Published online Sep 16, 2025. doi: 10.12998/wjcc.v13.i26.106999
Revised: April 29, 2025
Accepted: June 11, 2025
Published online: September 16, 2025
Processing time: 132 Days and 4.1 Hours
Ovarian sclerosing stromal tumors (OSSTs) are found most commonly in females at 20-30 years of age. They can also occur at any point during pre-puberty, puberty, or menopause. Clinical manifestations of OSSTs include abdominal pain, an abdominal mass, menstrual abnormalities, and infertility. Infrequently, patients will experience androgen-related manifestations of masculinization, such as increased hair, acne, or a low voice. Diagnosis must be confirmed by immunohistochemical analysis of the tissue as clinical symptoms and imaging studies are unreliable.
CASE SUMMARY
A 14-year-old female presented with amenorrhea. After a thorough medical examination, endocrine and tumor markers analysis, and imaging, a pelvic mass was discovered. The patient also exhibited endocrine dysfunction but was not positive for any tumor markers. The patient underwent surgery to remove the ovarian tumor. Immunohistochemical analysis of the resected specimen indicated an OSST. During the postoperative follow-up, the patient had attained menarche.
CONCLUSION
This case’s clinical manifestation of endocrine dysfunction due to OSST provides new insights that will assist clinicians in the diagnosis and treatment of this common tumor.
Core Tip: Ovarian sclerosing stromal tumor (OSSTs) are found most commonly in females at 20-30 years of age. OSST is a rare tumor and is even rarer during puberty or menopause. We report a case of a 14-year-old female who had not yet reached menarche. After thorough examination at our clinic, a pelvic mass was discovered. The patient underwent surgery and reached menarche. Immunohistochemical analysis of the resected specimen provided definitive diagnosis of an OSST. This case highlights the importance of a thorough examination and pathological analysis to properly diagnose and treat patients with OSST.
- Citation: Han X, Zhang B, Gao JC. Late menstruation with an ovarian sclerosing stromal tumor: A case report. World J Clin Cases 2025; 13(26): 106999
- URL: https://www.wjgnet.com/2307-8960/full/v13/i26/106999.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i26.106999
Ovarian sclerosing stromal tumors (OSSTs) were first reported by Chalvardjian and Scully[1] in 1973. The World Health Organization classified OSSTs as a simple mesenchymal tumor subtype among gonadal mesenchymal tumors. OSSTs, however, are relatively rare among ovarian gonadal mesenchymal tumors, accounting for only 6% of cases. Their etiology is still unclear, but they are known to be benign and may have endocrine function. OSSTs typically occur in females in the 3rd decade of life[2], with a mean age of 27.5 years[3]. Although more than 80% of OSST cases occur by the age of 30 years, OSSTs have also been reported during puberty, pre-puberty, and menopause[4,5]. The tumors present unilaterally, but there are reports of bilateral tumors in the literature[6].
Currently, there are few reports describing the diagnosis and treatment of OSSTs[7]. Here, we report a case of OSST during puberty. We describe the symptoms caused by endocrine dysfunction related to the tumor by detailing the entire course of the treatment and the follow-up.
A 14-year-old female of Han ethnicity presented to the Gynecology and Obstetrics Clinic due to amenorrhea.
The patient had been admitted to a local hospital 1 year prior to presentation due to the lack of menarche. At that time she was informed by a doctor that the gonadal axis might be underdeveloped. The doctor recommended follow-up to monitor the menstrual status, but the patient did not undergo any relevant examinations. One year later, the patient revisited the local hospital due to the persistent absence of menarche. Considering that she was already 14-years-old and pubertal, she underwent gynecological ultrasonography, which revealed a pelvic mass.
The height of the patient was 168 cm, and her weight was 82 kg (body mass index of 29.05 kg/m2). Hair distribution was normal, and there was breast development. Axillary hair, distribution of pubic hair, and morphology of the labia majora and minora and clitoris were normal. The vaginal opening and hymenal rim were visible. We observed local slight hirsutism and a normal voice with no obvious manifestations of masculinity.
The physical examination showed the female sexual characteristics of this patient, including uterus size, to be developed normally, indicating that the amenorrhea was not caused by reproductive tract malformations. However, a solid mass measuring approximately 13 cm × 12 cm × 10 cm was palpated in the posterior pelvis. It was hard, movable, and slightly uneven with a clear contour and no pressure pain.
