Published online Sep 16, 2025. doi: 10.12998/wjcc.v13.i26.107496
Revised: April 29, 2025
Accepted: June 7, 2025
Published online: September 16, 2025
Processing time: 114 Days and 19.5 Hours
Metastasis of breast cancer usually affects the lungs, bones, liver, and brain. It rarely spreads to the gastrointestinal tract, and cases with similar endoscopic ma
A 52-year-old woman presented with a history of invasive lobular carcinoma and experienced metastasis of breast cancer to the gastrointestinal tract. The patient underwent a left mastectomy and tumor cells were positive for estrogen receptor (ER) and progesterone receptor (PR), negative for human epidermal growth factor receptor 2 (HER2) and E-cadherin. She did not experience any local or distant recurrences for four years following the mastectomy, chemoradiotherapy, and hormone therapy. However, the patient complained of upper abdominal discom
Distinguishing metastatic breast cancer from primary gastrointestinal lesions is crucial for initiating the correct treatment and enhancing the quality of life for patients.
Core Tip: It is uncommon for breast cancer to spread simultaneously to the stomach and intestinal tract. We report a case of a 52-year-old woman with metastatic lobular carcinoma in the gastrointestinal tract. This case highlights the critical role of multidisciplinary collaboration. Endoscopic biopsy combined with immunohistochemistry is essential to differentiate metastatic breast cancer from primary gastrointestinal malignancies, guiding timely systemic therapy.
- Citation: Liu PP, Sun LL, Jing X. Gastrointestinal metastasis from invasive lobular carcinoma following breast cancer treatment: A case report. World J Clin Cases 2025; 13(26): 107496
- URL: https://www.wjgnet.com/2307-8960/full/v13/i26/107496.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i26.107496
Breast cancer is the most common malignant disease and the leading cause of cancer-related death in women[1]. The tissue form of breast cancer has the highest number of invasive ductal carcinoma (IDC), while invasive lobular carcinoma (ILC) accounting for 5%-15% of invasive breast cancers is the second most common subtype of breast cancer[2]. ILC can be difficult to be detected clinically and radiographically, and consequently further biopsies are necessary[3]. Histologically, ILC is made up of non-cohesive cells scattered in a single-file linear pattern in a stroma[4]. The positivity rate of estrogen receptor (ER) in ILC is 80%-95%, and that of progesterone receptor (PR) is approximately 70%, while the human epidermal growth factor receptor 2 (HER2) overexpression is rare in ILC[3]. The comprehensive treatment includes surgical treatment, hormonal therapy, radiotherapy, and chemotherapy. The mutations of germline cadherin 1 gene and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene and E-cadherin inactivation are considered significant in the development of lobular breast cancer[5]. The distant metastasis rate of ILC is higher, but there is no significant difference in overall survival rate compared to IDC[6]. Lobular carcinoma metastasizes to gastrointestinal tract more often than IDC[7]. However, similar endoscopic manifestations between the involved gastric and colorectal lesions are even more infrequent. Advanced patients often have a frequent recurrence and can die from the disease[8]. Our report describes a 52-year-old woman presenting with metastatic lobular carcinoma involving the gastrointestinal tract four years following a left mastectomy, chemoradiotherapy, and hormone therapy for lobular carcinoma of the breast.
A 52-year-old female patient was referred to our hospital with a chief complaint of upper abdominal discomfort on January 29, 2023.
On January 9, 2023, the patient experienced epigastric discomfort without obvious triggers, which became worse after meals, without nausea, vomiting, acid reflux, heartburn, abdominal pain, diarrhea, dry stool, and blood in the stool. Her general medical history was unremarkable.
The patient was diagnosed with a left breast cancer four years ago. The right side of the breast and other parts of the body showed no significant abnormalities on imaging examinations. She underwent a left mastectomy and axillary dissection on January 30, 2019. The histologic type of breast cancer was ILC (pT2, N2a, M0, luminal B). In postoperative immunohistochemistry (IHC), tumor cells were positive for ER and PR, and negative for HER2 and E-cadherin. She received adjuvant chemotherapy with epirubicin and cyclophosphamide every 21 days for four cycles and paclitaxel liposomal for injection every 21 days for another four cycles, starting on March 9, 2019. In addition, she received a total of 25 times of radiotherapy beginning on September 10, 2019, and commenced hormonal therapy with an aromatase inhibitor on the same day. Four years after chemotherapy, radiation, and hormone therapy, the patient did not experience any local or distant recurrences as confirmed by chest X-ray, computed tomography (CT), and bone scanning.
The patient had unremarkable familial or personal history, with no notable medical conditions or genetic predispositions.
On admission, physical examination revealed epigastric tenderness. No obvious rebound pain and muscle tension in abdomen.
