Published online Apr 26, 2025. doi: 10.12998/wjcc.v13.i12.100248
Revised: November 29, 2024
Accepted: December 19, 2024
Published online: April 26, 2025
Processing time: 149 Days and 1.7 Hours
This article discusses the coexistence of prostate adenocarcinoma and prostate urothelial carcinoma. Combining existing literature and research results, the potential mechanisms of the co-occurrence of these two cancers are explored, including the role of androgen receptor, gene mutations, and their complex interactions in cell signaling pathways, etc. Also, the hypothesis of prostate cancer transformation into urothelial carcinoma is explained from some perspectives, including tumor multipotent stem cell differentiation, epithelial-mesenchymal transition, mesenchymal-epithelial transition, and other mechanisms. Ultimately, the goal is to provide more accurate diagnoses and more personalized treatments in clinical practice, as well as to lay the foundation for improving patient pro
Core Tip: This article delves deeply into the mechanisms underlying the coexistence of prostate adenocarcinoma and urothelial carcinoma, encompassing the intricate interplay of androgen receptors, genetic mutations, and cellular signaling pathways. It elucidates the hypothesis that prostate cancer could potentially transition into urothelial carcinoma, offering fresh perspectives to inform clinical practice.
- Citation: Liu XC, Liu YX, Liu C. Concurrent occurrence of adenocarcinoma and urothelial carcinoma of the prostate: Coexistence mechanisms from multiple perspectives. World J Clin Cases 2025; 13(12): 100248
- URL: https://www.wjgnet.com/2307-8960/full/v13/i12/100248.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i12.100248
Prostate cancer ranks as the second most prevalent form of cancer among men globally, with its incidence rate escalating as they age[1]. The majority of prostate cancer cases are adenocarcinomas, classified using the Gleason grading system[2]. However, as a rare subtype, prostatic urothelial carcinoma typically originates from the prostatic urethra urothelium[3]. It may be associated with the proximal prostatic duct. Hsu et al's article details the case of an 82-year-old man diagnosed with both prostate adenocarcinoma and urothelial carcinoma[4]. It provides a comprehensive account of his diagnostic journey, treatment regimen, surgical intervention, and postoperative monitoring, while also delving into the mechanisms underlying the coexistence of these two malignancies. In clinical practice, the coexistence of prostate adenocarcinoma and urothelial carcinoma is exceedingly rare. This not only complicates the diagnostic and treatment processes but also presents new challenges to current strategies for managing prostate cancer. Drawing upon current literature and the most recent research findings, this article delves into the mechanisms underlying the coexistence of prostate adenocarcinoma and urothelial carcinoma.
The study of prostate adenocarcinoma in conjunction with urothelial carcinoma remains in its nascent stages. The majority of reports consist of individual cases or studies with small sample sizes, lacking the support of large-scale, multicenter data. Understanding the mechanisms behind their coexistence is crucial for clinical diagnosis, treatment, and prognosis evaluation.