The following laboratory parameters were analyzed and showed normal serological tumor markers [carcinoembryonic antigen: 1.48 ng/mL, alpha-fetoprotein: 1.91 IU/mL, carbohydrate antigen (CA) 125: 13.47 U/mL, CA 19-9: 5.34 U/mL, and human epididymis protein 4: 30.34 pmol/L] but variable hormone profile [testosterone: 2.35 ng/mL (normal range: < 0.10-0.55 ng/mL), progesterone: 3.04 ng/mL (normal range: 0.31-1.52 ng/mL), luteinizing hormone: 5.78 mIU/mL (normal range: 1.90-11.60 mIU/mL), follicle stimulating hormone: 3.69 mIU/mL (normal range: 3.50-12.50 mIU/mL), estradiol: 46.42 pg/mL (normal range: 15.16-127.81 pg/mL), and pituitary prolactin: 779.24 µIU/mL (normal range: 71.00-566.00 µIU/mL)].
Gynecological ultrasonography was performed at an outside hospital. It revealed a posterior uterus with regular morphology, a size of 37 mm × 33 mm × 24 mm, and an endometrial thickness of 2 mm. Both ovaries were not observed. A mass was observed in the pelvic cavity with a size of 97 mm × 92 mm × 52 mm. The mass was hypoechoic with dense strong light spots and an unclear boundary. The upper edge reached just below the umbilicus and no abundant blood flow signal was detected, with a resistive index of 0.67.
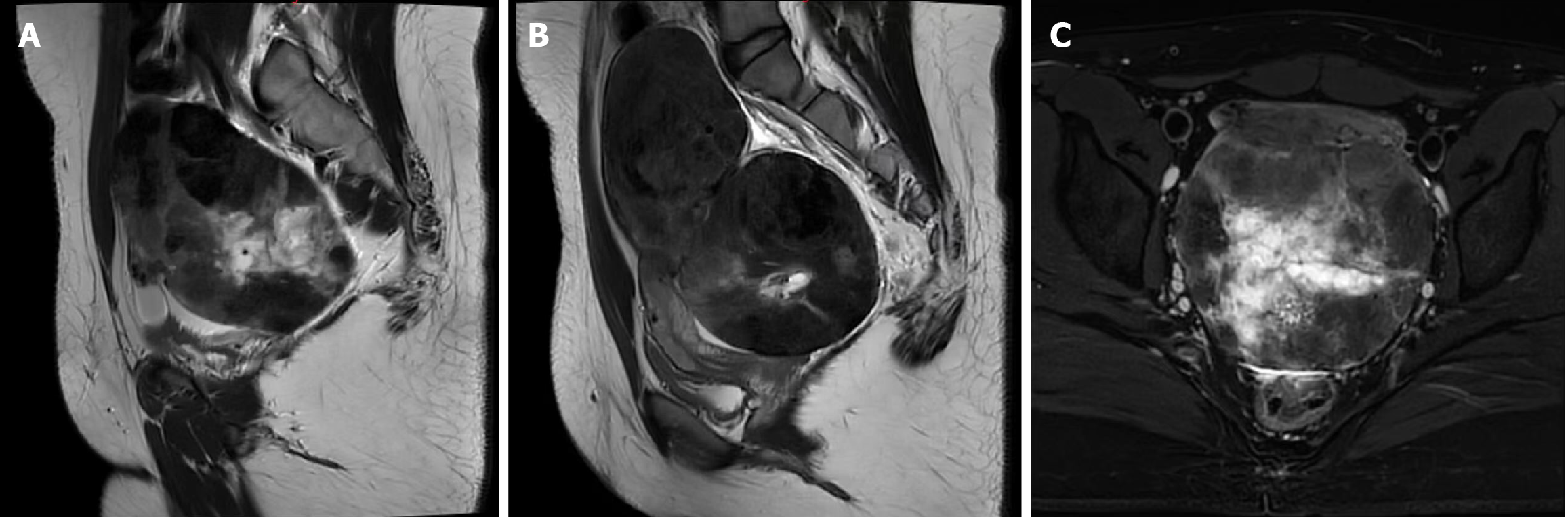
We performed pelvic magnetic resonance imaging (MRI) (Figure 1). It revealed a very large, round mass in the pelvis, with clear borders, a visible periphery, and an uneven signal. The mass had a T1-weighted imaging (WI) equal signal and a T2WI equal or slightly higher signal. These observations were strengthened on enhancement scanning. Within the mass there was a T1WI and a T2WI low-signal area and a T1WI low-signal and a T2WI high-signal area. The low signal was not strengthened on enhancement scanning and was closely related to the uterine fundus. The uterine compression showed change. The mass was closely related to the uterus, and the uterus was flattened and shifted anteriorly by compression. The mass was not clearly demarcated from the posterior wall of the uterine fundus, the thickness of the uterine cavity endothelium was normal, and the bilateral ovaries were not observed. The sigmoid colon was shifted to the right by compression. The mass was well demarcated from the bladder, which had a smooth, homogeneous bladder wall. The pelvic wall was structurally abnormal. No enlarged lymph nodes were observed in the pelvis, the boundary was clear, and homogeneous enhancement was observed.