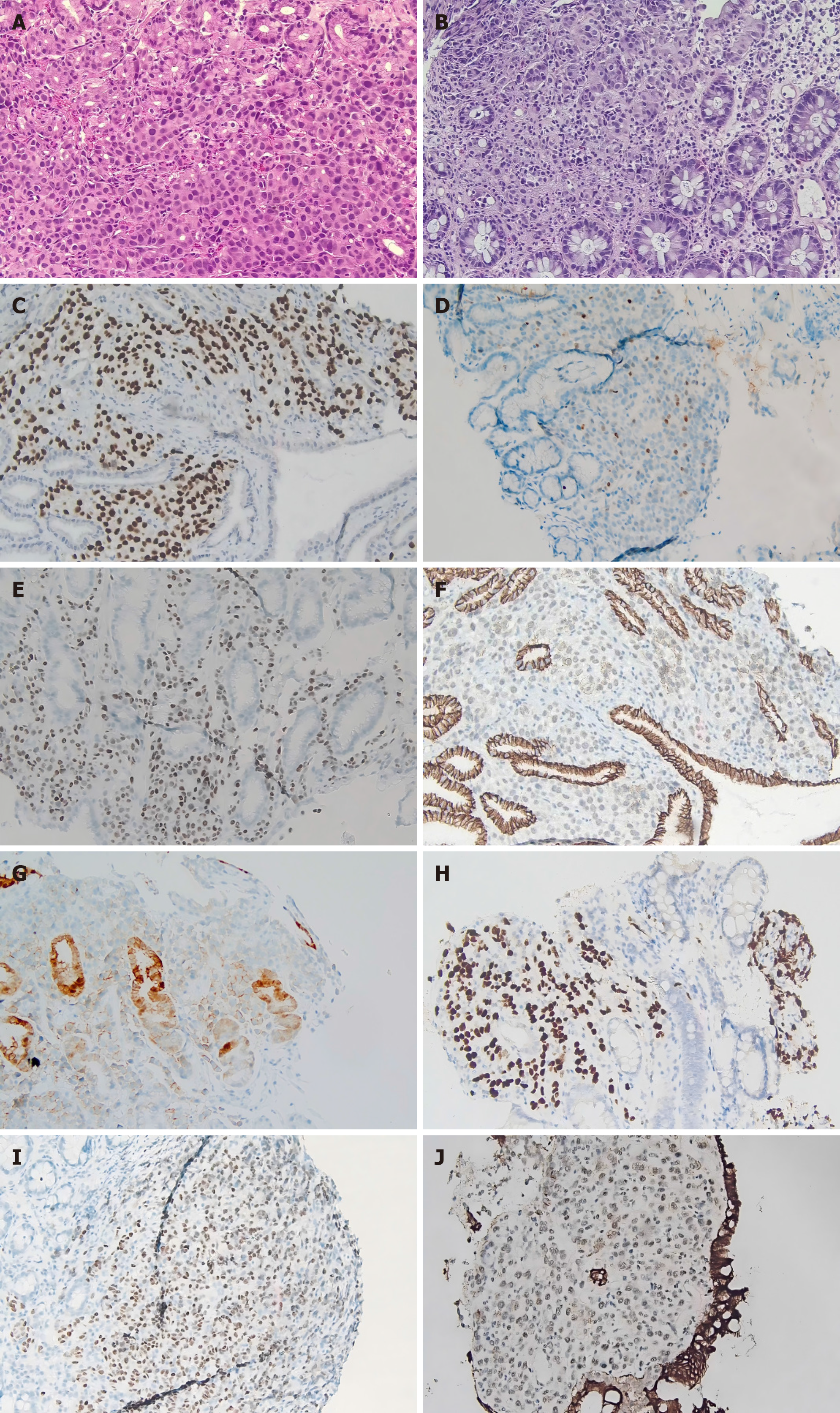
Routine laboratory tests showed no apparent abnormalities. Histological examination revealed poorly differentiated carcinoma with regular glandular structure formation by hematoxylin and eosin staining (Figure 1A and B). Immunohistochemical staining showed that gastric tumor cells were positive for GATA-binding protein 3 (GATA3) (Figure 1C), PR (Figure 1D), ER (Figure 1E), and negative for E-cadherin (Figure 1F), gross cystic disease fluid protein 15, and HER2 (Figure 1G). The colorectum tumor cells were positive for GATA3 (Figure 1H) and ER (Figure 1I) and negative for cytokeratin (CK) 20 and E-cadherin (Figure 1J).
