pancreatitis secondary to pancreatic metastases from small-cell lung cancer is an extremely rare entity, with few cases reported in the literature. This case highlights the importance of considering metastatic involvement in un
- Citation: Suárez M, Simón S, Martínez R, Crespo J. Acute pancreatitis secondary to small-cell lung cancer metastasis: A case report and literature review. World J Clin Cases 2025; 13(27): 107805
- URL: https://www.wjgnet.com/2307-8960/full/v13/i27/107805.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i27.107805
Acute pancreatitis (AP) is one of the most common conditions leading to hospitalization in gastroenterology services. The global incidence of AP ranges from 5 to 73.4 cases per 100000 inhabitants, with a prevalence of 76.2 cases per 100000 inhabitants[1,2]. This corresponds to an annual increase in AP incidence of approximately 3.07%[3]. Although most cases are not fatal, AP-related mortality is estimated at 1.4 to 1.6 cases per 100000 inhabitants, a non-negligible figure consi
AP accounts for approximately 30% of hospital admissions in gastroenterology units, with nearly 300000 hospitalizations annually in the United States alone[5]. The leading cause, by far, is biliary etiology, responsible for 42% to 56% of AP episodes. Other major causes include alcohol consumption, hypertriglyceridemia (over 1000 mg/dL), hypercalcemia, and idiopathic cases. Less common etiologies include post-endoscopic retrograde cholangiopancreatography (ERCP) pan
Neoplastic AP is estimated to account for approximately 1% of all AP cases. The most frequently associated tumors include primary pancreatic, biliary, and ampullary neoplasms[9,10]. Anecdotally, case reports in the literature have des
Due to its exceptionally rare origin, we present a case of AP secondary to metastasis from small cell lung cancer (SCLC).
A 49-year-old Caucasian male presented with continuous epigastric pain radiating in a belt-like pattern, with no clear triggering factor.
The patient reported a 5-day history of symptoms and had attempted to manage the pain at home with morphine and fentanyl, without improvement, after consulting his primary physician. Three days prior to the hospital admission described in this case, he visited the emergency department with the same symptoms, where his analgesic regimen was adjusted due to normal laboratory test results. He denied alcohol consumption or dietary indiscretions.
The patient was diagnosed 2 weeks prior with stage IV SCLC with infiltration of major vessels, bilateral mediastinal lymphadenopathy, bone and adrenal metastases, and pleural effusion. Additionally, nonspecific lymphadenopathy was noted at the hepatic hilum. The diagnosis was established following persistent dorsal pain that failed to improve despite various conservative treatments. Magnetic resonance imaging (MRI) revealed a left mediastinal mass involving the main pulmonary artery and 40% of the descending aorta, as well as the previously mentioned pleural effusion and an expan
His medical history was notable for a 30-year history of smoking, with a consumption of 30 cigarettes per day, as well as dyslipidemia, nephrolithiasis with recurrent renal colic, and hyperuricemia.
The patient had no prior history of oncological disease.
On physical examination, the patient had a performance status of 3 due to dorsal metastases. The pain secondary to this lesion limited her mobility and caused her to spend a significant amount of the day in bed. The patient weighed 54 kg and was 162 cm tall. His blood pressure was 138/90 mmHg, and his heart rate was 94 beats per minute. He was afebrile and required oxygen via nasal cannula at 2 L per minute to maintain an oxygen saturation above 92%. Pulmonary auscultation revealed decreased breath sounds in the left hemithorax. The abdomen was mildly distended, with tender
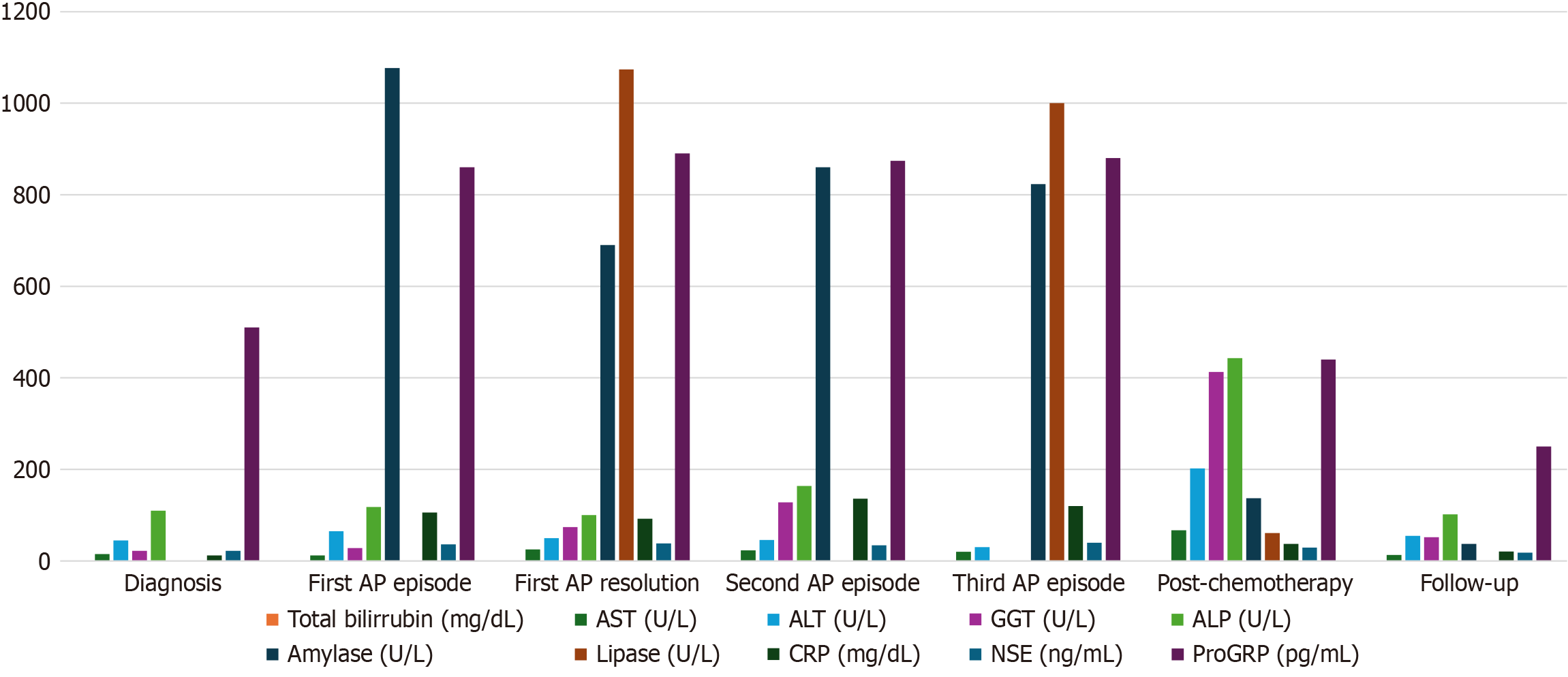
Upon presentation to the emergency department, laboratory tests revealed a leukocyte count of 27400 cells/mm³, with 95% neutrophils. Coagulation parameters were unremarkable, except for an elevated fibrinogen level of 707 mg/dL. Liver function tests showed a mild elevation of alanine aminotransferase (65 U/L) and an amylase level of 1077 U/L. C-reactive protein was elevated at 106 mg/L.