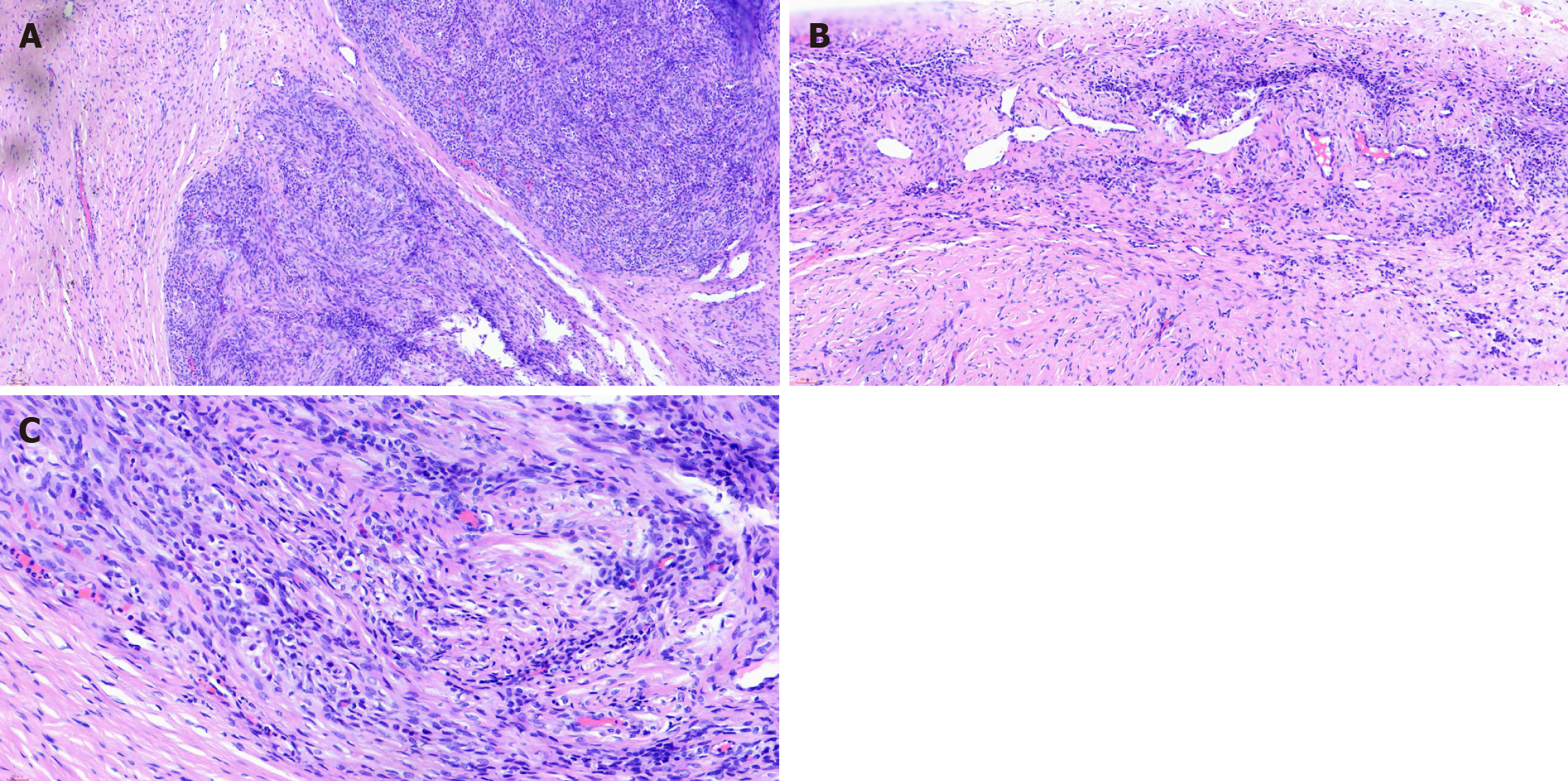
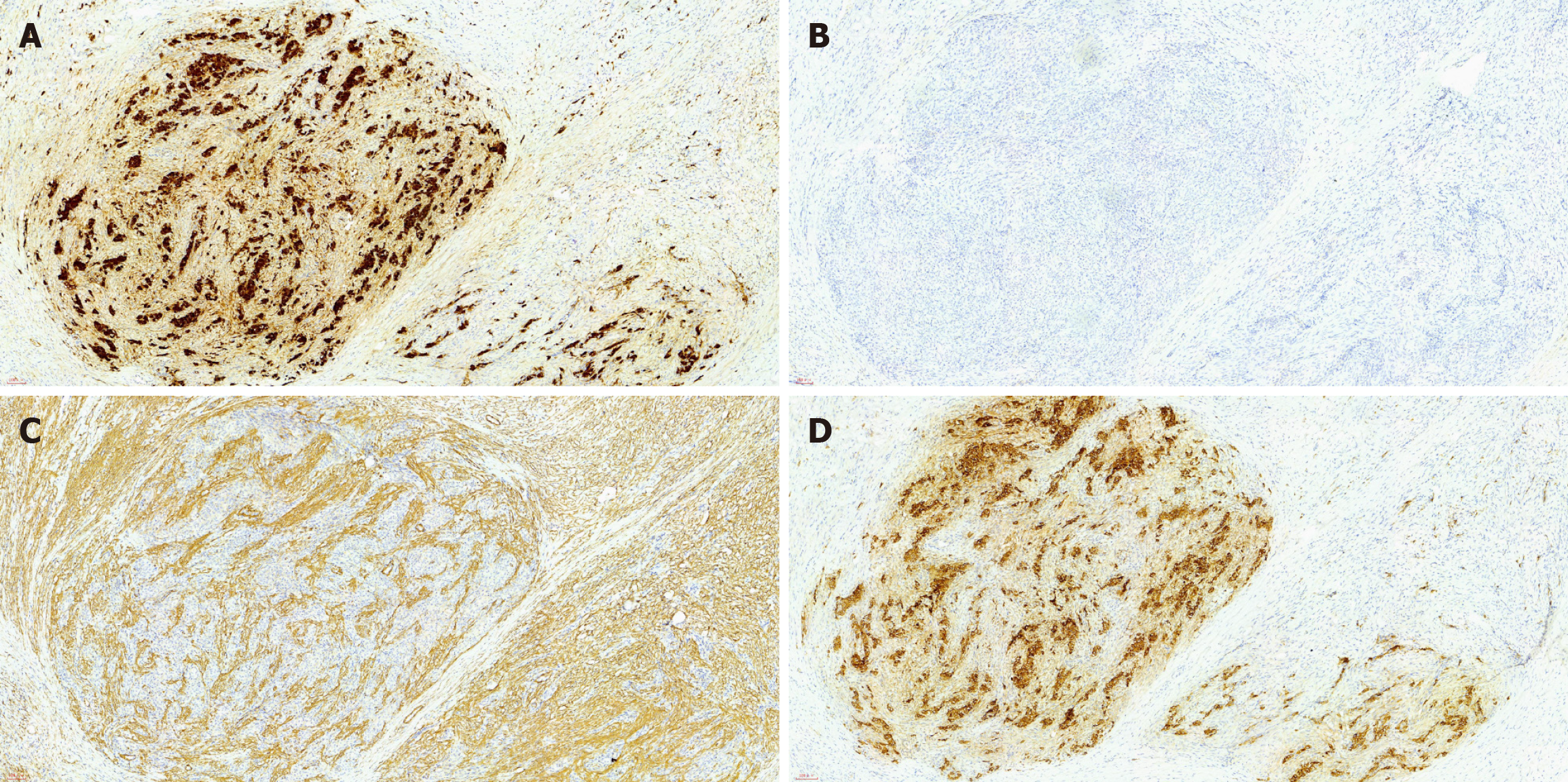
Immunohistochemical analysis of the mass revealed the following: (1) CD10 (-); (2) Cytokeratin (-); (3) Calretinin (+); (4) Epithelial membrane antigen (-); (5) Ki-67 (+10%); (6) Melan-A (-); (7) Progesterone receptor (+); (8) Smooth muscle actin (partial +); (9) Transcription factor E3 (+); (10) Wilms tumor-1 (-); and (11) Α-inhibin (+) (Figures 2 and 3).
