Published online Sep 6, 2025. doi: 10.12998/wjcc.v13.i25.106335
Revised: April 15, 2025
Accepted: May 13, 2025
Published online: September 6, 2025
Processing time: 134 Days and 21.5 Hours
Leptospirosis is a globally prevalent zoonotic disease with a significant burden in tropical and subtropical regions, including India. Despite its high fatality rate and endemic nature, the disease remains underreported in many areas, particularly in Northern India.
To analyze the demography, clinical presentation, complications, and mortality risk factors in presumptive leptospirosis patients admitted to a tertiary care hospital over the last 7 years from the Himalayan and Sub-Himalayan regions of Northern India.
A retrospective analysis was conducted on hospital records of patients admitted with leptospirosis at the All India Institute of Medical Sciences, Rishikesh, between January 2018 and December 2024. Diagnosis was based on the Modified Faine’s Criteria and laboratory confirmation via IgM enzyme-linked immu
A total of 62 patients were included in the study. The most common symptoms were fever (98.39%), myalgia (41.94%), and jaundice (20.97%). Thrombocytopenia was the most frequent complication, occurring in 72.58% of cases, followed by hepatic involvement (62.9%) and acute kidney injury (40.32%). Multiorgan dys
Leptospirosis remains a severe and often fatal disease in Himalayan and sub-Himalayan regions, particularly in hilly areas, where underreporting and delayed diagnosis contribute to poor outcomes. Mortality was highest (33.33%) in cases with multiorgan involvement, particularly affecting the liver, kidneys, and lungs. We did not identify any statistically significant mortality predictors. Although the study did not assess the impact of timely diagnosis, improving healthcare accessibility in hilly regions may facilitate earlier detection and intervention, potentially reducing mortality.
Core Tip: This study highlights the high burden of severe leptospirosis, with significant multiorgan dysfunction, intensive care unit needs, and a 20.97% overall mortality rate. Patients from the Himalayan region had more severe renal impairment, higher hemodialysis requirements, and nearly double the mortality of the Sub-Himalayan group. Jaundice and acute respiratory distress syndrome were potential contributors to mortality, while international normalized ratio showed a possible protective association. These findings emphasize regional variability in disease severity and the need for targeted risk stratification and resource allocation.
- Citation: Das D, Ponnampurathu S, Panda PK, Mathuria YP. Different clinical profile of leptospirosis in a tertiary care Indian hospital: A Himalayan experience. World J Clin Cases 2025; 13(25): 106335
- URL: https://www.wjgnet.com/2307-8960/full/v13/i25/106335.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i25.106335
Leptospirosis is a worldwide zoonotic infectious disease caused by pathogenic bacteria of the Leptospira genus from the family Leptospiraceae. This disease is known for its endemicity, primarily in countries with a humid tropical or subtropical climate. The classical description of leptospirosis is that of Weil’s disease, a dramatic acute febrile and sometimes epidemic illness characterized by jaundice, splenomegaly, and nephritis. Numerous cases of leptospirosis have been reported from the southern, central, eastern and western parts of India, where it has long been recognized as one of the foremost causes of acute febrile illness.
There are few reports of leptospirosis from Northern India, which may be due to a lack of awareness, clinical suspicion and active surveillance[1,2]. Although it can have high fatality and is recognized as the most common and widespread zoonotic disease, the worldwide burden of morbidity and mortality of leptospirosis is still unknown.
A systematic review and modelling exercise estimated that more than one million human cases occur worldwide annually, including almost 60000 deaths[3]. The global burden of leptospirosis was estimated at 2.90 million disability adjusted life-years (DALYs) per annum (uncertainty interval [UI], 1.25–4.54 million). This represents an incidence of 41.8 DALYs per 100000 populations per year (UI 18.1–65.5). It is more prevalent in tropical regions but also occurs in temperate regions. Regions with the highest incidence of infection include South and Southeast Asia, Oceania, the Caribbean, parts of sub-Saharan Africa, and parts of Latin America. In India, more than half a million instances of severe leptospirosis are diagnosed each year with a fatality rate of more than 10%. The Southern region has the highest positivity rate at 25.6% and 8.3%, 3.5%, 3.1%, and 3.3% in Northern, Western, Eastern and focal India, respectively. Leptospirosis has been endemic in India since the early 20th century. Significant numbers of outbreaks occur in the coastal regions, with particularly higher rates during July, August and September, coinciding with monsoon season[4,5]. Uttarakhand state is located in the Northern part of India and has 86% mountainous and 65% forest areas. It is home to a diversity of flora and fauna. The state experiences moderate to heavy rainfall throughout the year with frequent flooding of several districts in recent years. Agriculture is one of the most significant economic sectors of Uttarakhand. In spite of all these factors, it is not an endemic region compared to other endemic states like Gujarat, Maharashtra, Kerala, Tamil Nadu, Karnataka, and the union territory of Andaman & Nicobar Islands.