Once admitted to the ward, a comprehensive liver panel was performed, revealing only a mildly elevated gamma-glutamyl transferase of 74 U/L, with normal bilirubin levels. Other potential causes of AP were investigated, but cor
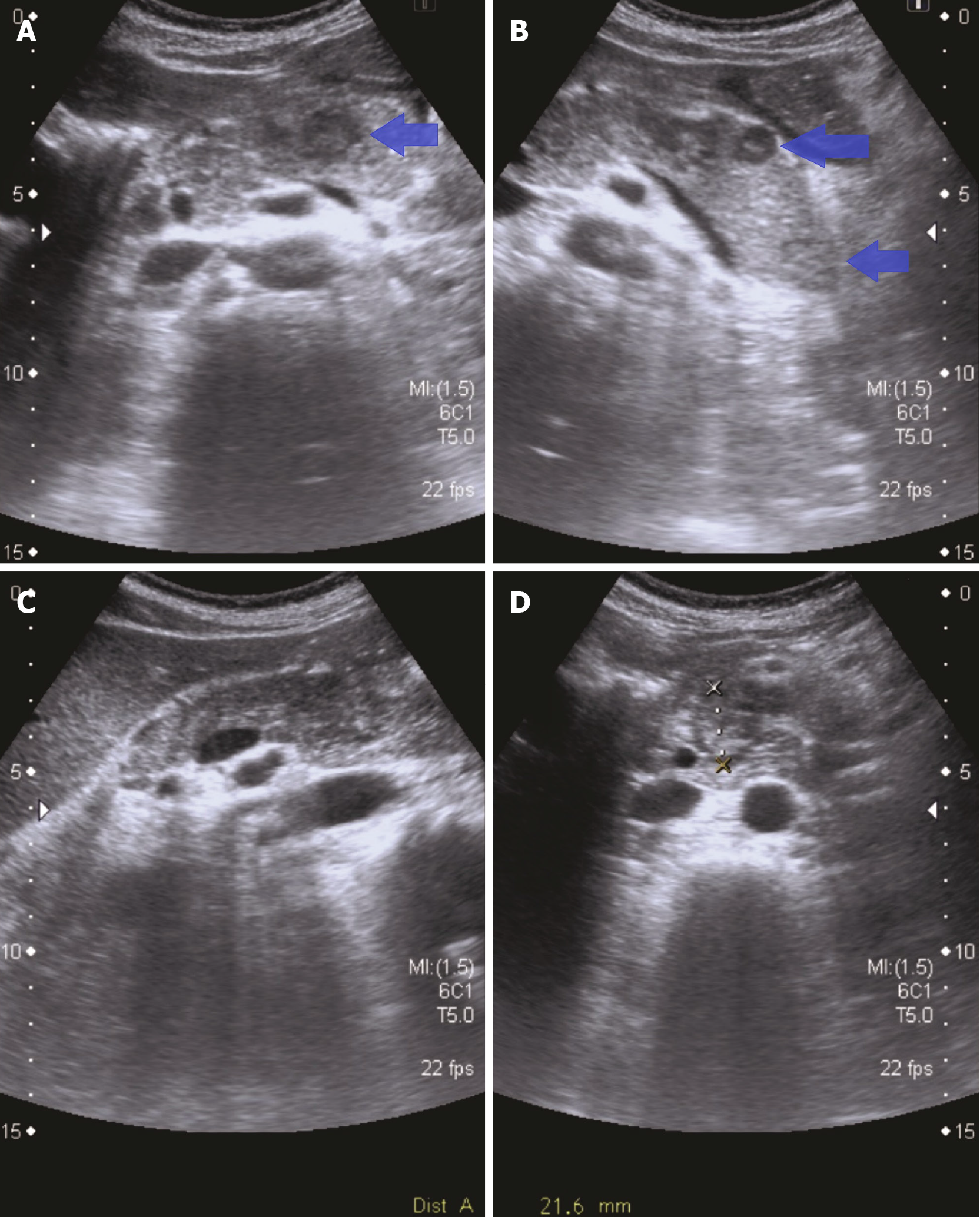
Given the recent whole-body computed tomography (CT) scan and the greater accessibility of ultrasound (US), an abdominal US was performed by an expert radiologist specialized in biliopancreatic pathology. This imaging study revealed multiple nodular lesions, most of them hypoechoic and approximately 2 cm in size, consistent with metastatic pancreatic lesions (Figure 2). No gallstones or abnormalities in the common bile duct were observed.