Considering the collective medical history and immunohistochemistry findings, the final diagnosis was OSST.
After communicating with the patient and her family, the patient underwent a reduction laparoscopic biopsy of the left ovarian mass, mid-rotation open left ovarian tumor debulking, left ovary molding, and removal of the right ovarian surface adhesions.
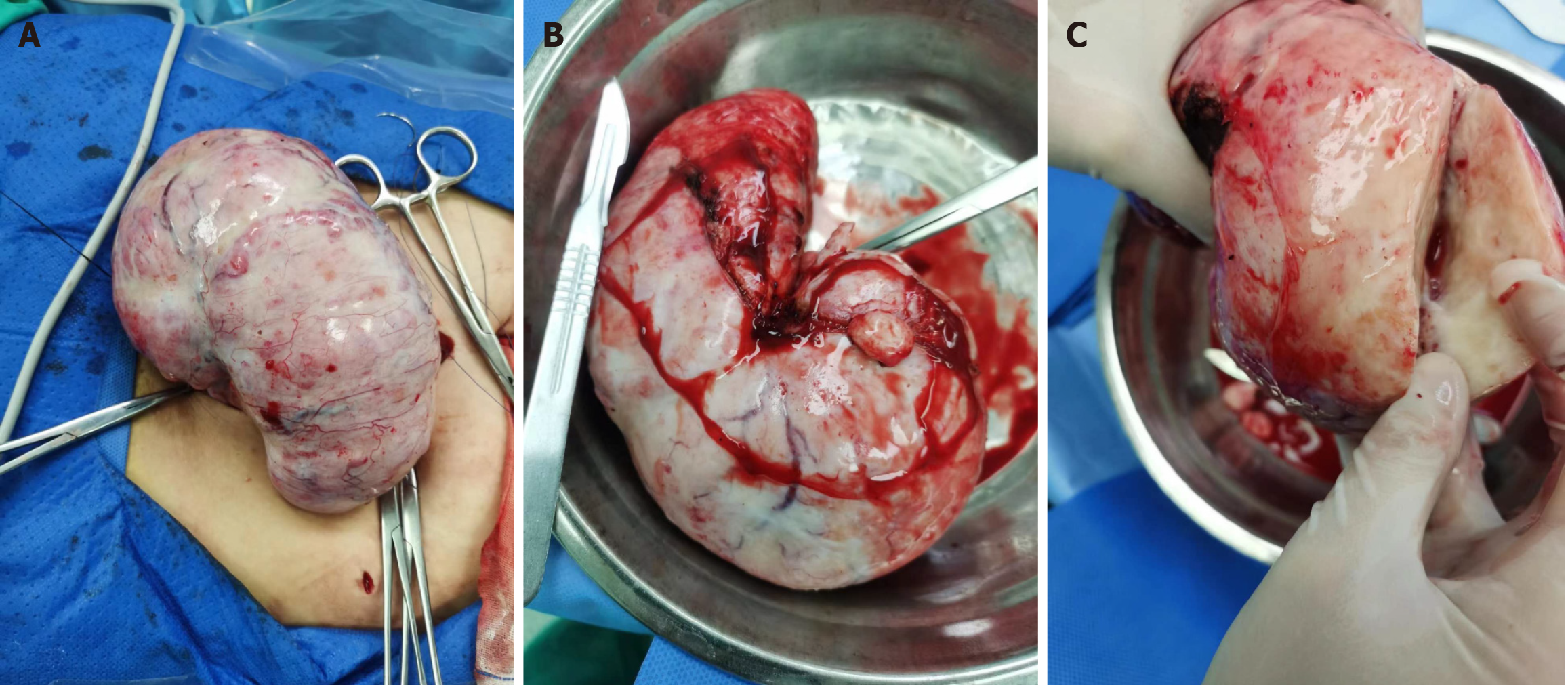
Intraoperatively, we observed no ascites. The left ovary was a huge solid mass, grayish-white, textured, and hard (Figure 4). It was 16 cm × 9 cm × 10 cm with an intact peritoneum, 180° degrees of torsion of the hilum, and very rich in surface blood vessels. We observed a smooth, slightly small, and child-like uterine surface. The right ovary was normal in appearance, and the bilateral fallopian tubes appeared normal. The surgery was successful in removing the mass.
