Published online Mar 16, 2024. doi: 10.12998/wjcc.v12.i8.1448
Peer-review started: November 13, 2023
First decision: December 18, 2023
Revised: January 11, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 16, 2024
Processing time: 120 Days and 5.7 Hours
Clear cell sarcoma (CCS) is a rare soft-tissue sarcoma. The most common metastatic sites for CCS are the lungs, bones and brain. CCS is highly invasive and mainly metastasizes to the lung, followed by the bone and brain; however, pancreatic metastasis is relatively rare.
We report on a rare case of CCS with pancreatic metastasis in a 47-year-old man. The patient had a relevant medical history 3 years ago, with abdominal pain as the main clinical manifestation. No abnormalities were observed on physical examination and the tumor was found on abdominal computed tomography. Based on the medical history and postoperative pathology, the patient was diagnosed with CCS with pancreatic metastasis. The patient was successfully treated with surgical interventions, including distal pancreatectomy and sple
This report summarizes the available treatment modalities for CCS and the importance of regular postoperative follow-up for patients with CCS.
Core Tip: Clear cell sarcoma (CCS) is a rare soft tissue sarcoma accounting for less than 1% of sarcomas and was originally reported in 1965. CCS is a type of highly invasive cancer which mainly metastasizes to the lungs, followed by the bones and brain, but pancreatic metastasis is relatively rare. Here, we give details of the clinical manifestations and imaging features of a case of metastatic CCS of the pancreas.