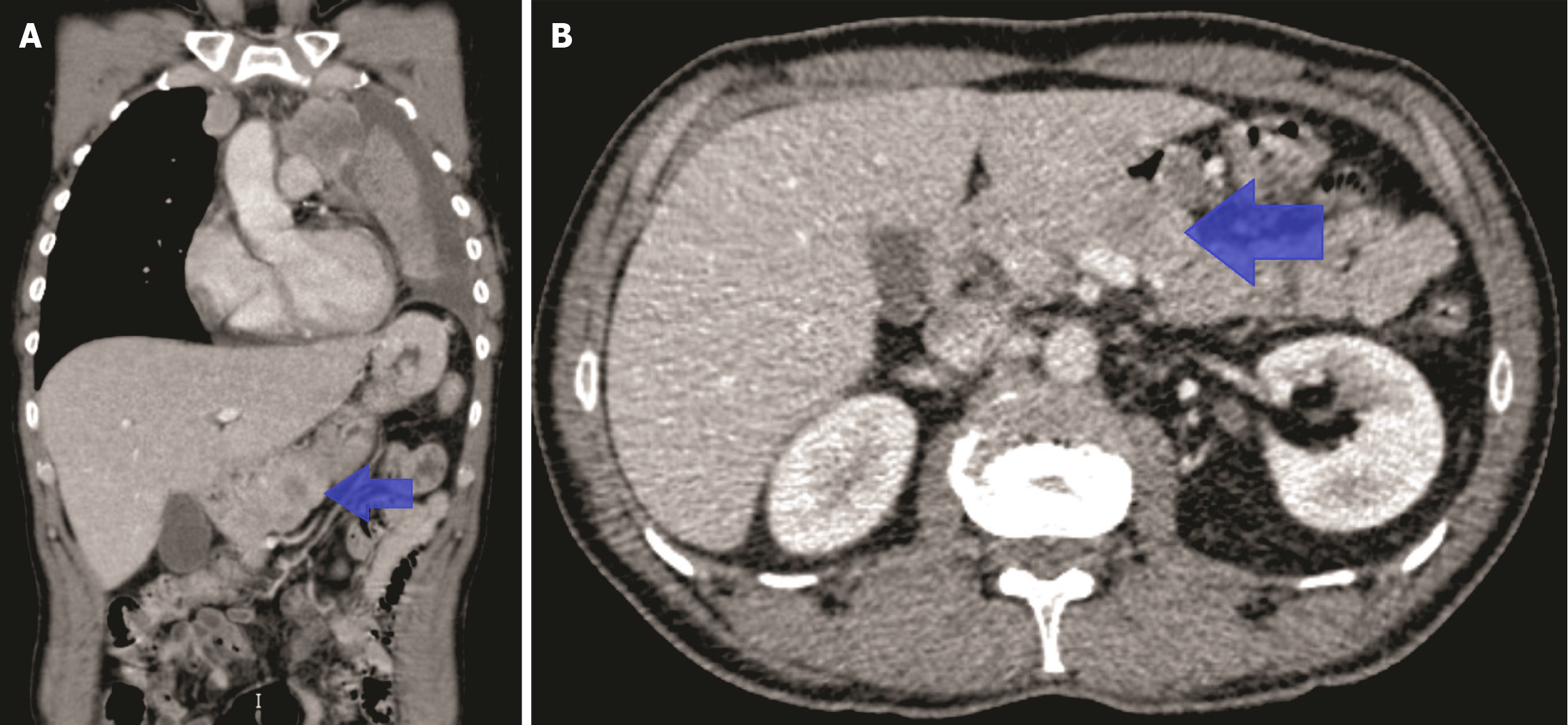
Due to the short time elapsed since the initial SCLC diagnosis, the previously performed CT scan from 1 month earlier was reviewed. That scan had shown a single hypodense area between the body and tail of the pancreas, initially considered nonspecific (Figure 3). Given the patient's clinical progression, this lesion was retrospectively interpreted as pancreatic metastasis.
Based on the clinical presentation, laboratory results, imaging studies, and medical history, the diagnosis was mild AP secondary to metastasis from SCLC.
Initially, conservative management was implemented, as with any case of mild AP. Several attempts were made to initiate oral intake once the pain subsided, but each time even a liquid diet was reintroduced, the pain reappeared.
Given the complexity of the clinical case and the inability to progress and the repeated recurrence of pain, the case was discussed in a multidisciplinary team meeting. Several options were considered. First, endoscopic US (EUS) with biopsy was proposed to confirm the suspected diagnosis prior to initiating treatment. Pancreatic MRI was also considered but ultimately dismissed as the radiologists deemed the US images conclusive for the etiological assessment. Given the need to initiate chemotherapy for the underlying SCLC, which had been delayed due to the current hospitalization and pending immunohistochemical results, this option was also evaluated. Concomitant radiotherapy was discussed but deferred in case abdominal pain persisted. All options were thoroughly discussed with the patient and his family. Along with all this information, the patient was also asked for permission to publish this clinical case due to its rarity and the limited evidence available in the literature. Informed consent was obtained. The patient declined invasive procedures unless strictly necessary; therefore, chemotherapy with carboplatin and etoposide at 80% of the standard dose was initiated. After the first cycle of treatment, the patient was able to advance with the diet without issues and was dis
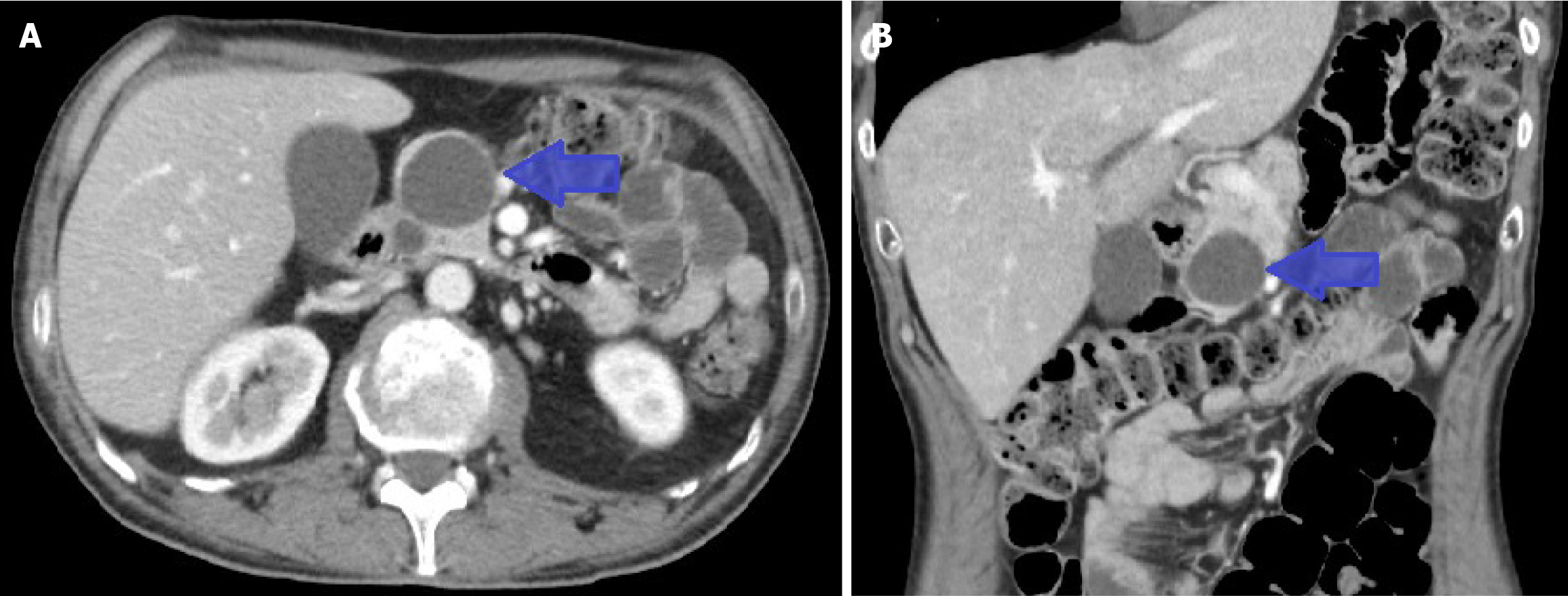
After discharge, the patient did not experience any further episodes of AP. Four cycles of chemotherapy were received, along with radiotherapy for analgesic control of pain due to dorsal bone metastasis. A follow-up CT scan showed a partial response of the SCLC. Pancreatic lesions were resolved, revealing a pancreatic cystic lesion consisting of an asymptomatic pseudocyst (Figure 4). Due to the clinical response, Atezolizumab was added to treatment based on the IMpower133 trial[15].