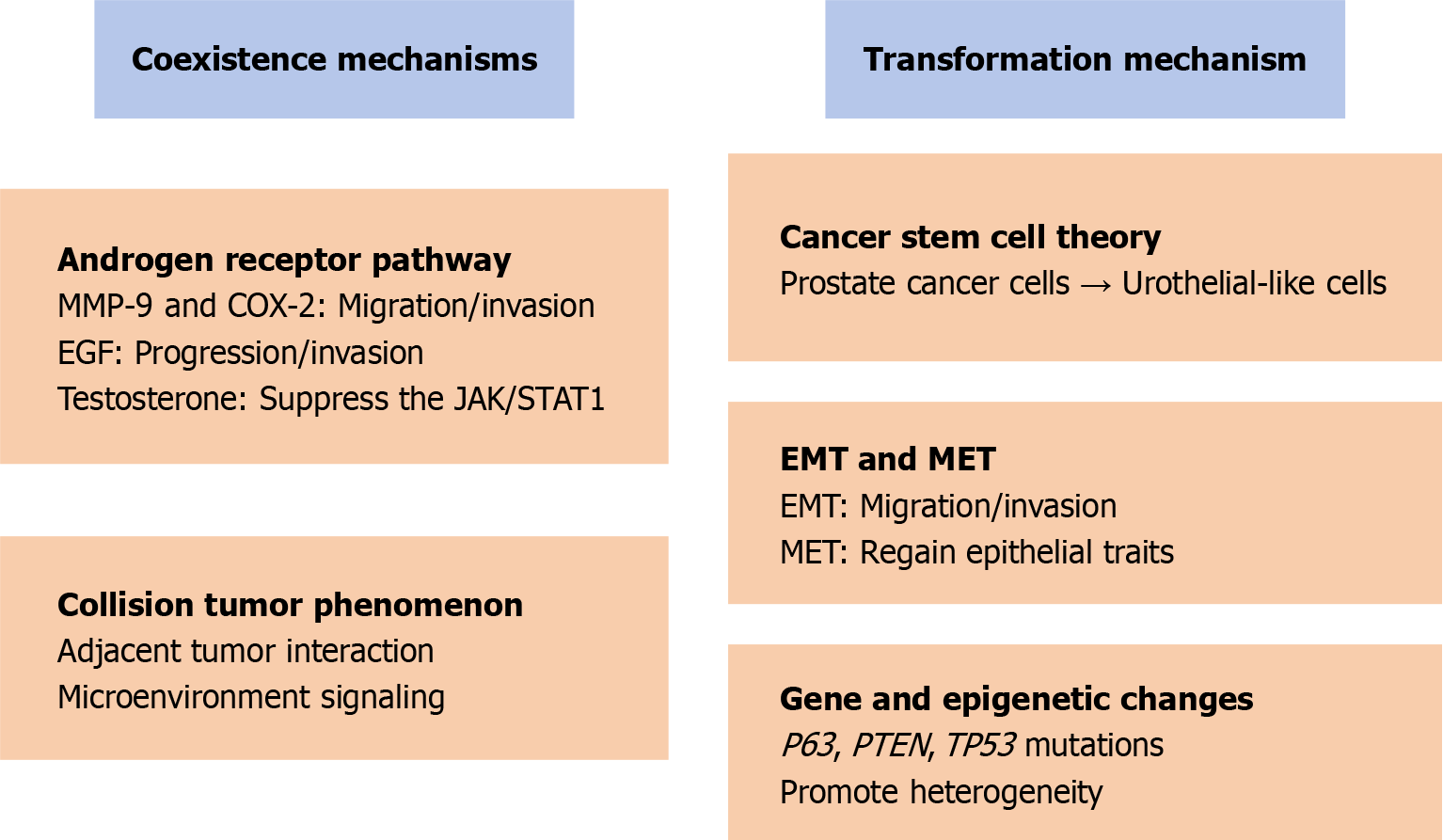
The case detailed in Hsu et al's article involved an elderly male patient[4]. Perhaps we can propose a hypothesis: Androgen receptor (AR) is the key to the co-occurrence of the two tumors. Androgens can bind to the AR and transport extracellular androgens into the nucleus via this receptor. The resulting androgen-AR complexes then attach to DNA, modulating gene expression. This process influences their function in the regulation of the reproductive, immune, and endocrine systems[5]. Activation of the AR and AR-driven transcriptional programs are central to prostate cancer pathophysiology. AR plays a key role in prostate cancer, especially castration-resistant prostate cancer, through mechanisms such as point mutation, overexpression, and altered androgen biosynthesis[6]. Likewise, AR activation promotes urothelial carcinogenesis and tumor growth[7]. A systematic review and meta-analysis have uncovered a high incidence of prostate cancer within cystoprostatectomy specimens from patients diagnosed with urothelial bladder cancer. This discovery could further support the notion that AR signaling serves as a common pathway in the development of these two types of cancer[8]. This phenomenon can be elucidated at the cellular pathway level. For instance, the AR synergizes with MMP-9 and COX-2 to facilitate the migration and invasion of urothelial carcinoma and prostate cancer, thereby contributing to the development of these two types of cancer[9,10]. MMP-9, a matrix metalloproteinase, is responsible for degrading collagen and extracellular matrix components, thereby facilitating the spread of cancer cells. COX-2, an enzyme that produces prostaglandins such as PGE2, plays a pivotal role in inflammation and is frequently overexpressed in prostate and urothelial cancers, correlating with cancer progression and resistance to apoptosis. The prostaglandins produced by COX-2 can stimulate MMP-9 through heightened inflammation, leading to elevated levels of MMP-9 within the tumor microenvironment. The expression of growth factors within the tumor microenvironment could play a significant role in the concurrent development of prostate adenocarcinoma and urothelial carcinoma. The functional interplay between AR and a cascade of growth factor signaling events, such as those involving epidermal growth factor (EGF), fibroblast growth factor, insulin-like growth factor 1, vascular endothelial growth factor, and transforming growth factor-beta, orchestrates