The patient recovered well postoperatively and commenced menstruation. In the 6 months of follow-up, the patient experienced regular menstruation and no recurrence of the OSST.
Ovarian gonadal mesenchymal tumors account for 5%-8% of all ovarian tumors[8]. They originate in the gonads and mesenchyme in the primitive gonads. Other gonadal tumors include granulosa cell tumors, fibromas, follicular membranous cell tumors, supportive cell-mesenchymal stromal cell tumors, and sclerosing mesenchymal tumors. Ovarian sclerosing mesenchymal tumors are relatively rare, accounting for approximately 6% of ovarian gonadal mesenchymal tumors, and often lack typical clinical manifestations. Common clinical manifestations include abdominal pain, abdominal mass found on physical examination, menstrual abnormalities (including abnormal uterine hemorrhage and amenorrhea), and infertility. Occasionally, manifestations of masculinization such as hirsutism, acne, and a low voice are observed.
Menstrual abnormalities are common clinical issues in obstetrics and gynecology, and menstrual disorders are relatively common in adolescents. Determining the etiology of pubertal amenorrhea requires clinical examinations. In this case, the patient had not reached menarche. We observed that the patient’s serum testosterone level was elevated, which suggested abnormal endocrine function. Gynecological ultrasonography and pelvic MRI revealed a very large pelvic mass.
The abnormal endocrine function can be caused by tumors, and there are several types of gonadal mesenchymal tumors that cause endocrine abnormalities. However, the pathological examination is required because the characteristics of these various tumors are similar. Therefore, preoperative diagnosis is difficult. However, imaging findings can identify OSST in some cases[9].
A very large pelvic mass requires multiple evaluations including medical history, gynecological examination, imaging, serological tests, specific gynecological examination including bimanual diagnosis (or anal diagnosis), and abdominal palpation, and imaging. Clinical manifestations and abdominal pain provide clinicians with enough information to begin an investigation[10]. In the current case, the patient developed pubertal amenorrhea and serological hyperandrogenism, which led to the discovery and removal of the OSST. After removal, natural and regular menstruation occurred.
Tumor markers are utilized to determine the malignancy of a tissue. Tumor markers are typically in the normal range for OSSTs, but some reports have cited an elevated CA 125 level[2].
Imaging examinations include gynecologic ultrasonography, enhanced computed tomography (CT) of the abdomen, and enhanced MRI of the abdomen. Lucchetti et al[11] reported that ultrasound images showed a solid mass with focal hypoechoic components, possibly due to necrosis. Zhou et al[12] retrospectively analyzed the MRIs of 14 patients with histopathologically confirmed OSST. They concluded that MRI T2WI had the “lake island” sign. Therefore, MRI/CT with dynamic enhancement may play a key role in facilitating a preoperative diagnosis.
The gold standard for OSST clinical evaluation is histopathological analysis. OSSTs have a distinctive pseudofollicular structure mainly composed of various cellular nodules, edema, collagen, or fibrous mesenchyme[13]. Immunohistochemistry provides the diagnosis for all ovarian tumors postoperatively. However, the accuracy of intraoperative frozen section histopathology is also very important for clinical decision-making. Due to the limitations of histopathology, immunohistochemistry is required on sections from paraffin-embedded tissues. However, there have been reports of accurate intraoperative diagnosis[14]. This enabled the clinician to determine that the tumor was benign. The ovary was then preserved to retain fertility and ovarian function.