This study aimed to assess the demography, clinical features, investigations, treatment, and outcomes of patients admitted with leptospirosis over the last 7 years at a tertiary care teaching hospital in Northern India. The study also aimed to highlight variations among leptospirosis patients in Himalayan and sub-Himalayan regions.
This study was a retrospective hospital-based study designed to assess the clinical profile of all admitted leptospirosis patients from their data records.
The study was conducted at a tertiary care teaching hospital in Uttarakhand, Northern India. Data were collected from the Department of General Medicine from January 2018 to December 2024. The hospital receives patients from both Himalayan and sub-Himalayan regions with diverse ethnic variation and variable clinical presentation. The study was approved by the Institute Ethics Committee (AIIMS Rishikesh), ensuring patient confidentiality, data protection, and adherence to ethical standards in research involving human participants.
Participants included adult patients aged 18 years or older admitted to the Department of General Medicine with a presumptive diagnosis of leptospirosis based on a clinical scoring system proposed by Faine (WHO Guideline, Table 1)[6]. Patients with incomplete data were excluded.
| Part A: Clinical features | Score | Part B: Epidemiological factors | Score |
| Headache | 2 | Contact with contaminated environment | 4 |
| Fever ≥ 39°C | 2 | Occupation involving animals or contaminated water | 4 |
| Conjunctival suffusion | 4 | Rainfall | 5 |
| Muscle pain | 4 | Part C: Laboratory findings | Score |
| Meningism | 4 | Positive IgM ELISA | 15 |
| Jaundice | 1 | Positive PCR | 15 |
| Albuminuria or nitrogen retention | 2 | Culture positive | 10 |
| Hemorrhages | 2 | MAT titer ≥ 1:400 | 15 |
| Cough, hemoptysis, or breathlessness | 2 | MAT titer ≥ 1:800 | 20 |
| Interpretation of scores | |||
| Presumptive diagnosis of leptospirosis: Total score (Parts A + B + C) ≥ 25; Total score (Parts A + B) ≥ 26 | Possible diagnosis of leptospirosis: Total score between 20 and 25 | ||
The study employed universal sampling due to the small number of leptospirosis patients in this non-endemic region. All eligible patients during the study period were included.
The study analyzed the demography, clinical presentation, investigation, treatment, complication, and outcomes of all leptospirosis patients admitted over the study period. For this, electronic hospital health records and available physical clinical records were the primary data sources for retrieving information, including gender, age, locality, month of admission, symptoms, signs, organ function tests, leptospira IgM antibody positivity, complications, given treatments, and outcome as organ failures, cured and death. Data were collected from patient records and medical charts using e-hospital (AIIMS Rishikesh) and Microsoft Excel.
Statistical analysis was performed and the data was entered into Microsoft Excel. The data were summarized using descriptive statistics. Continuous variables were presented as mean ± standard deviation (SD) or median depending on the normality of the data. Categorical variables were presented as frequencies and percentages. For continuous variables, most data were converted to categorical variables for easier application from the published data as follows: Total leukocyte counts > 12000/mm3, Hemoglobin < 10 mg/dL, total platelet counts < 100000/mm3, urea > 40 mg/dL, creatinine > 1.4 mg/dL, transaminase > 3x upper limit of normal, total bilirubin > 2 mg/dL, international normalized ratio (INR) > 1.5. The diagnosis of ARDS (acute respiratory distress syndrome) was based on the 2012 Berlin definition[7]. A two-sided significance level of 0.05 was used for all statistical tests. Results with a P value < 0.05 were considered statistically significant. All statistical analyses were conducted using the statistical software SPSS-25 (Statistical Package for the Social Sciences).