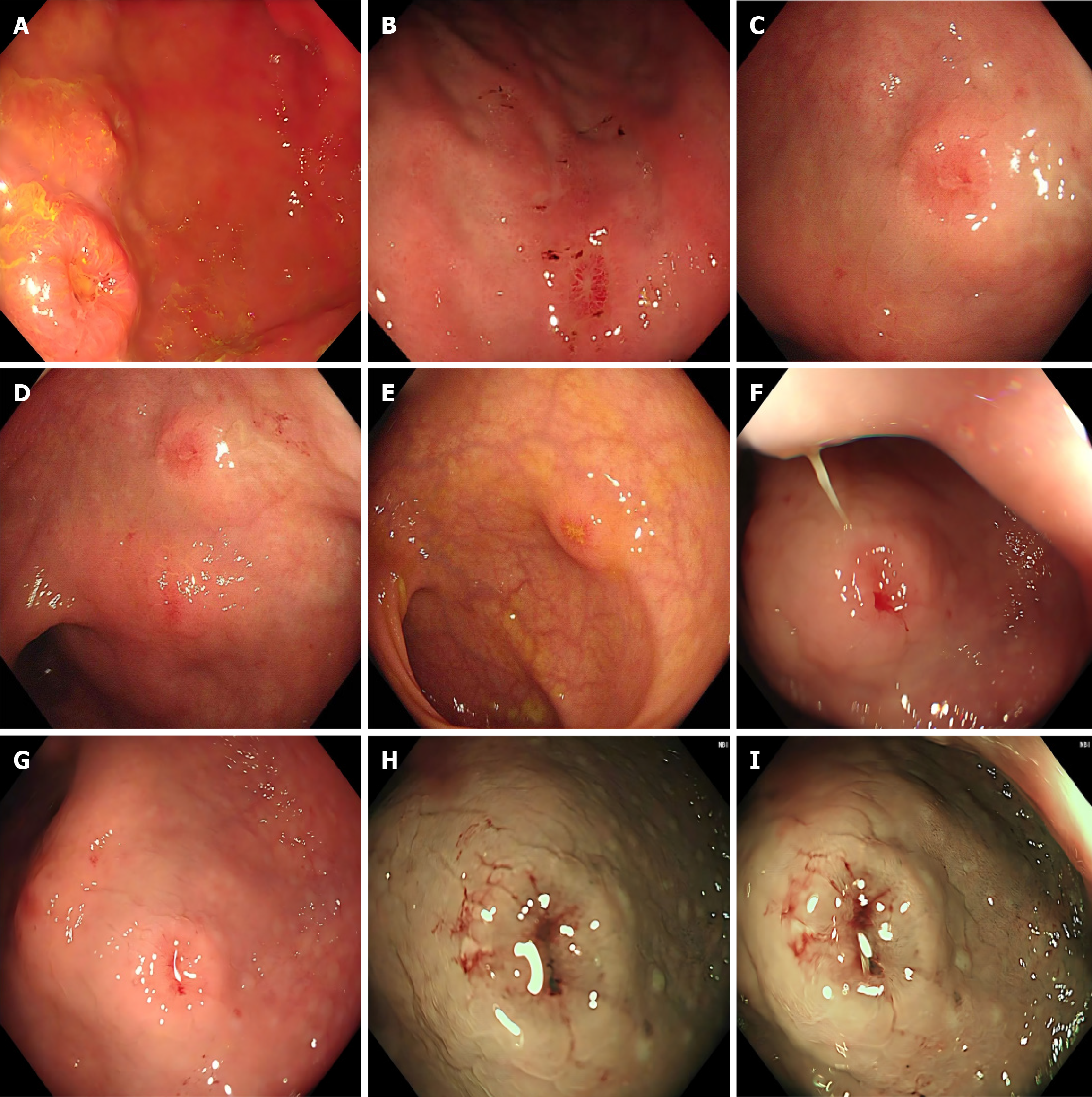
Abdominal dynamic contrast-enhanced CT did not reveal gastrointestinal related lesions. An upper gastrointestinal endoscopy revealed multiple crater-like ulcers scattered throughout the gastric body and antrum (Figure 2A and B). And a lower gastrointestinal endoscopy demonstrated numerous crater-like ulcers in the colorectum (Figure 2C-G). Narrow band imaging (NBI) displayed abnormal microvessels in the ulcer edges (Figure 2H and I), which is more common in malignant lesions. Due to multiple and conspicuous lesions, a biopsy was performed as a precaution.
Based on the patient’s past medical history, endoscopic findings, and histological and immunohistochemical examination results, a final diagnosis of gastrointestinal metastases of ILC of the breast was established.
The patient underwent left mastectomy, 8 cycles of chemotherapy, and 25 sessions of radiotherapy. She is currently on adjuvant toremifene therapy.