the cell cycle, apoptosis, and differentiation processes in prostate cancer cells[11]. The downregulation of AR can suppress the growth of urothelial carcinoma by inducing cellular apoptosis, decreasing cellular proliferation, and curtailing cellular migration[12]. For example, EGF can promote bladder cancer progression and invasion by enhancing AR transactivation[13]. Testosterone propels the growth of prostate cancer by activating AR through ligand binding. Initial serum testosterone levels are correlated with the risk of developing prostate cancer and the outcomes of the disease[14,15]. Testosterone has the potential to optimize its levels by modulating the expression of enzymes responsible for steroid metabolism, which convert androgen precursors into active androgens within prostate tumor cells. Research has indicated that testosterone exhibits an anti-inflammatory effect in LPS-induced prostate epithelial cells by suppressing the JAK/STAT1 signaling pathway. This suppression could explain why prostate urothelial carcinoma tends to remain confined to the prostate, rather than spreading to the bladder[16,17].
Prostate cancer combined with urothelial carcinoma may also involve tumor collision phenomenon. In tumor biology, collision tumors are a rare and complex phenomenon involving two different tumor cell populations that invade each other or grow adjacent to each other in the same tissue or organ, but without mixing of cellular components[18]. This phenomenon not only prompts an in-depth investigation into tumor biology but also offers a novel perspective on our comprehension of cellular lineage determination. The emergence of collision tumors induces substantial alterations in the microenvironment. Cells from distinct lineages, when interacting within the same microenvironment, may provoke changes in cellular signaling, thereby influencing cell fate selection[19]. For instance, a particular type of tumor cell might release specific factors that either suppress or stimulate the growth or differentiation of another type of tumor cell. Direct interaction between tumor cells and the cytokines they secrete can result in reciprocal signaling. These signals have the potential to modify gene expression within cells, thereby impacting their capacity to proliferate, migrate, and differentiate[20,21]. For instance, via contact-dependent signaling among cells, they can modify their differentiation state to accommodate alterations in their surrounding environment (Figure 1).