- Citation: Liu YJ, Zou C, Wu YY. Metastatic clear cell sarcoma of the pancreas: A rare case report. World J Clin Cases 2024; 12(8): 1448-1453
- URL: https://www.wjgnet.com/2307-8960/full/v12/i8/1448.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i8.1448
Clear cell sarcoma (CCS) is a rare soft-tissue sarcoma (STS) that accounts for less than 1% of all cases and was originally reported in 1965[1,2]. The incidence of CCS is estimated to be approximately 0.014/100000 depending on the surveillance, epidemiology and end results databases[3]. CCS originates from the tendon and aponeurosis, and is characterized by local invasive growth of the tendon and other soft tissues[4]. CCS is a highly invasive type that mainly metastasizes to the lungs, followed by the bones and brain; however, pancreatic metastasis is relatively rare[3-8]. To our knowledge, only two cases of primary pancreatic CCS have been reported[9,10]. Here, we provide details of the clinical manifestations and imaging features of a patient with metastatic CCS of the pancreas.
A 47-year-old male presented with abdominal pain and diarrhea.
Symptoms started more than a half month before presentation with abdominal pain and diarrhea.
No special notes.
No special notes.
No special notes.
No special notes.
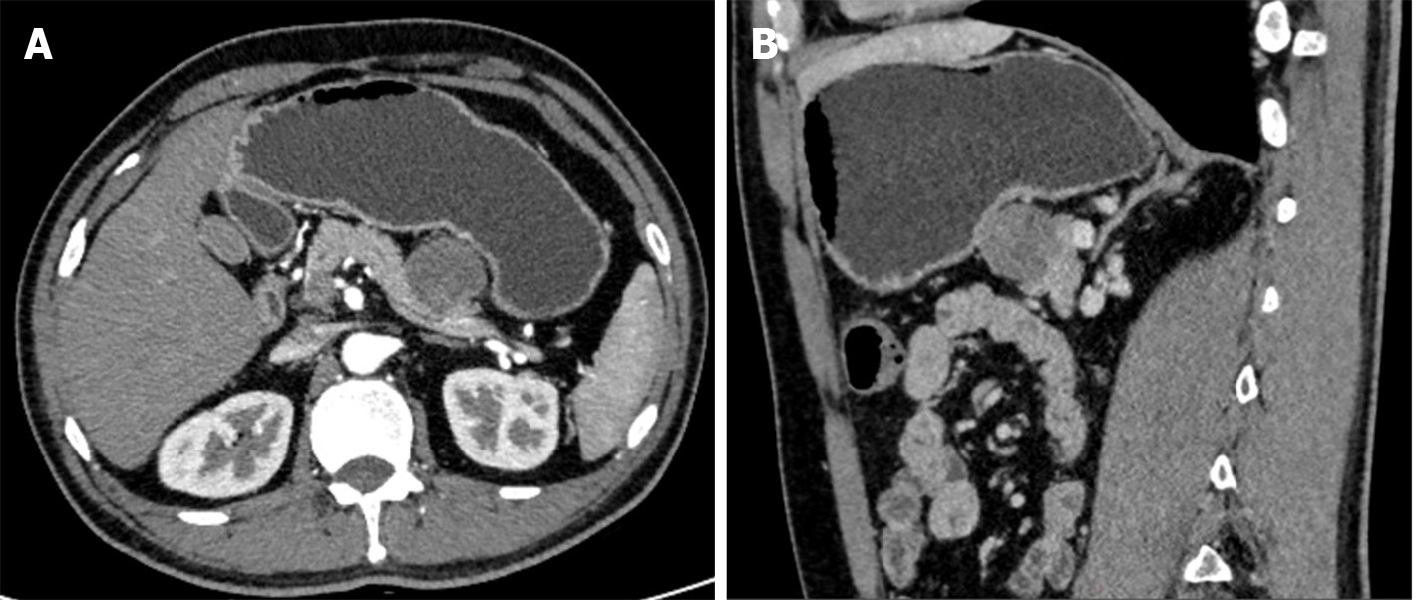
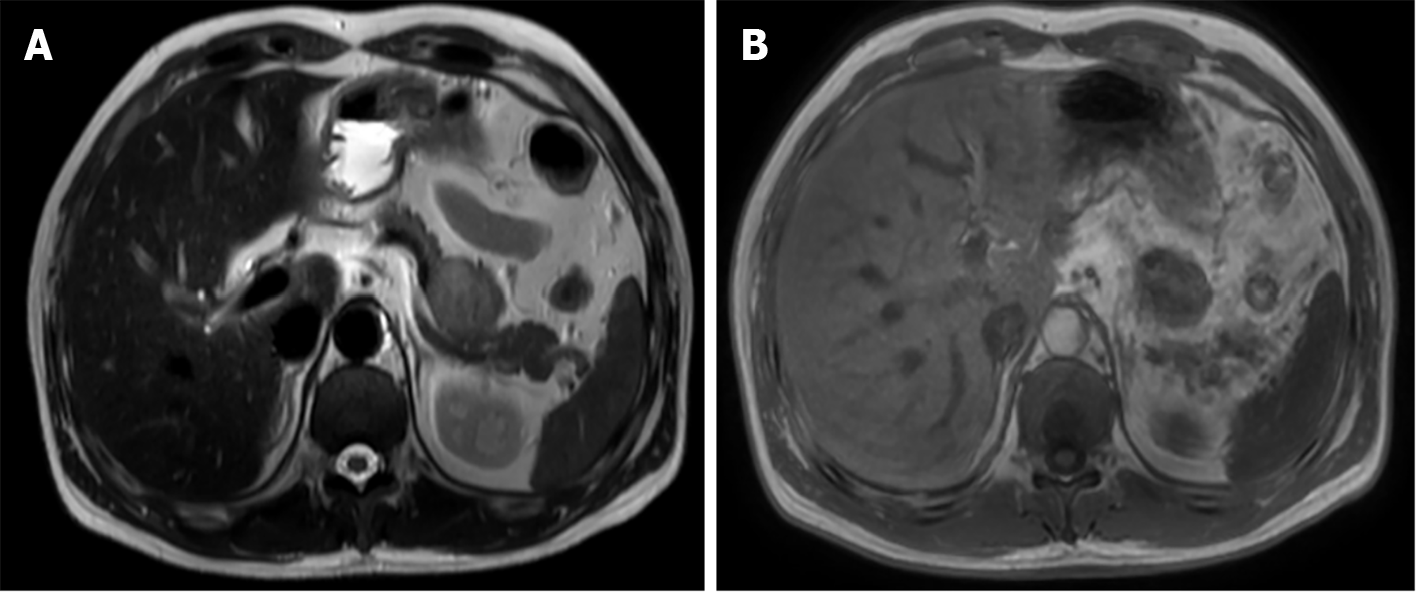
Computed tomography (CT) and magnetic resonance imaging (MRI) revealed an isolated lesion in the pancreatic tail without metastasis (Figures 1 and 2).