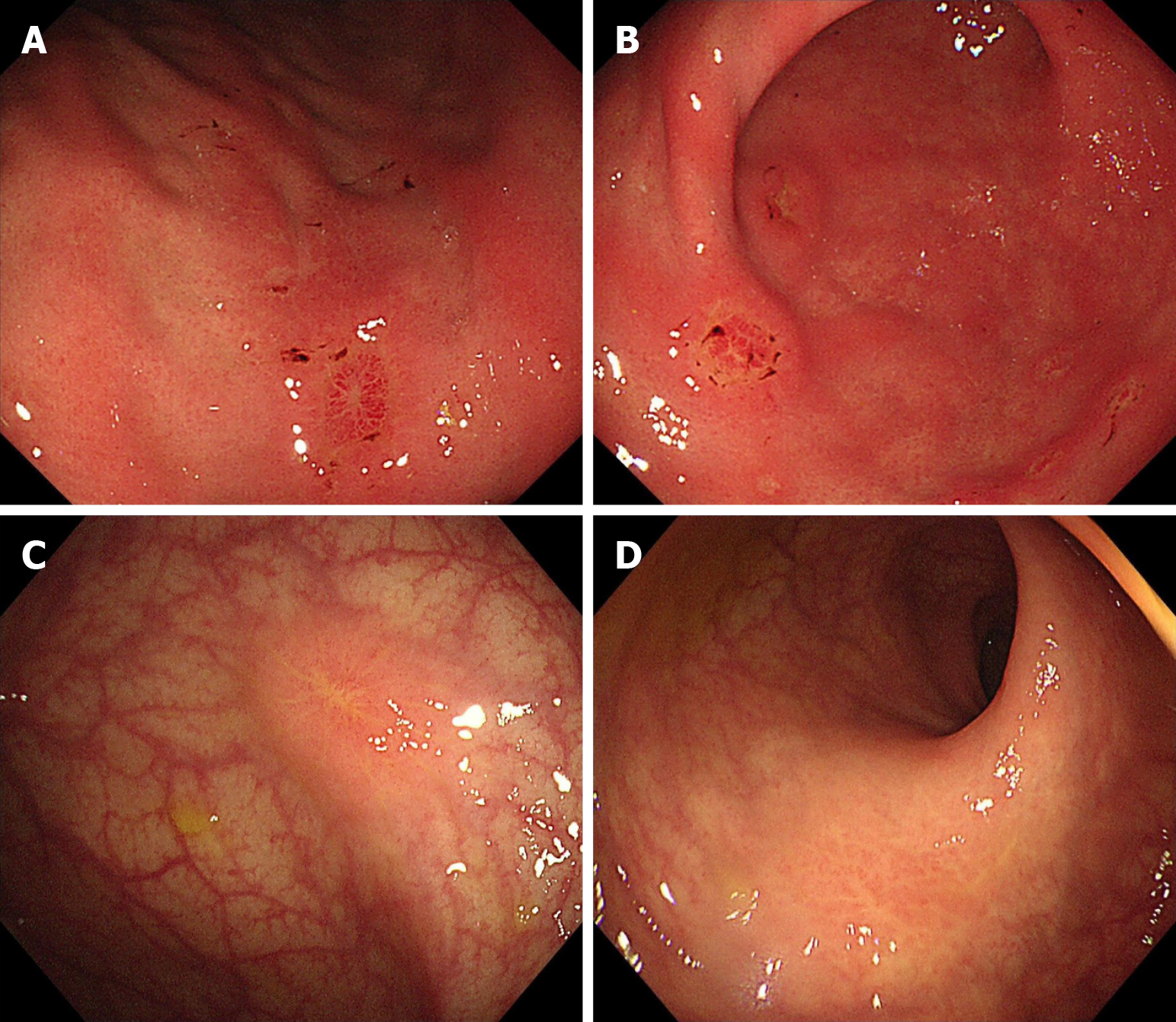
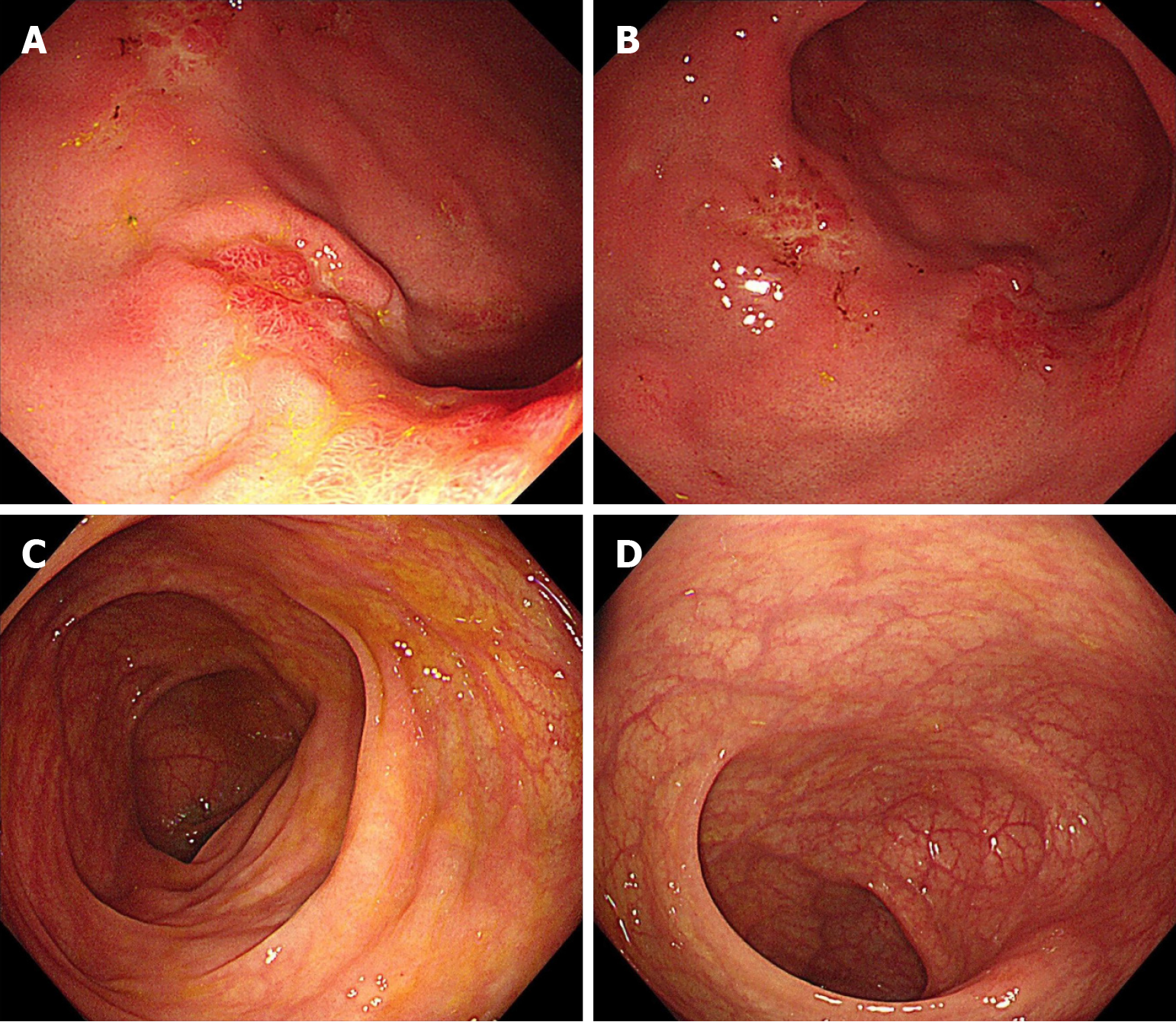
In February 2023, the patient was diagnosed with gastrointestinal metastasis from breast cancer by gastrointestinal endoscopy. Considering the progression of the disease, the patient's treatment with toremifene was discontinued. On February 14, 2023, the patient continued to receive combination therapy with letrozole and palbociclib. In May 2023, the patient's re-examination with gastrointestinal endoscopy suggested that the crater-like ulcers were improved (Figure 3). To achieve medical castration, the patient received a subcutaneous injection of goserelin acetate. To achieve more complete castration, the patient underwent laparoscopic bilateral salpingo-oophorectomy under general anesthesia on December 21, 2023. Histopathological examination of the bilateral ovaries and fallopian tubes revealed poorly differentiated adenocarcinoma. Combined with the patient's medical history and histopathological results, metastatic breast cancer is the most likely diagnosis. Follow-up endoscopy in August 2024 revealed scattered patchy areas of mucosal hyperplasia are observed in the gastric mucosa, accompanied by focal erosive changes, and the patient showed signs of disease progression (Figure 4). The patient received dual-agent therapy consisting of fulvestrant in combination with abemaciclib beginning August 26, 2024. The patient was readmitted in December 2024 due to abdominal distension. Dynamic contrast-enhanced abdominopelvic CT demonstrates patchy nodular thickening of the greater omentum with peritoneal effusion, highly suggestive of peritoneal carcinomatosis. On December 23, 2024, the patient initiated oral chemotherapy with capecitabine and vinorelbine. Dynamic contrast-enhanced lower abdominal CT on February 19, 2025 revealed peritoneal involvement. A therapeutic paracentesis with pigtail catheter placement was performed under ultrasound guidance on March 27, 2025 for ascites drainage. By histopathological examination, malignant tumor cells were found in the patient's ascites. Tumor cells were positive for ER and PR, and negative for HER2 by immunohistochemical staining. Based on comprehensive evaluation, the findings were consistent with peritoneal metastasis from breast cancer. The patient underwent intraperitoneal cisplatin perfusion therapy on April 1, 2025.