The transformation of prostate cancer into urothelial carcinoma is an exceedingly rare yet clinically significant phenomenon, attributed to multiple intricate mechanisms. Trans differentiation, which involves the direct conversion of one mature cell type into another, could also serve as a mechanism for this transformation in prostate cancer. This process may entail the reprogramming of particular genes and signaling pathways, rendering cancer cells highly plastic and adaptable. The theory of pluripotent cancer stem cells posits that prostate cancer cells can be effectively reprogrammed into induced pluripotent stem cells. These cells have the potential to differentiate into urothelial-like cells under a range of conditions. This process may entail the activation of particular microenvironmental signals and gene regulatory pathways[22]. Furthermore, this transition may encompass both the epithelial-mesenchymal transition (EMT) and the mesenchymal-epithelial transition (MET). The EMT is a cellular process wherein epithelial cells undergo molecular alterations to adopt characteristics akin to mesenchymal cells. MET represents the reversal of EMT, characterized by the reversion of cellular state from a mesenchymal phenotype back to an epithelial one. In the course of EMT, cancer cells boost their ability to invade and migrate by shedding epithelial traits and adopting those of mesenchymal cells. Subsequently, during MET, these cells have the potential to reacquire epithelial characteristics. Under certain conditions, a subset of stem cells can differentiate into urothelial-like cells, endowing them with features akin to urothelial carcinoma[23,24]. Gene mutations and epigenetic changes are also critical to this process. These changes may lead to abnormal cell cycle regulation and genomic instability, further promoting cancer cells' phenotypic transformation and malignant behavior. The tumor protein P63 (P63) serves as a marker for basal epithelial cells and is essential for the proper development of various epithelial tissues, such as the bladder and prostate. P63 exhibits both tumor suppressive and oncogenic characteristics and plays a pivotal role in the differentiation of prostate and urothelial cells. Dysregulation of P63 could result in aberrant differentiation, potentially elevating the risk of cancer coexistence. Prostate progenitor cells are believed to be the origin of prostate cancer tumors. PTEN and TP53 are pivotal in governing the self-renewal and differentiation processes of prostate progenitor cells. The absence of PTEN/TP53 in the prostate epithelium triggers the transformation of multipotent progenitor cells and induces an epithelial-to-mesenchymal transition, which in turn fosters tumor heterogeneity and enhances metastatic potential[25] (Figure 1).
Although the co-occurrence of prostate adenocarcinoma and urothelial carcinoma is rare, it highlights the potential diversity and complexity of cancer evolution. This coexistence not only challenges traditional cancer classification and treatment options but also emphasizes the importance of monitoring and managing heterogeneous tumors in prostate cancer patients. Although current studies have not yet fully revealed the exact mechanism of the co-development of the two cancers, existing findings suggest that they may involve a series of complex gene regulation and cell signaling networks. Future research should focus on large-scale clinical data collection and more in-depth mechanistic exploration to better understand this phenomenon and ultimately improve patients' treatment outcomes and quality of life.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12804] [Article Influence: 6402.0] [Reference Citation Analysis (8)] |
| 2. | Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1272] [Article Influence: 127.2] [Reference Citation Analysis (2)] |
| 3. | Mazzucchelli R, Lopez-Beltran A, Cheng L, Scarpelli M, Kirkali Z, Montironi R. Rare and unusual histological variants of prostatic carcinoma: clinical significance. BJU Int. 2008;102:1369-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Hsu JY, Lin YS, Huang LH, Tsao TY, Hsu CY, Ou YC, Tung MC. Concurrent occurrence of adenocarcinoma and urothelial carcinoma of the prostate gland: A case report. World J Clin Cases. 2024;12:5952-5959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36:3-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 664] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 6. | Fujita K, Nonomura N. Role of Androgen Receptor in Prostate Cancer: A Review. World J Mens Health. 2019;37:288-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (2)] |