The preoperative diagnosis did not rule out the possibility of CCS recurrence combined with the patient's disease history.
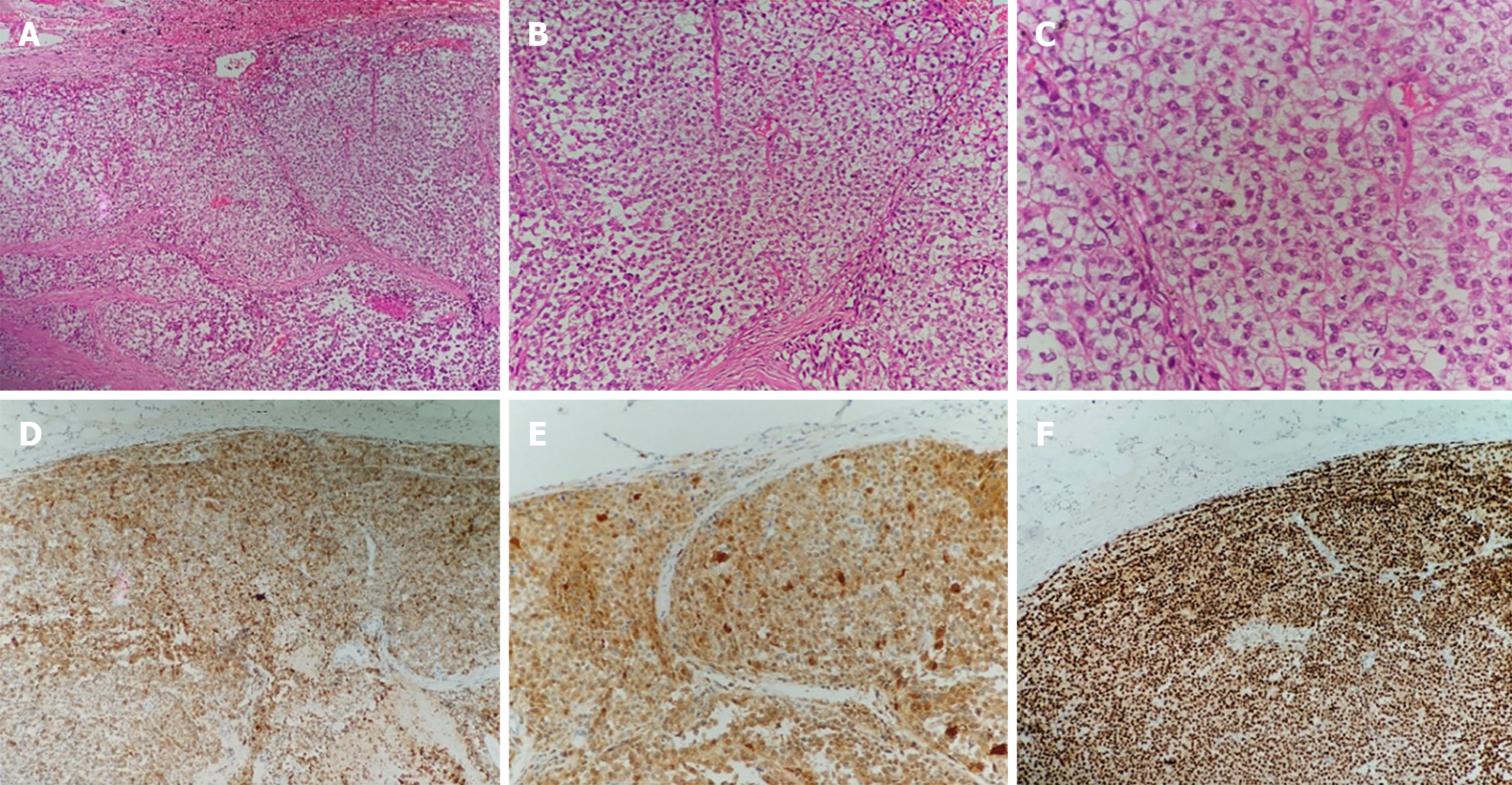
The patient underwent surgical treatment. Under general anesthesia, the abdomen was prepped and draped in the usual fashion, with the patient in the supine position. A midline incision of the abdomen was made, and the peritoneal cavity was entered. Generalized abdominal exploration revealed no metastases to the liver, spleen, or peritoneal omentum of the abdominopelvic cavity. The gastrocolic ligament was opened in the avascular zone, and the stomach was turned upward to reveal the pancreas. A solid tumor measuring 3 cm × 3 cm × 4 cm with a hard texture and smooth surface located in the pancreatic body was found during surgery. A distal pancreatectomy and splenectomy were successfully performed. The posterior, transverse, caudal, and dorsal pancreatic arteries and pancreatic freeing were ligated. The pancreatic tissues were gradually dissected by suturing 2 cm away from the tumor, close to the neck of the pancreas. The pancreatic body and tail, and the spleen were excised. No tumor residue was found in the pancreatic margins or the spleen (Figure 3).
The patient recovered well after surgery without complications and was free of any discomfort until the last follow-up (6 mo postoperatively), with refusal of any other therapy or examination.
CCS is a malignant tumor arising from the neural spinal cells caused by the EWSR1-ATF1 gene fusion resulting from t(12;22) (q13;q12) translocation[11]. CCS often occurs in the second to fourth decades of life and affects the lower extremities. CCS metastasis to the gastrointestinal system (CCSLGT) is rare and was first reported by Zambrano et al[12] in 2003. CCSLGT is prevalent in the walls of the small intestine, stomach, colon, and peritoneum, but is rare in the pancreas[13,14]. The manifestations of CCSLGT include abdominal pain, intestinal obstruction, anemia, nausea, and vomiting. The clinical presentation in this case was abdominal pain with nausea, which is consistent with the literature.
