INTRODUCTION
Sotos syndrome is characterized by overgrowth conditions with clinical features including advanced bone age, distinctive facial appearance, macrocephaly and learning abnormalities in children, while mutations and deletions of nuclear receptor binding SET domain protein 1 (NSD1) were identified to be diagnostic markers in this disease[1,2]. Recently, it has been shown that Sotos syndrome may be associated with not only verbal ability but also cognitive and intellectual function[3]. Moreover, several early neuropsychiatric features have been observed in children with Sotos syndrome following neurodevelopmental examination.
Yang et al[4] indicated that attention-deficit/hyperactivity disorder (ADHD) can be present in children with Sotos syndrome showing clinical and genetic features. It has been demonstrated that neuropsychiatric symptoms such as autistic behavior, ADHD, and phobia-mediated anxiety are present in children and adolescents with Sotos syndrome, which are indicated as bridges between Sotos syndrome and ADHD in the clinic[5]. ADHD is considered a neurodevelopmental and psychiatric disorder during childhood with a multifactorial pathogenesis accompanied by social difficulties in adult life[6]. Several health-associated impairments including smoking, drug abuse, obesity, hypertension, diabetes, suicide, etc. have previously been demonstrated in patients with ADHD[7]. Although there is a possible connection in terms of clinical symptoms between these two disorders, there is also an obvious gap between Sotos syndrome and ADHD. Hence, it is meaningful to explore the effect of NSD1 in the association between Sotos syndrome and ADHD during childhood and in subsequent maturity stage.
NSD1 GENETIC FEATURES AND EPIGENETICS IN SOTOS SYNDROME
Sotos syndrome was first demonstrated by Sotos et al[8] and observed in five patients with excessive overgrowth, and non-progressive cerebral disorder. NSD1 which is located in the chromosome 5q35 region has been identified as a transcription coactivator and stimulates transcriptional programs in the development of Sotos syndrome and contributes to embryonic stem cell differentiation or final transition[9,10]. The definition of NSD1-positive individuals was used to evaluate the association with Sotos syndrome in which 93% of patients with Sotos syndrome were identified as having NSD1 abnormalities including 83% intragenic mutations and 10% 5q35 microdeletions[11]. Phenotype is characterized by short stature, microcephaly, and delayed bone maturation has been demonstrated in three cases of Sotos syndrome with a NSD1 mutation involving 5q35 microduplication[12].
In addition, epigenetic inactivation of NSD1 via CpG island-promoter hyper- methylation can reduce methylation of H4-K20 and H3-K36 in histone lysine residues which were considered to predict the outcomes of human neuroblastomas and gliomas in patients with Sotos syndrome[13]. Although NSD1 gene alterations were associated with Sotos syndrome in some cases, mutations of NSD1 were absent in other overgrowth phenotypes such as weaver syndrome, and unclassified overgrowth/mental retardation in 20 patients[14]. However, NSD1 intragenic mutations were examined in patients with Weaver syndrome and were found in 3/6 patients, while NSD1 intragenic mutations and deletions were present in 22/23 Sotos syndrome patients[15]. These differences in genetics may have been the result of small candidate samples, and the specific mechanism should be investigated in further studies. In the study by Yang et al[4], the 4605C>T point mutation in NSD1 was determined to be present in the proband’s father but absent in her mother, and this relationship can provide a clue for exploring the genetic mechanism of NSD1 in patients with Sotos syndrome or other overgrowth phenotypes.
NSD1 GENETIC FEATURES IN ADHD
ADHD has been observed during the developmental period in children with three symptoms including inattentiveness, impulsivity and motor unrest occurring at around six months old[16]. These impairments including emotional and cognitive disorders could be the result of abnormal neurobehavior in ADHD patients as mentioned by Yang et al[4]. Moreover, concurrent cognitive, language, learning and mental disorders could also exist in children and adolescents with ADHD. Compared to Sotos syndrome, the relationship between genes and the environment has been investigated in the pathogenesis of ADHD, but research on genetic risk factors and the molecular basis of ADHD has been rarely reported[17]. The genetic structure of ADHD was described as complex under heritable conditions while several genes including: (1) Dopamine D4 receptor; (2) Dopamine D5 receptor; (3) Dopamine transporter; (4) Dopamine-hydroxylase; and (5) Serotonin transporter, etc. were associated with ADHD with a pooled odds ratio ranging from 1.13 to 1.45[18]. Based on previous studies, dysfunction of the dopamine system at the genetic and neuropsychological levels may be involved in the pathogenesis of ADHD and mood disorders[19]. In addition, the etiology of ADHD may be related to gene-environment in view of its multifactorial and genetic pathogenesis[20]. Compared to the association with Sotos syndrome, NSD1 gene alterations are rarely involved in ADHD symptoms. It was reported that NSD1 gene mutation could represent a risk in patients with Sotos syndrome in terms of social competence, communication and life skills, but a consistent association with ADHD was not observed in 29 participants divided into the NSD1 mutation group (n = 12) and the NSD1 non-mutation group (n = 17)[21]. It was noted that the pediatric patient with both Sotos syndrome and ADHD had a synonymous mutation of the NSD1 gene by genetic testing according to the report by Yang et al[4]. Certainly, there could be to display that prevalence of behavioral features was overlapped to present by clinical findings of between Sotos syndrome and ADHD in patients[22]. Based on these findings, it is suggested that NSD1 gene mutation is highly related to Sotos syndrome, but is rarely found in ADHD patients.