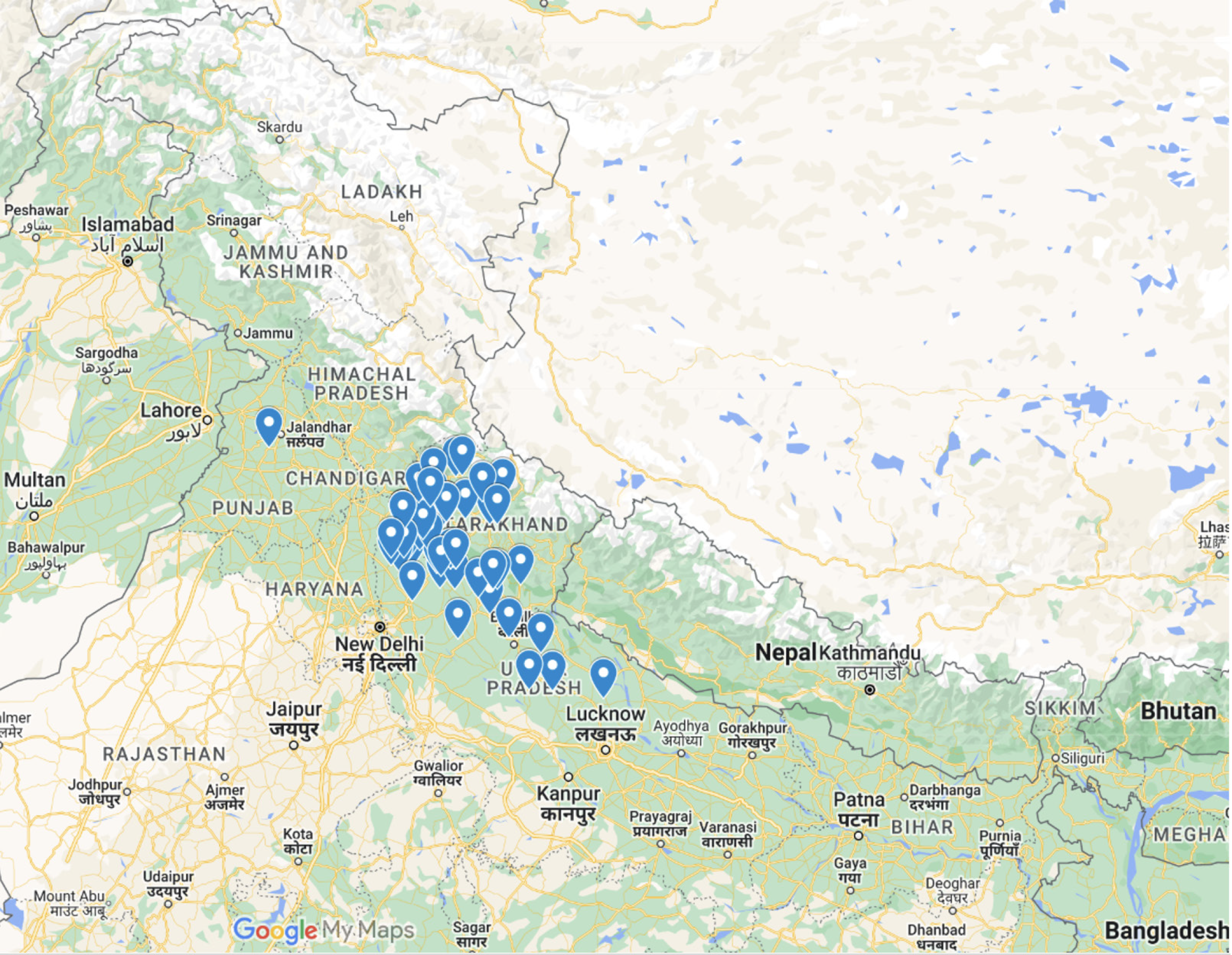
A total of 62 out of 68 patients were included in the study, with 20 (32.26%) from the Himalayan region and 42 (67.74%) from the Sub-Himalayan region (Figure 1). Fever (> 39°C) was the most prevalent symptom, observed in 98.39% of patients. Other common symptoms included myalgia (41.94%), abdominal pain (43.55%), and thrombocytopenia (72.58%). Conjunctival suffusion was present in 14.52% of patients, with a slightly higher proportion in the Himalayan region (20.00%) than in the Sub-Himalayan region (11.90%). Notably, patients from the Himalayan region had a higher prevalence of dyspnea (35.00% vs 19.05%, P = 0.29) and jaundice (30.00% vs 16.67%, P = 0.38) (Table 2).