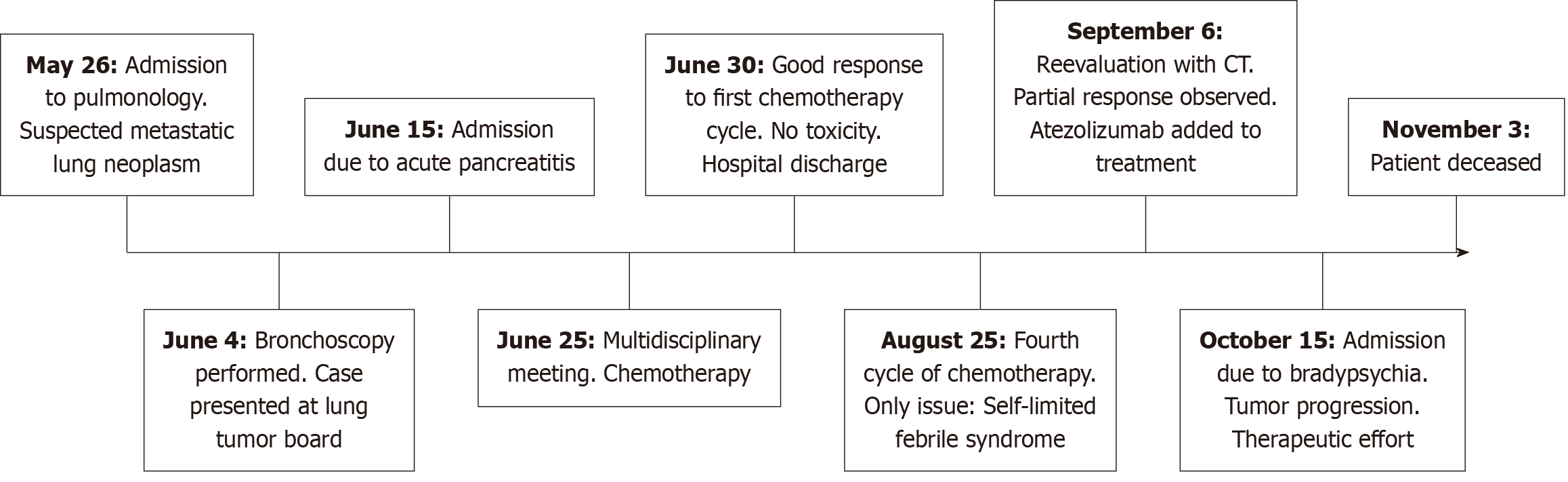
One month later, the patient was admitted due to bradyphrenia and paralytic ileus. Brain progression was diagnosed through MRI. The patient and family were informed about the disease progression. In agreement with them, a limitation of therapeutic effort was decided. The patient was discharged home after receiving whole-brain radiotherapy with an infusion pump for symptom control, and follow-up by the palliative care team. Eventually, the patient passed away at home. A timeline has been developed to summarize this section (Figure 5).
SCLC are highly aggressive tumors primarily associated with smoking. They are characterized by rapid growth and a high tendency to metastasize, which often results in an advanced stage at the time of diagnosis[16,17]. Fortunately, the decline in tobacco use in recent decades has led to a decrease in their incidence, dropping from 15.3 per 100000 people in the 1980s to 6.5 per 100000 people in 2019[18].
The ability of these tumors to metastasize is influenced by various factors, including the following: Their high proliferation rate and cellular plasticity, which allow them to change phenotypes and facilitate their spread; the expression of adhesion molecules that enable them to adhere to the vascular endothelium and extravasate into other tissues; evasion of the innate immune system, especially natural killer cells; and their intratumoral heterogeneity, which favors the dissemination of specific tumor subpopulations through the bloodstream[19,20].
Although it remains a tumor with poor prognosis, recent years have witnessed significant advances in its treatment, particularly with the incorporation of immunotherapy and the identification of novel molecular targets are offering renewed hope in the management of SCLC. The disease remains a significant clinical challenge due to its aggressive behavior and rapid development of treatment resistance.
The combination of chemotherapy with immune checkpoint inhibitors (ICIs) has shifted the treatment paradigm for extensive-stage SCLC (ES-SCLC). The combination of platinum–etoposide chemotherapy with ICIs, such as atezolizumab or durvalumab, has transformed the therapeutic landscape. This strategy has shown a significant improvement in overall survival, with an increase in median survival from 8.1 to 11.1 months (hazard ratio = 0.62, P < 0.001)[21]. Moreover, com
In limited-stage disease (LS-SCLC), concurrent chemoradiotherapy remains the cornerstone of treatment, often followed by thoracic radiotherapy and prophylactic cranial irradiation in patients achieving complete response to initial therapy[19,25]. Recently, durvalumab has been explored as consolidation therapy after chemoradiation, showing long-term survival benefits[26]. Furthermore, ongoing clinical trials are evaluating the synergistic effects of immunotherapy and radiotherapy in both ES-SCLC and LS-SCLC[27].
Regarding second-line therapies, topotecan and amrubicin (the latter primarily used in Japan) remain traditional options. However, lurbinectedin, a transcriptional inhibitor with alkylating activity, has been recently approved for its efficacy in patients with relapsed metastatic SCLC, also demonstrating promising results in combination with immunotherapy[19,28,29].