FUTURE PERSPECTIVES
Based on the patient described by Yang et al[4], the sample size of patients presenting with ADHD and Sotos syndrome is expected to be enlarged whether NSD1 mutations are examined or not. The genetic mechanism of NSD1 mutation should be investigated in association with the clinical features of both Sotos syndrome and ADHD in future studies. Moreover, transcriptome profiling is being used to evaluate the gene expression status in multiple brain tissues and whole blood in ADHD and Sotos syndrome patients[23,24]. During these investigations, one report indicated that five probands were examined by short-read genome sequencing (GS) and long-read GS to identify three variants of NSD1, Kelch-like protein 7 and H3 lysine 4 methylase in neurodevelopmental disorders including Sotos syndrome, Perching and Kabuki syndromes as well as assessment by peripheral blood transcriptome sequencing. In another report, a transcriptome-wide association study with meta-analysis was carried out to determine the relationship between ADHD and transcriptomic risk scores from multiple brain tissues and blood samples of 14 gene-expression reference panels in 38691 patients with ADHD and 186843 controls. These findings may provide potential clinical applications in the previously mentioned neurodevelopmental disorders. Hence, use of transcriptome profiles for discovering more potential candidates to explore the pathogenesis of Sotos syndrome patients with ADHD is necessary in the future studies.
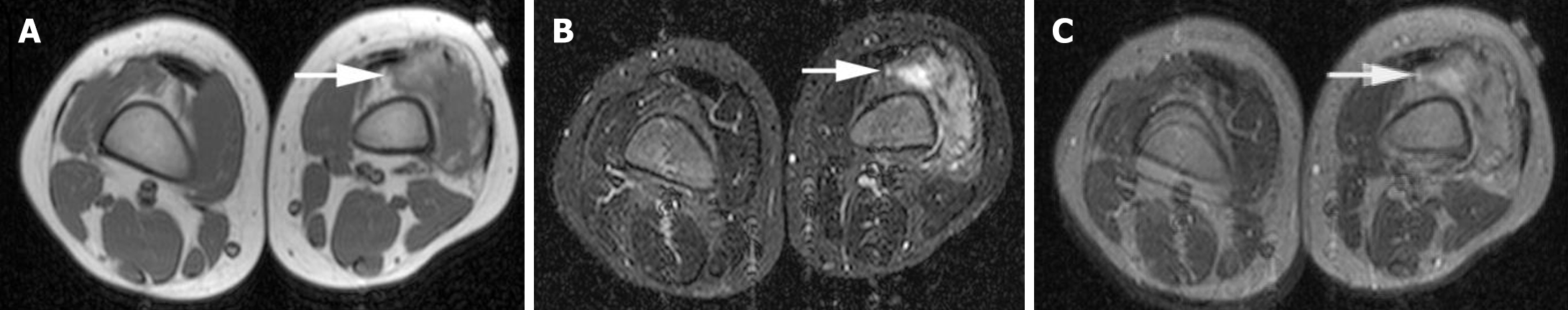
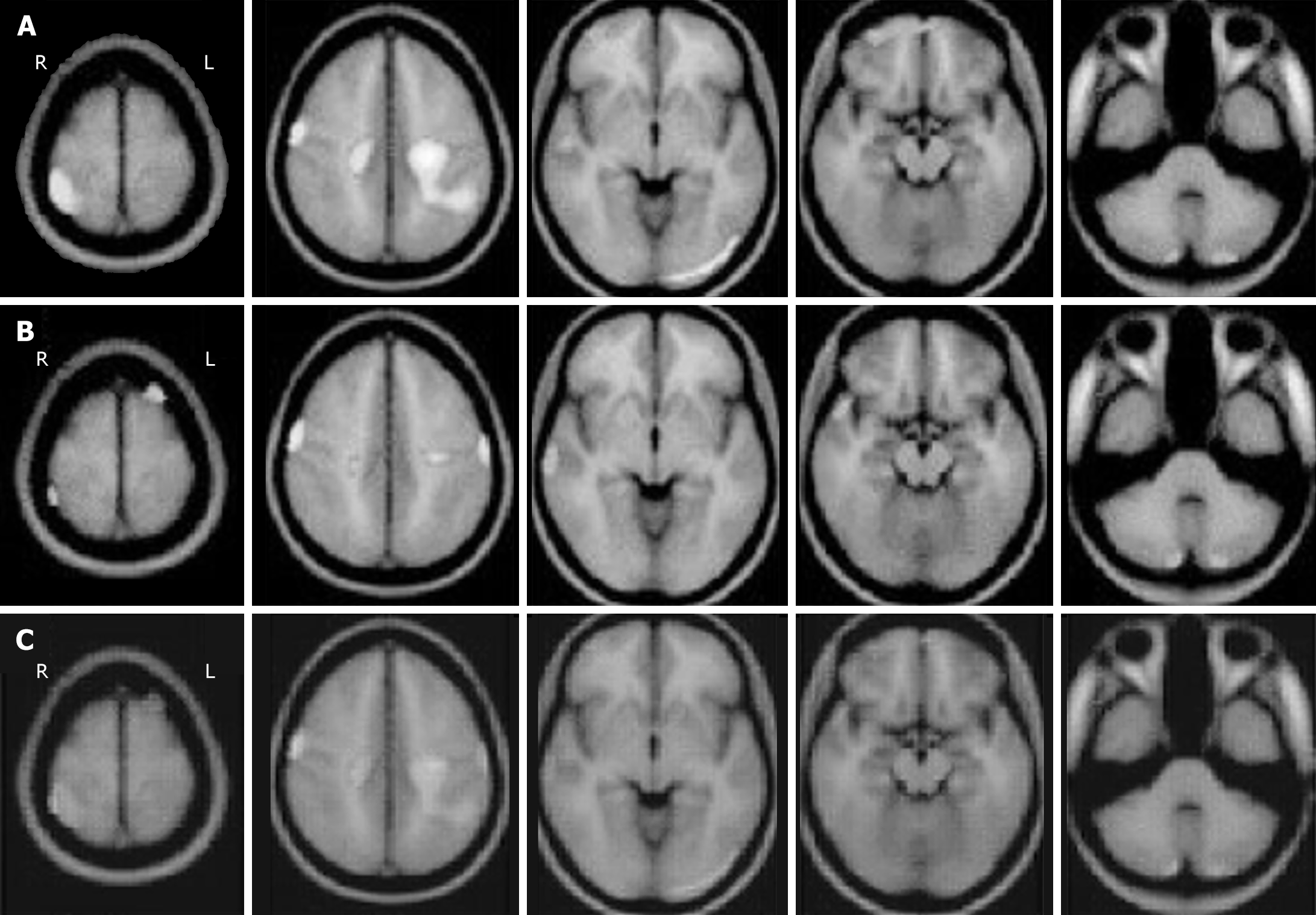
Medical imaging has been used to display the features of patients to aid diagnosis by doctors in the clinic. Although the phenotypic and genotypic profile of patients with Sotos syndrome and ADHD were previously characterized by clinical and genomic examinations, medical imaging such as magnetic resonance imaging (MRI) and functional MRI have been used to identify pathogenic factors in these patients[25,26]. As presented by Yang et al[4], the left hand of the Sotos syndrome patient with ADHD was examined by X-ray radiographs and the patient’s pituitary gland was evaluated by MRI