| Clinical variable | Frequency, n = 62 | No. of patients from Himalayan region, n = 20 | No. of patients from Sub-Himalayan region, n = 42 | P value |
| Fever (> 39 °C) | 61 (98.39) | 20 (100.00) | 41 (97.62) | 1 |
| Headache | 20 (32.26) | 7 (35.00) | 13 (30.95) | 0.977565281 |
| Conjunctival suffusion | 9 (14.52) | 4 (20.00) | 5 (11.90) | 0.452540931 |
| Neck rigidity | 4 (6.45) | 0 (0.00) | 4 (9.52) | 0.295162635 |
| Myalgia | 26 (41.94) | 9 (45.00) | 17 (40.48) | 0.950434811 |
| Jaundice | 13 (20.97) | 6 (30.00) | 7 (16.67) | 0.383257647 |
| Dyspnea | 15 (24.19) | 7 (35.00) | 8 (19.05) | 0.291929299 |
| Increased liver enzyme (> 3 × upper limit) | 39 (62.90) | 14 (70.00) | 25 (59.52) | 0.60511935 |
| Thrombocytopenia | 45 (72.58) | 14 (70.00) | 31 (73.81) | 0.992162843 |
| Edema | 7 (11.29) | 4 (20.00) | 3 (7.14) | 0.198564612 |
| Abdominal pain | 27 (43.55) | 11 (55.00) | 16 (38.10) | 0.326599126 |
| AKI | 25 (40.32) | 10 (50.00) | 15 (35.71) | 0.426604756 |
| Hemodialysis Required | 14 (22.58) | 8 (40.00) | 6 (14.29) | 0.052520105 |
| Altered sensorium | 16 (25.81) | 6 (30.00) | 10 (23.81) | 0.833434549 |
| ARDS | 12 (19.35) | 7 (35.00) | 5 (11.90) | 0.070626099 |
| CKD | 4 (6.45) | 2 (10.00) | 2 (4.76) | 0.588416137 |
| DIC | 8 (12.90) | 2 (10.00) | 5 (11.90) | 1 |
| MODS | 41 (66.13) | 13 (65.00) | 28 (66.67) | 1 |
| HDU or ICU requirement | 37 (59.67) | 13 (65.00) | 24 (57.14) | 0.754549351 |
At admission, the mean pulse rate was 102.19 ± 11.97 bpm. The mean systolic and diastolic blood pressures were also comparable between groups. Oxygen saturation at room air was lower in the Himalayan group (91.05 ± 7.28% vs 94.73 ± 4.38%, P = 0.052), bordering statistical significance. Modified Faine’s Score was 34.34 ± 14.61 without major variations between Himalayan vs sub-Himalayan groups (36.15 ± 16.21 vs 33.48 ± 13.90 respectively, P = 0.52). Laboratory findings showed elevated liver enzymes, with lower serum glutamic pyruvic transaminase (SGPT) and serum glutamic oxaloacetic transaminase (SGOT) levels in the Himalayan group (226.45 ± 194.48 and 263.90 ± 220.05 vs 294.02 ± 380.96 and 300.36 ± 388.44 U/L respectively, P = 0.3591 and 0.6400). Direct Bil (mg/dL) was 1.37 ± 1.56. Serum creatinine levels were higher in the Himalayan group (2.36 ± 1.91 mg/dL vs 1.52 ± 1.26 mg/dL, P = 0.08), suggesting more severe renal impairment in this subset. Platelet counts were lower in the Himalayan group (103.95 ± 77.44 × 10³/µL vs 135.40 ± 136.70 × 10³/µL).
A total of 37 patients (59.67%) required admission to a high-dependency unit or intensive care unit (ICU), with a comparable proportion in the Himalayan (65.00%) and Sub-Himalayan groups (57.14%). Hemodialysis was required in 22.58% of patients, with a higher proportion in the Himalayan group (40.00%) compared to the Sub-Himalayan group (14.29%), suggesting a possible association between geographical region and renal dysfunction severity (Tables 3 and 4, Figure 2).
| Clinical variable | Frequency, n = 62 | No. of patients from Himalayan region, n = 20 | No. of patients from sub-Himalayan region, n = 42 | P value |
| Mortality | 13 (20.97) | 7 (35.00) | 6 (14.29) | 0.094 |
| System involvement | Discharged | Expired | Total | Mortality (%) |
| Hematological + renal | 20 | 7 | 27 | 25.93 |
| Hematological + renal + hepatic | 16 | 7 | 23 | 30.43 |
| Hematological + renal + hepatic + respiratory | 6 | 3 | 9 | 33.33 |
The overall mortality rate was 20.97% (13/62). Female patients had a higher mortality rate (26.67%) compared to males (15.62%). Mortality was higher among patients from the Himalayan region (35.00%) than the Sub-Himalayan region (14.29%). Mortality increased with the extent of multiorgan involvement, reaching 33.33% in those with hematological, renal, hepatic, and respiratory involvement.