In parallel, translational research is actively exploring novel therapeutic targets and personalized strategies. Notable approaches include inhibitors of aurora kinase A, cyclin-dependent kinase 7, and poly (ADP-ribose) polymerase, as well as agents targeting delta-like ligand 3 such as the bispecific T-cell engager tarlatamab[30-32]. In addition, emerging modalities such as antibody–drug conjugates and cellular therapies, including CAR-T cells targeting SCLC-specific antigens, are currently under preclinical and clinical investigation[33,34]. These novel strategies hold promises for expanding the therapeutic arsenal and improving outcomes for patients with SCLC.
The most common organs for metastasis are the brain, bone tissue, liver, adrenal glands, and lymph nodes[35]. Gastrointestinal tract metastases are rare but can occur in advanced stages, particularly in the stomach, where they may cause both nonspecific symptoms and upper gastrointestinal bleeding[36,37].
Pancreatic metastases are extremely rare, at least in the published scientific literature. In our literature search, we found only about 50 cases to date, including a case series of 14 patients[38]. This highly atypical situation means that clinical guidelines do not mention this condition, making its management particularly challenging. The different cases reported in our literature research have been summarized in Table 1.
| Ref. | Year | Article type | Diagnosis | Treatment | Outcomes |
| Levine and Danovitch[55] | 1973 | Case report | SCLC with pancreatic metastasis | Conservative management | Resolution of AP with supportive care; SCLC diagnosis confirmed later |
| Yeung et al[56] | 1979 | Case report | SCLC with metastasis-induced AP | Conservative management | Resolution of AP after conservative treatment |
| Schmitt[57] | 1985 | Case report | Metastatic SCLC with pancreatitis and diabetes | Supportive care | Clinical presentation of both pancreatitis and diabetes |
| Allan et al[58] | 1985 | Case report | SCLC with associated AP | Conservative management | Resolution of AP with conservative therapy |
| Hall et al[59] | 1987 | Case report | Oat cell carcinoma with AP | Symptomatic treatment | Diagnosis of AP revealed metastatic SCLC |
| Noseda et al[40] | 1987 | Case report | AP as sole manifestation of SCLC | Conservative management | AP resolved with supportive care |
| Evans et al[60] | 1988 | Case report | Necrotizing pancreatitis and diabetes due to metastatic SCLC | Supportive care | Associated with disseminated disease |
| Chowhan et al[61] | 1990 | Case report | Metastasis-induced AP | Chemotherapy | Symptom resolution after treatment |
| Maclennan et al[62] | 1993 | Case report | SCLC-induced AP | Chemotherapy | Symptom resolution |
| Stewart et al[63] | 1993 | Case report | SCLC presenting as AP | Conservative management | Symptom resolution; SCLC diagnosis confirmed |
| Huang et al[64] | 2005 | Case report | AP and Budd-Chiari syndrome due to SCLC | Supportive and systemic therapy | Initial manifestation of SCLC |
| Tanaka et al[65] | 2009 | Case report | Metastasis-induced AP from SCLC | Conservative management | Resolution of AP |
| Wurm Johansson et al[41] | 2012 | Case report | Pancreatic metastases from SCLC causing AP | Conservative management | AP resolved; detected by EUS |
| Hussain et al[66] | 2012 | Case report | SCLC presenting as AP | Conservative management | Resolution of AP |
| Leung et al[67] | 2013 | Case report | SCLC metastasis-induced pancreatitis | Supportive care | AP resolved with management |
| Khan et al[68] | 2014 | Case report | SCLC with AP as initial manifestation | Conservative management | Improved with supportive care |
| Okutur et al[51] | 2015 | Case report | Metastasis-induced AP in SCLC | Chemotherapy (cisplatin and irinotecan) + radiotherapy | Resolution of AP; tumor control at 6 months |
| Hernanz et al[69] | 2016 | Case report | Pancreatitis as first manifestation of SCLC | Conservative management | Favorable outcome |
| Yu et al[38] | 2019 | Retrospective study | SCLC with AP due to metastases | Systemic chemotherapy vs supportive care alone | Chemotherapy improved survival |
| Zhang et al[50] | 2023 | Case report | Recurrent SCLC transformation with AP after adenocarcinoma surgery | Radiotherapy | Resolution of AP; tumor control at 6 months |
| Jing et al[46] | 2024 | Case report | Lung adenocarcinoma with SCLC transformation causing AP | Chemotherapy (etoposide + cisplatin) | Resolution of AP; transformation confirmed |
| Xiao and Gu[47] | 2025 | Case report | Refractory SCLC with pancreatic metastasis | Chemotherapy + PD-1 (albumin with paclitaxel + atezolizumab) | Resolution of AP; tumor control at 3 months |
In this case, as in the reviewed literature, the clinical presentation is indistinguishable from any other type of pan
Once other potential causes were ruled out, and considering the limitations in performing additional tests as outlined later, imaging played a pivotal role in guiding the diagnostic process. In our patient, the performance of the US and its comparison with the previously performed CT scan was considered enough for establishing diagnosis. However, other tests may be required, as outlined in the literature. For example, the case presented by Noseda et al[40] required the use of ERCP for diagnosis[40]. Another report by Wurm Johansson et al[41] required the use of EUS to establish the diagnosis. The choice of one technique over the other will always depend on the clinical context, as well as the need for biopsy if there is diagnostic uncertainty and the necessity of performing therapeutic procedures.
Due to the unusual nature of this etiology of AP, its management is not addressed in the current clinical guidelines[5,42-45]. In the literature, only case reports are published with the management deemed most appropriate. According to our scientific literature research, there is only a retrospective case series of 14 patients in which chemotherapy plus palliative treatment was compared to palliative care alone. Although it lacks significant statistical power, this study showed that patients treated with chemotherapy had better outcomes[38]. The results align with most published clinical cases, in which chemotherapy treatment yields favorable results. However, the regimens used are often not detailed in the reported cases. The regimens employed are typically platinum-based, such as etoposide and cisplatin[46]; or iri
Radiotherapy has also been described as an adjunct to chemotherapy with promising results. Its use is documented in both SCLC treatment regimens and secondary metastases[35,48,49]. Although not commonly used, in cases of AP where low-dose radiotherapy has been combined with chemotherapy, as well as in subsequent consolidation treatment, positive results have been reported, including a reduction in lesion size[47,50,51].