The most frequent metastatic sites of breast cancer are the bones, lungs, liver, and brain[9]. It's rare for breast cancer to spread to the gastrointestinal tract, but in autopsies related to breast cancer, it has been reported to occur in about 6%-18% of cases[10]. ILC of the breast is more likely to spread to the gastrointestinal tract than IDC, possibly related to deletion of a protein called E-cadherin[7,10]. E-cadherin is a master regulator contributing to adherence junctions between epithelial cells and has an impact on suppressing tumorigenesis. Some studies have confirmed that the absence of E-cadherin expression has an important connection with the development of lobular lesions[11]. The stomach is the most common site for breast cancer to metastasize in the gastrointestinal tract, accounting for about 60% of cases[12], while colorectal metastasis from breast cancer is less common. Simultaneous metastasis to both the stomach and colorectum is even rarer and is typically reported as individual case studies. The present case is a 52-year-old female patient presenting with metastatic lobular carcinoma involving the gastrointestinal tract. We suggest that doctors performing endoscopy should be vigilant against simultaneous scattered ulcer-like lesions in the gastrointestinal tract. Distinguishing the benign and malignant nature of ulcer lesions is essential. And if the lesions are malignant, it is important to determine whether the tumor is primary or secondary.
We retrieved a case of a 78-year-old woman with gastric metastasis from ILC found a decade after breast cancer surgery[13]. We also retrieved a case of a 40-year-old woman with gastric metastasis from grade 2 ILC two years fo
Patients with gastrointestinal metastasis of breast cancer may not show any symptoms, or their symptoms may be nonspecific. They might experience dyspepsia, loss of appetite, nausea, vomiting, and abdominal pain[15]. These symp
In gastrointestinal endoscopic examinations, we suggest to pay special attention to the following points. Firstly, gastrointestinal lesions often appear as crater-like ulcers when viewed under the endoscope. It is worth noting that these ulcer-like lesions can be either benign or malignant. They can be distinguished based on the characteristics of the ulcer's edge and bottom, the appearance of the base, the shape of gastric movements, and the size and location of the ulcer[16]. Secondly, technologies like high-resolution micro endoscopes, NBI, and magnifying gastroscopes help differentiate between different ulcer properties. When dealing with definite malignant ulcer cases, it's essential to distinguish between primary carcinoma and secondary carcinoma. Adenocarcinoma accounts for over 95% of primary gastric cancers. Among these, the intestinal type, which often forms distinct tumor masses, is composed of highly differentiated tumor cells arranged in tubular or glandular structures, with scattered goblet cells present. The diffuse type, characterized by cells with signet-ring or non-signet-ring morphology, typically exhibits infiltrative growth patterns with diffuse invasion of the gastric wall[17]. Intestinal metastasis of breast cancer is typically diffuse and similar to primary diffuse cancer. Gastric metastases are often described as parietal nodules, polypoid masses, or ulcerated lesions[18]. Thus endoscopic findings are not specific, which can lead to misdiagnosis as primary gastrointestinal tumors.
The endoscopic examination followed by multiple and deep biopsies will drastically improve diagnostic accuracy. Moreover, IHC can help distinguish between primary cancers and breast cancer metastasis. GATA3 expression has a diagnostic sensitivity above 90% in primary breast cancers. If CK7 is strongly expressed, it often represents breast meta
Despite differential diagnosis between primary and metastatic gastric carcinoma, additional factors were considered during the diagnosis of this case. Distinguishing between peptic ulcers and malignant ulcerations should be the diagnostic priority. The pathognomonic symptom of peptic ulcer disease is rhythmic epigastric pain with distinct chronobiological patterns: Gastric ulcer pain typically exacerbates 15-30 minpostprandially, while duodenal ulcer pain characteristically ameliorates with food intake[21]. Endoscopically, peptic ulcers typically present as well-circumscribed round or oval lesions with smooth, clean bases and regular margins[21]. In contrast, malignant ulcers demonstrate irregular, heaped-up borders, frequently associated with exophytic masses and convergence of distorted mucosal folds toward the ulcer bed[22]. Gastric ulcers typically occur in the lesser curvature side of the antrum, and duodenal ulcers are most commonly located in the duodenal bulb[21]. Malignant gastric ulcers exhibit a predilection for the gastric body and are characteristically larger in diameter compared to their benign counterparts[22]. Crohn's disease represents another benign