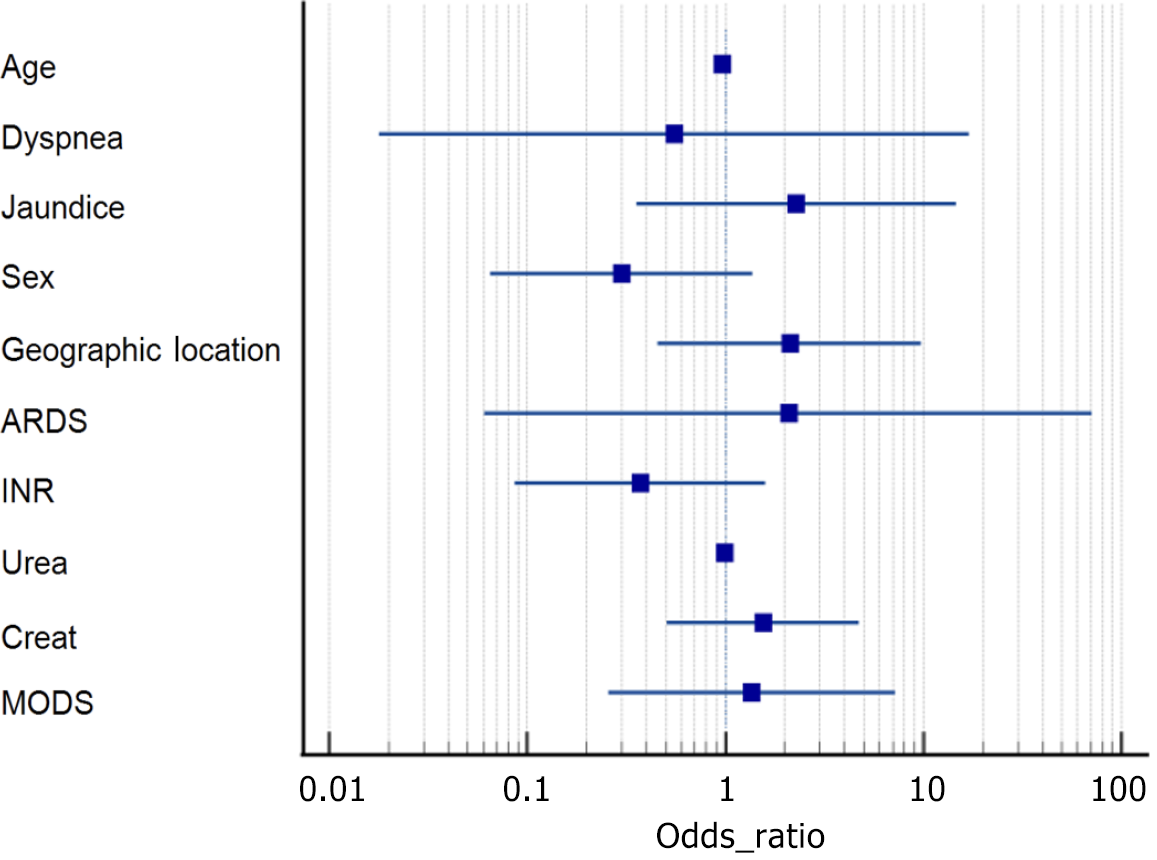
Logistic regression analysis did not identify any statistically significant predictors of mortality. ARDS had the highest odds ratio (OR = 2.10) but was highly variable (P = 0.68). Jaundice showed a potential trend toward increased mortality (OR = 2.28, P = 0.38), though it did not reach statistical significance. Multivariate regression indicated that INR (OR = 0.33, P = 0.066) trended toward significance, suggesting a possible protective effect, while elevated creatinine levels (OR = 2.12, P = 0.10) suggested a potential association with higher mortality, though not statistically significant. Age and urea levels were not significant predictors.