In our case, we initially opted for a conservative management with absolute diet and analgesia. The response was rapid with the disappearance of pain. Once we attempted to reintroduce oral intake, pain and analytical alterations secondary to AP were observed. After several attempts, due to suspicion of the origin of the AP, a consultation with the oncology team was made. The case was discussed in a multidisciplinary committee. Two options were considered to confirm the diagnosis. One was a pancreatic MRI, but the US image was considered sufficient for diagnostic purposes. Another option was EUS with the intent to confirm the diagnosis. However, when this option was presented to the patient, it was rejected as they did not want to undergo invasive procedures unless absolutely necessary. Additionally, the anesthetist also assessed the case and commented that the anesthetic risk was very high, so unless it was essential for diagnosis and subsequent treatment, it would be better to avoid any invasive procedure.
Given the suspicion of AP secondary to pancreatic metastases and the need to initiate treatment for SCLC, che
If the patient had not responded, the consensus plan would have been to perform EUS to confirm the diagnosis of pancreatic metastases from a pulmonary origin. After confirmation, both radiotherapy and atezolizumab, a programmed death-ligand 1 (PD-L1) inhibitor approved for use in SCLC in combination with chemotherapy, would have been added. Atezolizumab was initiated later, so the plan would have been to advance this therapeutic option.
Other therapeutic options may be explored in the future for the management of these patients. The emergence and development of ICIs could be a possibility. In addition to atezolizumab, other PD-L1 inhibitors such as durvalumab, nivolumab, or pembrolizumab have been used in the management of SCLC[35,52]. Additionally, improvements in the precision and doses administered through stereotactic body radiation therapy may also serve as an alternative to conventional radiotherapy. It has not yet been used in this context. The number of pancreatic lesions, their location, and pro
AP secondary to metastases from SCLC is an extremely rare condition, and its management is not yet clearly defined. Oncological treatments, especially chemotherapy, may be considered among the therapeutic options for the management of these patients. However, further studies are needed to establish a standardized management protocol for this entity.
This article was sponsored by Virgen de la Luz Hospital and Chair of Artificial Intelligence by Bayer.
| 1. | Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial Medical Treatment of Acute Pancreatitis: American Gastroenterological Association Institute Technical Review. Gastroenterology. 2018;154:1103-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (1)] |
| 2. | Ouyang G, Pan G, Liu Q, Wu Y, Liu Z, Lu W, Li S, Zhou Z, Wen Y. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. BMC Med. 2020;18:388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, Coward S, Forbes N, Heitman SJ, Shaheen AA, Swain M, Buie M, Underwood FE, Kaplan GG. Global Incidence of Acute Pancreatitis Is Increasing Over Time: A Systematic Review and Meta-Analysis. Gastroenterology. 2022;162:122-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 483] [Article Influence: 120.8] [Reference Citation Analysis (1)] |
| 4. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 526] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 5. | Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, Yadlapati RH, Gardner TB. American College of Gastroenterology Guidelines: Management of Acute Pancreatitis. Am J Gastroenterol. 2024;119:419-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 201] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 6. | Pu W, Luo G, Chen T, Jing L, Hu Q, Li X, Xia H, Deng M, Lü M, Chen X. A 5-Year Retrospective Cohort Study: Epidemiology, Etiology, Severity, and Outcomes of Acute Pancreatitis. Pancreas. 2020;49:1161-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Zilio MB, Eyff TF, Azeredo-Da-Silva ALF, Bersch VP, Osvaldt AB. A systematic review and meta-analysis of the aetiology of acute pancreatitis. HPB (Oxford). 2019;21:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 8. | Lai T, Li J, Zhou Z, Rao J, Zhu Y, Xia L, Lei Y, Huang X, Ke H, Wu Y, Liu P, Zeng H, Xiong H, Luo L, Chen Y, He W, Zhu Y, Lu N. Etiological Changes and Prognosis of Hospitalized Patients with Acute Pancreatitis Over a 15-Year Period. Dig Dis Sci. 2024;69:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Cui YF. Severe acute pancreatitis complicated with intra-abdominal infection secondary to trauma: A case report. World J Clin Cases. 2024;12:5821-5831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 10. | Zheng L, Zhao P, Peng X, Zhou Y, Bao Y, Sun Y, Zhou L. Clinical characteristic and pathogenesis of tumor-induced acute pancreatitis: a predictive model. BMC Gastroenterol. 2022;22:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Tejedor Bravo M, Justo LM, Lasala JP, Moreira Vicente VF, Ruiz AC, Scapa Mde L. Acute pancreatitis secondary to neuroendocrine pancreatic tumors: report of 3 cases and literature review. Pancreas. 2012;41:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Niccolini DG, Graham JH, Banks PA. Tumor-induced acute pancreatitis. Gastroenterology. 1976;71:142-145. [PubMed] [DOI] [Full Text] |
| 13. | Akpinar B, Obuch J, Fukami N, Pokharel SS. Unusual presentation of a pancreatic cyst resulting from osteosarcoma metastasis. World J Gastroenterol. 2015;21:8452-8457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Mori T, Kondo H, Naitoh I, Koyama T, Takenaka Y, Komai H, Araki S, Kitagawa M, Nishigaki N, Tanaka Y, Itoh K, Hasegawa C, Kawai T, Hayashi K. Endoscopic Ultrasonography-guided Fine-needle Aspiration Revealed Metastasis-induced Acute Pancreatitis in a Patient with Adrenocortical Carcinoma. Intern Med. 2019;58:2645-2649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J, Califano R, Nishio M, Orlandi F, Alatorre-Alexander J, Leal T, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol. 2021;39:619-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 621] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 16. | Megyesfalvi Z, Gay CM, Popper H, Pirker R, Ostoros G, Heeke S, Lang C, Hoetzenecker K, Schwendenwein A, Boettiger K, Bunn PA Jr, Renyi-Vamos F, Schelch K, Prosch H, Byers LA, Hirsch FR, Dome B. Clinical insights into small cell lung cancer: Tumor heterogeneity, diagnosis, therapy, and future directions. CA Cancer J Clin. 2023;73:620-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 224] [Article Influence: 74.7] [Reference Citation Analysis (1)] |