| 7. | Inoue S, Mizushima T, Miyamoto H. Role of the androgen receptor in urothelial cancer. Mol Cell Endocrinol. 2018;465:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Fahmy O, Khairul-Asri MG, Schubert T, Renninger M, Stenzl A, Gakis G. Clinicopathological Features and Prognostic Value of Incidental Prostatic Adenocarcinoma in Radical Cystoprostatectomy Specimens: A Systematic Review and Meta-Analysis of 13,140 Patients. J Urol. 2017;197:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Mandel A, Larsson P, Sarwar M, Semenas J, Syed Khaja AS, Persson JL. The interplay between AR, EGF receptor and MMP-9 signaling pathways in invasive prostate cancer. Mol Med. 2018;24:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Chen CC, Hsieh TF, Chang CH, Ma WL, Hung XF, Tsai YR, Lin MH, Zhang C, Chang C, Shyr CR. Androgen receptor promotes the migration and invasion of upper urinary tract urothelial carcinoma cells through the upregulation of MMP-9 and COX-2. Oncol Rep. 2013;30:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (2)] |
| 11. | Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15:841-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Bourlon MT, Flaig TW. The Epidemiological, Mechanistic and Potential Clinical Role of Androgen Receptor (AR) in Urothelial Carcinoma. Curr Drug Targets. 2016;17:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Hsieh TF, Chen CC, Ma WL, Chuang WM, Hung XF, Tsai YR, Lin MH, Zhang Q, Zhang C, Chang C, Shyr CR. Epidermal growth factor enhances androgen receptormediated bladder cancer progression and invasion via potentiation of AR transactivation. Oncol Rep. 2013;30:2917-2922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Fiamegos A, Varkarakis J, Kontraros M, Karagiannis A, Chrisofos M, Barbalias D, Deliveliotis C. Serum testosterone as a biomarker for second prostatic biopsy in men with negative first biopsy for prostatic cancer and PSA>4ng/mL, or with PIN biopsy result. Int Braz J Urol. 2016;42:925-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (4)] |
| 15. | Capogrosso P, Ventimiglia E, Moschini M, Boeri L, Farina E, Finocchio N, Gandaglia G, Fossati N, Briganti A, Montorsi F, Salonia A. Testosterone Levels Correlate With Grade Group 5 Prostate Cancer: Another Step Toward Personalized Medicine. Prostate. 2017;77:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Ho CH, Fan CK, Yu HJ, Wu CC, Chen KC, Liu SP, Cheng PC. Testosterone suppresses uropathogenic Escherichia coli invasion and colonization within prostate cells and inhibits inflammatory responses through JAK/STAT-1 signaling pathway. PLoS One. 2017;12:e0180244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Kaipainen A, Zhang A, Gil da Costa RM, Lucas J, Marck B, Matsumoto AM, Morrissey C, True LD, Mostaghel EA, Nelson PS. Testosterone accumulation in prostate cancer cells is enhanced by facilitated diffusion. Prostate. 2019;79:1530-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 18. | Bulte CA, Hoegler KM, Khachemoune A. Collision tumors: A review of their types, pathogenesis, and diagnostic challenges. Dermatol Ther. 2020;33:e14236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Yang C, Li S, Liang Z, Jiang L. Case Report: The first case of primary pulmonary collision tumor comprising mixed squamous cell and glandular papilloma and glomus tumor. Front Oncol. 2022;12:1050220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 20. | Abdullah AM, Qaradakhy AJ, Ahmed MM, Salih AM, Omar SS, Kakamad FH, Rahim HM, Abdulla BA, Mohammed SH, Ahmed SF, Baba HO, Ishaac RH. Thyroid collision tumors; A case series with literature review. Ann Med Surg (Lond). 2022;76:103444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Zapata Palomino M, Caicedo-Holguín I, Pardo S, Mera AT, Gómez AP, Zorrilla JO. Case report: Tumor collision in the colon, adenocarcinoma - lymphoma. Int J Surg Case Rep. 2022;98:107573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 22. | Zhang Y, Chen B, Xu P, Liu C, Huang P. Reprogramming Prostate Cancer Cells into Induced Pluripotent Stem Cells: a Promising Model of Prostate Cancer Stem Cell Research. Cell Reprogram. 2020;22:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Lo UG, Lee CF, Lee MS, Hsieh JT. The Role and Mechanism of Epithelial-to-Mesenchymal Transition in Prostate Cancer Progression. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 24. | Pei D, Shu X, Gassama-Diagne A, Thiery JP. Mesenchymal-epithelial transition in development and reprogramming. Nat Cell Biol. 2019;21:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 25. | Martin P, Liu YN, Pierce R, Abou-Kheir W, Casey O, Seng V, Camacho D, Simpson RM, Kelly K. Prostate epithelial Pten/TP53 loss leads to transformation of multipotential progenitors and epithelial to mesenchymal transition. Am J Pathol. 2011;179:422-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/