To avoid recurrence, aggressive surgery is performed to resect the ovary on the affected side[11]. If intraoperative frozen-section histopathological examination cannot determine malignancy, the ovary is still preserved. However, the patient must be informed that a second operation may be necessary. By understanding OSSTs in a comprehensive manner it will be possible to better preserve ovarian function and reduce overtreatment. The patient is satisfied with the treatment.
OSSTs are benign ovarian gonadal mesenchymal tumors that are rare in puberty and menopause. Some OSSTs contribute to endocrine dysfunction. When menstrual abnormalities occur, it is necessary to comprehensively determine the nature of the tumor by analyzing endocrine levels and tumor markers and performing imaging examinations and intraoperative frozen-section histopathology to support an immunohistochemically confirmed diagnosis. Individualized treatment with a more precise surgical approach can reduce the number of ovary resections and increase the likelihood of preserving fertility and ovarian function in young females.
| 1. | Chalvardjian A, Scully RE. Sclerosing stromal tumors of the ovary. Cancer. 1973;31:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Chen Q, Chen YH, Tang HY, Shen YM, Tan X. Sclerosing stromal tumor of the ovary with masculinization, Meig's syndrome and CA125 elevation in an adolescent girl: A case report. World J Clin Cases. 2020;8:6364-6372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Peng HH, Chang TC, Hsueh S. Sclerosing stromal tumor of ovary. Chang Gung Med J. 2003;26:444-448. [PubMed] |
| 4. | Lee CM, Lim S, Cho HY, Lee JS, Shin JW. Erratum to: Sclerosing Stromal Tumor of the Ovary in Postmenopausal Women: A Report of Two Cases. J Menopausal Med. 2015;21:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Gao C, Han Y, Fan X, Dong H, Xue F, Wang Y. A case of unilateral sclerosing stromal tumor of the ovary in a postmenopausal female. Int J Gynaecol Obstet. 2024;164:1226-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Chang W, Oiseth SJ, Orentlicher R, Agarwal G, Yahr LJ, Cayten CG. Bilateral sclerosing stromal tumor of the ovaries in a premenarchal girl. Gynecol Oncol. 2006;101:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Wang M, Zhang XW, Yang SL. Sclerosing Stromal Tumor and Fibroma Occuring Simultaneously in Both-sided Ovaries: A Rare Occurrence. J Coll Physicians Surg Pak. 2022;32:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Nguyen M, Soumit N, Waheed A, Sees J, Azhar E. A Rare Case of Sclerosing Stromal Tumor of the Ovary Presenting in Pregnancy: A Diagnostic Dilemma on Presentation. Case Rep Obstet Gynecol. 2019;2019:3927971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Palmeiro MM, Cunha TM, Loureiro AL, Esteves G. A rare benign ovarian tumour. BMJ Case Rep. 2016;2016:bcr2015214101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Cui X, Zhang X, Wang Y, Wang L. A rare case of sclerosing stromal tumor of the ovary presenting in pregnancy: a case report and review of the literature. J Int Med Res. 2023;51:3000605231152385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Lucchetti MC, Diomedi-Camassei F, Orazi C, Tassi A. A Rare Ovarian Tumor: The Sclerosing Stromal You Do Not Expect-A Case Series in the Adolescent Population and a Literature Review. Pediatr Rep. 2023;15:20-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Park CK, Kim HS. Clinicopathological Characteristics of Ovarian Sclerosing Stromal Tumor with an Emphasis on TFE3 Overexpression. Anticancer Res. 2017;37:5441-5447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Zhou Z, Chen X, Zeng Z, Zhang F, Yan J, Hou G. Imaging characteristics of ovarian sclerosing stromal tumor. BMC Womens Health. 2022;22:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Ayyanar P, Sable MN, Sethi P, Prasad N, Mishra P. An unreported case of intraoperative frozen section diagnosis of a sclerosing stromal tumor of the ovary- A report with a review. Indian J Pathol Microbiol. 2024;67:684-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/