| 17. | Uprety D, Seaton R, Niroula A, Hadid T, Parikh K, Ruterbusch JJ. Trends in the Incidence and Survival Outcomes in Patients With Small Cell Lung Cancer in the United States: An Analysis of the SEER Database. Cancer Med. 2025;14:e70608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Cittolin-Santos GF, Knapp B, Ganesh B, Gao F, Waqar S, Stinchcombe TE, Govindan R, Morgensztern D. The changing landscape of small cell lung cancer. Cancer. 2024;130:2453-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 1053] [Article Influence: 210.6] [Reference Citation Analysis (1)] |
| 20. | Zhu M, Huang Y, Bender ME, Girard L, Kollipara R, Eglenen-Polat B, Naito Y, Savage TK, Huffman KE, Koyama S, Kumanogoh A, Minna JD, Johnson JE, Akbay EA. Evasion of Innate Immunity Contributes to Small Cell Lung Cancer Progression and Metastasis. Cancer Res. 2021;81:1813-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 21. | Falchero L, Meyer N, Molinier O, Al Freijat F, Pegliasco H, Lecuyer E, Stoven L, Belmont L, Loutski S, Maincent C, Blanchet-Legens AS, Mairovitz A, Meniai F, Hominal S, Letierce A, Morel H, Debieuvre D. Real-life nationwide characteristics and outcomes of small cell lung cancer over the last 20 years: Impact of immunotherapy on overall survival in a real-life setting. Eur J Cancer. 2024;210:114277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Erciyestepe M, Ekinci ÖB, Doğan HGY, Öztürk AE, Aydın O, Büyükkuşcu A, Atasever T, Uslu BS, Akkaya K, Çelik E, Ertürk K, Atcı MM. Factors affecting survival and prognosis in extensive stage small cell lung cancer. BMC Pulm Med. 2025;25:160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW; CASPIAN investigators. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1584] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 24. | National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. 2008. [cited 13 June 2025]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/occult.pdf. |
| 25. | Shields MD, Chiang AC, Byers LA. Top advances of the year: Small cell lung cancer. Cancer. 2025;131:e35770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Park S, Noh JM, Choi YL, Chi SA, Kim K, Jung HA, Lee SH, Ahn JS, Ahn MJ, Sun JM. Durvalumab with chemoradiotherapy for limited-stage small-cell lung cancer. Eur J Cancer. 2022;169:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, Bezjak A, Cardenal F, Fournel P, Harden S, Le Pechoux C, McMenemin R, Mohammed N, O'Brien M, Pantarotto J, Surmont V, Van Meerbeeck JP, Woll PJ, Lorigan P, Blackhall F; CONVERT Study Team. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 432] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 28. | Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin Proc. 2019;94:1599-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 29. | Trigo J, Subbiah V, Besse B, Moreno V, López R, Sala MA, Peters S, Ponce S, Fernández C, Alfaro V, Gómez J, Kahatt C, Zeaiter A, Zaman K, Boni V, Arrondeau J, Martínez M, Delord JP, Awada A, Kristeleit R, Olmedo ME, Wannesson L, Valdivia J, Rubio MJ, Anton A, Sarantopoulos J, Chawla SP, Mosquera-Martinez J, D'Arcangelo M, Santoro A, Villalobos VM, Sands J, Paz-Ares L. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 330] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 30. | Petty WJ, Paz-Ares L. Emerging Strategies for the Treatment of Small Cell Lung Cancer: A Review. JAMA Oncol. 2023;9:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 118] [Reference Citation Analysis (0)] |
| 31. | Rudin CM, Reck M, Johnson ML, Blackhall F, Hann CL, Yang JC, Bailis JM, Bebb G, Goldrick A, Umejiego J, Paz-Ares L. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J Hematol Oncol. 2023;16:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 32. | Grenda A, Krawczyk P, Obara A, Gajek Ł, Łomża-Łaba A, Milanowski J. Transitioning to a Personalized Approach in Molecularly Subtyped Small-Cell Lung Cancer (SCLC). Int J Mol Sci. 2024;25:4208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 33. | Guaitoli G, Neri G, Cabitza E, Natalizio S, Mastrodomenico L, Talerico S, Trudu L, Lauro C, Chiavelli C, Baschieri MC, Bruni A, Dominici M, Bertolini F. Dissecting Immunotherapy Strategies for Small Cell Lung Cancer: Antibodies, Ionizing Radiation and CAR-T. Int J Mol Sci. 2022;23:12728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Patel SR, Das M. Small Cell Lung Cancer: Emerging Targets and Strategies for Precision Therapy. Cancers (Basel). 2023;15:4016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M, Florsheim C, Gold KA, Goldman JW, Grecula JC, Hann C, Iams W, Iyengar P, Kelly K, Khalil M, Koczywas M, Merritt RE, Mohindra N, Molina J, Moran C, Pokharel S, Puri S, Qin A, Rusthoven C, Sands J, Santana-Davila R, Shafique M, Waqar SN, Gregory KM, Hughes M. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:1441-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 317] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 36. | Gao S, Hu XD, Wang SZ, Liu N, Zhao W, Yu QX, Hou WH, Yuan SH. Gastric metastasis from small cell lung cancer: a case report. World J Gastroenterol. 2015;21:1684-1688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 37. | Nguyen NTY, Luong TV, Nguyen DX, Le LD, Dang HNN. Understanding gastric metastasis of small cell lung carcinoma: Insights from case reports and clinical implications. World J Gastroenterol. 2024;30:5092-5096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Yu M, Zheng L, Han D, Wang Y, Ren L, Lu Y, Zhang S. Systemic Chemotherapy in Metastasis-Induced Acute Pancreatitis Patients With Small Cell Lung Cancer. Pancreas. 2019;48:1303-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Jiang C, Zhao M, Hou S, Hu X, Huang J, Wang H, Ren C, Pan X, Zhang T, Wu S, Zhang S, Sun B. The Indicative Value of Serum Tumor Markers for Metastasis and Stage of Non-Small Cell Lung Cancer. Cancers (Basel). 2022;14:5064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Noseda A, Gangji D, Cremer M. Acute pancreatitis as presenting symptom and sole manifestation of small cell lung carcinoma. Dig Dis Sci. 1987;32:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Wurm Johansson G, Toth E, Torp J, Ehinger A, Andersson L, Thorlacius H. Acute pancreatitis evoked by small-cell lung carcinoma metastases and detected by endoscopic ultrasound. Endoscopy. 2012;44 Suppl 2 UCTN:E45-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1093] [Article Influence: 84.1] [Reference Citation Analysis (10)] |