This single-center observational study conducted at a tertiary care teaching hospital highlights the significant burden of severe leptospirosis, with high rates of multiorgan dysfunction, intensive care requirements, and mortality. Notably, patients from the Himalayan region exhibited more severe renal impairment and oxygen desaturation, with trends toward higher serum creatinine levels and increased hemodialysis requirements. The overall mortality rate was 20.97%, but it was nearly twice as high in the Himalayan group compared to the Sub-Himalayan group. Jaundice and ARDS emerged as potential contributors to mortality, whereas INR showed a possible protective association. These findings underscore the regional variability in disease severity and highlight the importance of risk stratification and resource allocation in critically ill patients.
Leptospirosis is primarily transmitted through contact with contaminated food, water, or via skin abrasions and mucous membranes. Environmental factors such as soil moisture, surface water, temperature, and humidity influence leptospiral survival[2]. Increased rainfall and frequent flooding due to climate change in Himalayan regions may have contributed to the rising incidence of leptospirosis. Studies have shown that variations in rainfall, land temperature, and frequent floods are associated with increased leptospirosis cases[8].
Our study observed a nearly equal distribution of cases among males (51.61%) and females (48.38%), with a mean age of 34.34 ± 14.61 years. A meta-analysis by Naing et al[9] reported a higher incidence of leptospirosis in young adult males due to occupational exposure, though our study did not find a significant gender-based difference. Leptospirosis typically progresses in two phases: The leptospiremic (acute) phase and the immune phase. The leptospiremic phase lasts 3-9 days and presents as a non-specific febrile illness with fever, chills, myalgia, and headache[10]. Severe myalgia may mimic an acute abdomen or cause meningismus. The immune phase follows, marked by the appearance of IgM antibodies in the blood and the excretion of organisms in urine. Thrombocytopenia was the most frequent hematological complication, affecting 72.58% of patients, with a mean platelet count of 125.26 k/μL. No significant difference was noted between patients from the Himalayan and Sub-Himalayan regions. This finding aligns with prior studies by Daher et al[11] (53.5%) and Sharma et al[12] (56%). The thrombocytopenia observed in leptospirosis is attributed to platelet consumption, immune-mediated destruction, and bone marrow suppression[13].
Liver involvement was the second most frequent complication in this cohort, observed in 62.90% of patients, with mean SGPT and SGOT levels of 272.23 U/L and 288.23 U/L, respectively. Jaundice was present in 20.97% of cases. Previous studies have reported similar results, with raised total bilirubin in 34% of cases and average bilirubin, SGOT and SGPT were 2.7-14.6 g/dL, 58–524 IU/L and 58–503 IU/L, respectively[14]. Cuaño et al[15] identified jaundice and transaminitis in 39.5% of cases. Hepatic dysfunction in leptospirosis results from bile leakage due to disruption of hepatocyte junctions, along with glycogen depletion and mitochondrial alterations[16]. Interestingly, elevated INR demonstrated a paradoxical protective association with mortality[17]. While INR is traditionally linked to coagulation dysfunction and worse outcomes, our findings suggest a possible antithrombotic advantage that warrants further investigation. This may reflect a compensatory anticoagulant response, moderate hepatic dysfunction with protective effects, or regional variations in coagulation adaptation. Further mechanistic studies are needed to clarify this observation.
Acute kidney injury (AKI) was observed in 40.32% of cases, with 22.58% requiring dialysis. We did not identify any significant difference between patients from different geographical regions. Previous studies have reported a non-oliguric form of AKI in 41-45% of leptospirosis patients, with a mortality rate of 22%[18]. In our study, AKI-related mortality was 28%, consistent with findings by Daher et al[18]. Leptospiral nephropathy results from bacterial invasion, inflammatory processes, and hemodynamic compromise, leading to damage of the brush border epithelium and glomerular alterations[16,19].
Pulmonary involvement in leptospirosis is rare but severe, often presenting as alveolar hemorrhage with dyspnea and hemoptysis[20]. Vandroux et al[21] reported ARDS in 23% of leptospirosis patients. In our study, 19.35% of patients developed ARDS, with a higher prevalence (35%) in the Himalayan group, suggesting a greater risk of respiratory complications in these patients. Studies have identified dyspnea as an independent predictor of severe leptospirosis mortality[22].