pathology requiring exclusion in the differential diagnosis. The clinical presentation of Crohn's disease encompasses right lower quadrant abdominal pain, involuntary weight loss, chronic non-bloody diarrhea, and complications including enteric fistulae and intra-abdominal abscess formation. Crohn's disease is pathognomonically characterized by longitudinal ulcerations that may involve any segment of the gastrointestinal tract, accompanied by distinctive cobblestone mucosa and non-caseating granulomas on histopathology[23]. Jejunoileal neuroendocrine tumors (NETs) represent a distinct subtype of gastrointestinal neuroendocrine neoplasms characterized by frequent multifocality[24]. Endoscopic findings of Jejunoileal NETs are sometimes similar to this case. However, it has the characteristics of NETs, which is helpful for further differential diagnosis. Carcinoid syndrome is a specific manifestation of NETs, including episodic flushing, watery diarrhea, and wheezing[25]. Monomorphic round cells with abundant eosinophilic cytoplasm and salt and pepper chromatin with rate mitotic figures may be seen in NETs. IHC is positive for cytokeratins, chromogranin A, synaptophysin, somatostatin receptors, and various hormonal receptors in functional tumors[26]. Additionally, the presence of multiple intestinal tract ulcers can be a feature of ischemic enteritis. Multiple ulcers with varied morphologies–including geographic, linear, and coin-like configurations–were observed in the intestinal tract. In contrast, ischemic enteritis results from acute insufficiency of mesenteric arterial blood flow. Computed tomographic angiography is valuable for differential diagnosis[27]. Chronic nonspecific multiple ulcers of the small intestine (CNSU) predominantly affect the ileum, typically presenting with over 20 ulcerative lesions. Gross examination reveals well-demarcated ulcers with shallow, flat bases, exhibiting linear, tall triangular, or geographic configurations. Histopathological evaluation of CNSU demonstrated ulcerations confined to the submucosal layer, associated with mild chronic inflammatory infiltrates[28]. Based on the patient's history of breast carcinoma, endoscopic characteristics, and immunohistochemical profile of the tumor, we conclusively diagnosed the gastrointestinal ulcerative lesions as metastatic breast cancer to the gastroin
It is uncommon for breast cancer to spread simultaneously to the stomach and intestinal tract. Endoscopic biopsy com
| 1. | Newman L. Oncologic anthropology: Global variations in breast cancer risk, biology, and outcome. J Surg Oncol. 2023;128:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 2. | Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, Wei W, Zhang Y, Sun S. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget. 2017;8:27990-27996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 3. | Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6:R149-R156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 610] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 4. | Thomas M, Kelly ED, Abraham J, Kruse M. Invasive lobular breast cancer: A review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin Oncol. 2019;46:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Harrison BT, Nakhlis F, Dillon DA, Soong TR, Garcia EP, Schnitt SJ, King TA. Genomic profiling of pleomorphic and florid lobular carcinoma in situ reveals highly recurrent ERBB2 and ERRB3 alterations. Mod Pathol. 2020;33:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Orvieto E, Maiorano E, Bottiglieri L, Maisonneuve P, Rotmensz N, Galimberti V, Luini A, Brenelli F, Gatti G, Viale G. Clinicopathologic characteristics of invasive lobular carcinoma of the breast: results of an analysis of 530 cases from a single institution. Cancer. 2008;113:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Fernandes GS, Corrêa TS, Carvalho EP, Katz A, Hoff PM. Gastric and endobronchial metastases in a case of lobular breast cancer. Case Rep Oncol. 2013;6:555-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Mersin H, Yildirim E, Gülben K, Berberoğlu U. Is invasive lobular carcinoma different from invasive ductal carcinoma? Eur J Surg Oncol. 2003;29:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Tahara RK, Brewer TM, Theriault RL, Ueno NT. Bone Metastasis of Breast Cancer. Adv Exp Med Biol. 2019;1152:105-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Invento A, Mirandola S, Pellini F, Pollini GP, Grigolato D. Breast cancer and gastrointestinal metastasis. A case report and review of the literature. Ann Ital Chir. 2018;89:153-156. [PubMed] |