| 43. | Crockett SD, Wani S, Gardner TB, Falck-Ytter Y, Barkun AN; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Initial Management of Acute Pancreatitis. Gastroenterology. 2018;154:1096-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 594] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 44. | Takada T, Isaji S, Mayumi T, Yoshida M, Takeyama Y, Itoi T, Sano K, Iizawa Y, Masamune A, Hirota M, Okamoto K, Inoue D, Kitamura N, Mori Y, Mukai S, Kiriyama S, Shirai K, Tsuchiya A, Higuchi R, Hirashita T. JPN clinical practice guidelines 2021 with easy-to-understand explanations for the management of acute pancreatitis. J Hepatobiliary Pancreat Sci. 2022;29:1057-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 45. | Beij A, Verdonk RC, van Santvoort HC, de-Madaria E, Voermans RP. Acute Pancreatitis: An Update of Evidence-Based Management and Recent Trends in Treatment Strategies. United European Gastroenterol J. 2025;13:97-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 46. | Jing Y, Li X, Sun X, Ren M, Xiao R, Zhao J, Liu Z. Case report: Acute pancreatitis in lung adenocarcinoma with small cell transformation after multiple line targeted therapy. Front Oncol. 2024;14:1274034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 47. | Xiao Z, Gu Y. Refractory small cell lung cancer with pancreatic metastasis: A case report. Medicine (Baltimore). 2025;104:e41167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 48. | Chun SG, Simone CB 2nd, Amini A, Chetty IJ, Donington J, Edelman MJ, Higgins KA, Kestin LL, Movsas B, Rodrigues GB, Rosenzweig KE, Slotman BJ, Rybkin II, Wolf A, Chang JY. American Radium Society Appropriate Use Criteria: Radiation Therapy for Limited-Stage SCLC 2020. J Thorac Oncol. 2021;16:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Daly ME, Ismaila N, Decker RH, Higgins K, Owen D, Saxena A, Franklin GE, Donaldson D, Schneider BJ. Radiation Therapy for Small-Cell Lung Cancer: ASCO Guideline Endorsement of an ASTRO Guideline. J Clin Oncol. 2021;39:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 50. | Zhang S, Guo N, Zhang Q, Wang Y, Yang S, Chen X. Case report: Clinical management of recurrent small cell lung cancer transformation complicated with lung cancer-induced acute pancreatitis after lung adenocarcinoma surgery. Front Pharmacol. 2023;14:1259221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 51. | Okutur K, Bozkurt M, Korkmaz T, Karaaslan E, Guner L, Goksel S, Demir G. Metastasis-Induced Acute Pancreatitis Successfully Treated with Chemotherapy and Radiotherapy in a Patient with Small Cell Lung Cancer. Case Rep Oncol Med. 2015;2015:304279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Zhou F, Zhao W, Gong X, Ren S, Su C, Jiang T, Zhou C. Immune-checkpoint inhibitors plus chemotherapy versus chemotherapy as first-line treatment for patients with extensive-stage small cell lung cancer. J Immunother Cancer. 2020;8:e001300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Dahiya DS, Chandan S, Ali H, Pinnam BSM, Gangwani MK, Al Bunni H, Canakis A, Gopakumar H, Vohra I, Bapaye J, Al-Haddad M, Sharma NR. Role of Therapeutic Endoscopic Ultrasound in Management of Pancreatic Cancer: An Endoscopic Oncologist Perspective. Cancers (Basel). 2023;15:3235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Cazacu IM, Singh BS, Saftoiu A, Bhutani MS. Endoscopic Ultrasound-Guided Treatment of Pancreatic Cancer. Curr Gastroenterol Rep. 2020;22:27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Levine M, Danovitch SH. Metastatic carcinoma to the pancreas. Another cause for acute pancreatitis. Am J Gastroenterol. 1973;60:290-294. [PubMed] |
| 56. | Yeung KY, Haidak DJ, Brown JA, Anderson D. Metastasis-induced acute pancreatitis in small cell bronchogenic carcinoma. Arch Intern Med. 1979;139:552-554. [PubMed] |
| 57. | Schmitt JK. Pancreatitis and diabetes mellitus with metastatic pulmonary oat-cell carcinoma. Ann Intern Med. 1985;103:638-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Allan SG, Bundred N, Eremin O, Leonard RC. Acute pancreatitis in association with small cell lung carcinoma: potential pitfall in diagnosis and management. Postgrad Med J. 1985;61:643-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Hall M, Bundred NJ, Hall AW. Oat cell carcinoma of the bronchus and acute pancreatitis. Eur J Surg Oncol. 1987;13:371-372. [PubMed] |
| 60. | Evans AT. Necrotising pancreatitis and diabetes associated with disseminated small cell carcinoma of lung. Scott Med J. 1988;33:377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Chowhan NM, Madajewicz S. Management of metastases-induced acute pancreatitis in small cell carcinoma of the lung. Cancer. 1990;65:1445-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 62. | Maclennan AC, Macleod IA. Case report: small cell carcinoma induced acute pancreatitis. Br J Radiol. 1993;66:161-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 63. | Stewart KC, Dickout WJ, Urschel JD. Metastasis-induced acute pancreatitis as the initial manifestation of bronchogenic carcinoma. Chest. 1993;104:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Huang YW, Yang JC, Chang YL, Tsang YM, Wang TH. Acute pancreatitis combined with acute Budd-Chiari syndrome as the initial manifestation of small cell lung cancer. J Formos Med Assoc. 2005;104:431-435. [PubMed] |
| 65. | Tanaka H, Nakazawa T, Yoshida M, Miyabe K, Okumura F, Naitoh I, Hayashi K, Ando T, Ohara H, Joh T. Metastasis-induced acute pancreatitis in a patient with small cell carcinoma of the lungs. JOP. 2009;10:557-561. [PubMed] |
| 66. | Hussain A, Adnan A, El-Hasani S. Small cell carcinoma of the lung presented as acute pancreatitis. Case report and review of the literature. JOP. 2012;13:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 67. | Leung E, Prasher A, Francombe J, Brocklebank A, Joy H. Metastasis-induced pancreatitis: case report. Prague Med Rep. 2013;114:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Khan M, Luni F, Kamal S, Alastal Y, Alwardia A, Bieszczad J, Casas L, Yoon Y. Acute Pancreatitis: First and Sole Manifestation of Small Cell Carcinoma of Lung. J Med Cases. 2014;5:525-528. |
| 69. | Hernanz Ruiz N, Téllez Villajos L, Ferre Aracil C, Martínez González J. Metastasis-induced Acute pancreatitis as the initial presentation of a small cell lung carcinoma. Rev Colomb Cancerol. 2016;20:37-39. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/