Multiorgan dysfunction syndrome (MODS) was observed in 66% of patients, and 59.67% required ICU support, reflecting the severity of the illness. Among patients with hematological and renal involvement, the mortality rate was 25.93%, whereas those with hematological, renal, hepatic, and respiratory involvement had the highest mortality (33.33%). Higher mortality rates in hilly regions likely reflect underreporting and delayed diagnosis; however, statistical significance was not observed (P = 0.094). Previous studies have shown that MODS is a strong predictor of mortality in leptospirosis, with ICU mortality rates ranging from 30% to 50% in severe cases[23,24]. Risk factors contributing to mortality include delayed presentation, organ failure, and the need for invasive ventilation[25]. Studies have also highlighted that early administration of antibiotics and aggressive supportive care can improve survival outcomes in critically ill patients with leptospirosis[26]. Even various expanded Leptospira syndromes without classical MODS have recently been reported[27].
Routine serological tests such as the microscopic agglutination test (MAT) and enzyme-linked immunosorbent assay (ELISA) often yield negative results in the early disease phase due to delayed antibody production[4,28]. MAT, the gold standard for serological diagnosis, requires a four-fold increase in antibody titer, which may not be evident in early-stage cases. ELISA demonstrates high sensitivity (90%) and specificity (95%) but may be less effective in early infection when antibody levels are low. Polymerase chain reaction (PCR)-based rapid tests offer early detection but were not available in the center, limiting their utility in our study.
This study has several limitations. First, it was a single-center observational study, which may limit the generalizability of the findings to other regions with different environmental and healthcare settings. Second, the retrospective nature of data collection could introduce selection and reporting biases. Third, the absence of molecular diagnostic methods such as PCR during the study period may have led to underdiagnosis of early cases. Finally, the small sample size, particularly in subgroup analyses, may have limited the statistical power to detect significant associations with mortality risk factors.
Our study confirms the significant burden of leptospirosis, with high rates of thrombocytopenia (72.58%), liver involvement (62.90%), and acute kidney injury (40.32%) in North-India. Patients from the Himalayan region exhibited more severe disease, with higher ICU admissions and a trend toward increased mortality. Notably, jaundice and ARDS were identified as major predictors of mortality. Conversely, higher INR had a potential protective association, warranting further investigation. Early detection remains a challenge due to the limitations of routine serological tests, emphasizing the need for expanded access to PCR-based diagnostics in endemic regions. Given the observed regional variability in disease severity, improved surveillance, timely supportive care, and risk-based ICU resource allocation are essential. Future prospective studies should explore geographic variations in leptospirosis severity and validate prognostic markers to refine clinical management strategies, risk-based ICU resource allocation is essential.
We acknowledge the contributions of our mentors and colleagues whose insights and guidance significantly enriched this research. Our heartfelt thanks go to the study participants for their cooperation and contribution. Finally, we recognize the role of anonymous reviewers and prior researchers whose work laid the foundation for this study. Their collective efforts were instrumental in the successful completion of this work.
| 1. | Mehta V, Bhasi A, Panda PK, Gupta P. A coinfection of severe leptospirosis and scrub typhus in Indian Himalayas. J Family Med Prim Care. 2019;8:3416-3418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (2)] |
| 2. | Sethi S, Sharma N, Kakkar N, Taneja J, Chatterjee SS, Banga SS, Sharma M. Increasing trends of leptospirosis in northern India: a clinico-epidemiological study. PLoS Negl Trop Dis. 2010;4:e579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
| 3. | Antima, Banerjee S. Modeling the dynamics of leptospirosis in India. Sci Rep. 2023;13:19791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (36)] |
| 4. | Bhatia M, Kumar P, Gupta P, Gupta PK, Dhar M, Kalita D. Serological evidence of human leptospirosis in patients with acute undifferentiated febrile illness from Uttarakhand, India: A pilot study. J Lab Physicians. 2019;11:11-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Samrot AV, Sean TC, Bhavya KS, Sahithya CS, Chan-Drasekaran S, Palanisamy R, Robinson ER, Subbiah SK, Mok PL. Leptospiral Infection, Pathogenesis and Its Diagnosis-A Review. Pathogens. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 6. | Guidelines for the control of leptospirosis. WHO Offset Publ. 1982;1-171. [PubMed] |