| 11. | Schipper K, Seinstra D, Paulien Drenth A, van der Burg E, Ramovs V, Sonnenberg A, van Rheenen J, Nethe M, Jonkers J. Rebalancing of actomyosin contractility enables mammary tumor formation upon loss of E-cadherin. Nat Commun. 2019;10:3800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Ambroggi M, Stroppa EM, Mordenti P, Biasini C, Zangrandi A, Michieletti E, Belloni E, Cavanna L. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer. 2012;2012:439023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Zhao Q, Zhang D, Wang X. Case report: Gastric metastasis of breast cancer. Front Oncol. 2024;14:1430881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Teixeira S, Sousa C, Castro M, Preto AS, Cardoso A, Madureira A. Gastric metastases from invasive lobular carcinoma of the breast: Case report. Radiol Case Rep. 2021;16:372-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Koike K, Kitahara K, Higaki M, Urata M, Yamazaki F, Noshiro H. Clinicopathological features of gastric metastasis from breast cancer in three cases. Breast Cancer. 2014;21:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Koçak E, Kılıç F, Akbal E, Taş A, Köklü S, Filik L, Bıyıkoğlu I, Ergül B. The usefulness of ulcer size and location in the differential diagnosis of benign and malignant gastric ulcer. Wien Klin Wochenschr. 2013;125:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | 17 Ajani JA, D'Amico TA, Bentrem DJ, Corvera CU, Das P, Enzinger PC, Enzler T, Gerdes H, Gibson MK, Grierson P, Gupta G, Hofstetter WL, Ilson DH, Jalal S, Kim S, Kleinberg LR, Klempner S, Lacy J, Lee B, Licciardi F, Lloyd S, Ly QP, Matsukuma K, McNamara M, Merkow RP, Miller AM, Mukherjee S, Mulcahy MF, Perry KA, Pimiento JM, Reddi DM, Reznik S, Roses RE, Strong VE, Su S, Uboha N, Wainberg ZA, Willett CG, Woo Y, Yoon HH, McMillian NR, Stein M. Gastric Cancer, Version 2.2025, NCCN Clinical Practice Guidelines In Oncology. J Natl Compr Canc Netw. 2025;23:169-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 18. | Inoue H, Arita T, Kuriu Y, Shimizu H, Kiuchi J, Yamamoto Y, Konishi H, Morimura R, Shiozaki A, Ikoma H, Kubota T, Fujiwara H, Okamoto K, Otsuji E. Colonic Metastasis from Breast Cancer: A Case Report and Review of the Literature. In Vivo. 2022;36:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Kaufmann O, Deidesheimer T, Muehlenberg M, Deicke P, Dietel M. Immunohistochemical differentiation of metastatic breast carcinomas from metastatic adenocarcinomas of other common primary sites. Histopathology. 1996;29:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Hong J, Kim Y, Cho J, Lim SW, Park SE, Kim HK, Lee H, Cho SY, Kim JY, Ahn JS, Im YH, Park YH. Clinical features and prognosis of breast cancer with gastric metastasis. Oncol Lett. 2019;17:1833-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | ASGE Standards of Practice Committee; Banerjee S, Cash BD, Dominitz JA, Baron TH, Anderson MA, Ben-Menachem T, Fisher L, Fukami N, Harrison ME, Ikenberry SO, Khan K, Krinsky ML, Maple J, Fanelli RD, Strohmeyer L. The role of endoscopy in the management of patients with peptic ulcer disease. Gastrointest Endosc. 2010;71:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Dinis-Ribeiro M, Libânio D, Uchima H, Spaander MCW, Bornschein J, Matysiak-Budnik T, Tziatzios G, Santos-Antunes J, Areia M, Chapelle N, Esposito G, Fernandez-Esparrach G, Kunovsky L, Garrido M, Tacheci I, Link A, Marcos P, Marcos-Pinto R, Moreira L, Pereira AC, Pimentel-Nunes P, Romanczyk M, Fontes F, Hassan C, Bisschops R, Feakins R, Schulz C, Triantafyllou K, Carneiro F, Kuipers EJ. Management of epithelial precancerous conditions and early neoplasia of the stomach (MAPS III): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG) and European Society of Pathology (ESP) Guideline update 2025. Endoscopy. 2025;57:504-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 50] [Article Influence: 50.0] [Reference Citation Analysis (2)] |
| 23. | 23 ASGE IBD Endoscopy Consensus Panel; Shen B, Abreu MT, Cohen ER, Farraye FA, Fischer M, Feuerstadt P, Kapur S, Ko HM, Kochhar GS, Liu X, Mahadevan U, McBride DL, Navaneethan U, Regueiro M, Ritter T, Sharma P, Lichtenstein GR. Endoscopic diagnosis and management of adult inflammatory bowel disease: a consensus document from the American Society for Gastrointestinal Endoscopy IBD Endoscopy Consensus Panel. Gastrointest Endosc. 2025;101:295-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 24. | Keck KJ, Maxwell JE, Utria AF, Bellizzi AM, Dillon JS, O'Dorisio TM, Howe JR. The Distal Predilection of Small Bowel Neuroendocrine Tumors. Ann Surg Oncol. 2018;25:3207-3213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 529] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 26. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 616] [Article Influence: 154.0] [Reference Citation Analysis (2)] |
| 27. | Hashimoto Y, Endo Y, Kuroki Y, Yoshikumi H, Yoshiba M. Transient ischemic small-bowel ulcers secondary to acute superior mesenteric artery branch thromboembolism diagnosed by double balloon enteroscopy. Endoscopy. 2008;40 Suppl 2:E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Matsumoto T, Iida M, Matsui T, Yao T. Chronic nonspecific multiple ulcers of the small intestine: a proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc. 2007;66:S99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/