scans. Image processing via artificial intelligence should be introduced in patients with Sotos syndrome and/or ADHD examined by MRI. For example, wavelet fusion can integrate the characteristics from two distinct images and provide more useful information for radiologists. The wavelet fusion method has been investigated by our computer resources to present the results in Figure 1 and 2, while gray Figure 1A and B from primary images[27] as well as gray Figure 2A and B treated by our modified method from primary images[28] were respectively integrated via wavelet fusion into the final results in Figure 1C and 2C. These fused images showed more in-depth information and less background compared to the single image and could provide better diagnostics in Sotos syndrome and ADHD.
Figure 1 Magnetic resonance imaging of soft-tissue sarcoma in a female child with Sotos syndrome.
A: Axial T1-weighted magnetic resonance imaging (MRI) with gray level showed isointense in the muscle with a high signal; B: Short tau inversion recovery MRI with gray level showed a heterogeneous mass with a high signal; C: Wavelet fusion was performed by integrating both A and B. Arrows show an ill-defined mass.
Figure 2 Magnetic resonance imaging of brain areas with cerebral blood flow in children with attention-deficit/hyperactivity disorder.
A and B: Lines of magnetic resonance imaging with gray level was applied to represent distinct baselines of cerebral blood flow at high or low density; C: Wavelet fusion was used to display the different cerebral blood flow in the pooled image. R: Right; L: Left.
CONCLUSION
NSD1 gene mutation has an important role in the pathogenesis of Sotos syndrome, and its function is associated with ADHD which could be an interesting topic for future investigation. MRI is a critical technique in the diagnosis of Sotos syndrome and/or ADHD. Medical image processing should be applied in the MRI in the future diagnosis.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade D
Novelty: Grade C, Grade C
Creativity or Innovation: Grade B, Grade C,
Scientific Significance: Grade B, Grade B
P-Reviewer: Nguyen PD S-Editor: Luo ML L-Editor: A P-Editor: Chen YX