| 7. | ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526-2533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 4413] [Article Influence: 315.2] [Reference Citation Analysis (0)] |
| 8. | Ab Kadir MA, Abdul Manaf R, Mokhtar SA, Ismail LI. Spatio-Temporal Analysis of Leptospirosis Hotspot Areas and Its Association With Hydroclimatic Factors in Selangor, Malaysia: Protocol for an Ecological Cross-sectional Study. JMIR Res Protoc. 2023;12:e43712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Naing C, Reid SA, Aye SN, Htet NH, Ambu S. Risk factors for human leptospirosis following flooding: A meta-analysis of observational studies. PLoS One. 2019;14:e0217643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Rajapakse S. Leptospirosis: clinical aspects. Clin Med (Lond). 2022;22:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 11. | Daher EF, Silva GB, Silveira CO, Falcão FS, Alves MP, Mota JA, Lima JB, Mota RM, Vieira AP, Pires Rda J, Libório AB. Factors associated with thrombocytopenia in severe leptospirosis (Weil's disease). Clinics (Sao Paulo). 2014;69:106-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Sharma J, Suryavanshi M. Thrombocytopenia in leptospirosis and role of platelet transfusion. Asian J Transfus Sci. 2007;1:52-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Nicodemo AC, Del Negro G, Amato Neto V. Thrombocytopenia and leptospirosis. Rev Inst Med Trop Sao Paulo. 1990;32:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Gupta N, Wilson W, Ravindra P. Leptospirosis in India: a systematic review and meta-analysis of clinical profile, treatment and outcomes. Infez Med. 2023;31:290-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 15. | Cuaño CRG, Cuaño PMGM, Ong JP, Borlongan MAB, Torres JMK, Hernandez ARB, Chua AV Jr. Determination of Liver Function Tests and Liver Ultrasonographic Findings in Patients with Leptospirosis in a Tertiary Hospital. Acta Med Philipp. 2024;58:17-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | De Brito T, Silva AMGD, Abreu PAE. Pathology and pathogenesis of human leptospirosis: a commented review. Rev Inst Med Trop Sao Paulo. 2018;60:e23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Wagenaar JF, Goris MG, Partiningrum DL, Isbandrio B, Hartskeerl RA, Brandjes DP, Meijers JC, Gasem MH, van Gorp EC. Coagulation disorders in patients with severe leptospirosis are associated with severe bleeding and mortality. Trop Med Int Health. 2010;15:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Daher EDF, Abreu KLSD, Silva Junior GBD. Insuficiência renal aguda associada à leptospirose. J Bras Nefrol. 2010;32:408-415. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 19. | Sitprija V, Losuwanrak K, Kanjanabuch T. Leptospiral nephropathy. Semin Nephrol. 2003;23:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Dolhnikoff M, Mauad T, Bethlem EP, Carvalho CR. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Braz J Infect Dis. 2007;11:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Vandroux D, Chanareille P, Delmas B, Gaüzère BA, Allou N, Raffray L, Jaffar-Bandjee MC, Martinet O, Ferdynus C, Jabot J. Acute respiratory distress syndrome in leptospirosis. J Crit Care. 2019;51:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Li D, Liang H, Yi R, Xiao Q, Zhu Y, Chang Q, Zhou L, Liu B, He J, Liu T, Fan Z, Cheng W, Wang W, Zhang Y, Pan P. Clinical characteristics and prognosis of patient with leptospirosis: A multicenter retrospective analysis in south of China. Front Cell Infect Microbiol. 2022;12:1014530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 23. | Goarant C. Leptospirosis: risk factors and management challenges in developing countries. Res Rep Trop Med. 2016;7:49-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Abela-Ridder B, Sikkema R, Hartskeerl RA. Estimating the burden of human leptospirosis. Int J Antimicrob Agents. 2010;36 Suppl 1:S5-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 473] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 26. | Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015;9:e0003898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1095] [Cited by in RCA: 1214] [Article Influence: 110.4] [Reference Citation Analysis (4)] |
| 27. | Dehkordi AG, Al-Tamary SRS, Al-Tamary ARS, Al-Tamary HRS, Albarari SSA, Assad AMZ, Hamdan GMO, Mikadze I. Atypical Cases of Leptospirosis: Insights From Georgia. Case Rep Infect Dis. 2024;2024:1414417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Musso D, La Scola B. Laboratory diagnosis of leptospirosis: a challenge. J Microbiol Immunol Infect. 2013;46:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/