Currently, there is no unified management method for advanced CCS, and surgery remains the standard treatment, similar to most STSs[4,7,15,16]. Ensuring that the margins of the tumor are clean and retaining a ring of normal tissue around the tumor is essential. Preoperative imaging assessment in conjunction with patient history is appropriate. CT provides initial postoperative follow-up and evaluation. MRI is an important modality that can reveal the location and extent of tumor invasion[17,18]. In this case, the patient was initially diagnosed with metastatic CCS on preoperative MRI, thus providing a good plan for surgery and ensuring clean surgical margins.
CCS has a poor prognosis, with limited effectiveness of other postoperative treatments. According to the literature, chemotherapy and radiotherapy for CCS are unsatisfactory[19-22]. Some studies have demonstrated that targeted therapy or immunotherapy may be effective; however, no confirmatory clinical trials have been conducted[23-27].
Despite optimal management of local disease, CCS has a high probability of recurrence or metastasis, and when it progresses to stages III and IV, the 5-year survival rate significantly reduces to 15%-35%[3]. Long-term follow-up is necessary for patients with CCS, because new metastatic lesions may appear after a longer period[28]. Postoperative management is important in CCS to effectively detect early metastases. In this case, the patient was not followed up after the first operation until symptoms appeared, leading to delayed treatment. At this time, the patient resisted follow-ups and examinations that would have facilitated disease monitoring.
A rare case of CCS in a patient who developed pancreatic metastases 3 years after surgery has been reported. Regularized follow-up and re-examination were not performed after the first operation, resulting in the patient being seen only after the onset of symptoms, which may have delayed the treatment. Therefore, regular postoperative follow-up is important for patients with CCS because recurrence has a long-term course.
| 1. | Dim DC, Cooley LD, Miranda RN. Clear cell sarcoma of tendons and aponeuroses: a review. Arch Pathol Lab Med. 2007;131:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Enzinger FM. Clear-cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. 1965;18:1163-1174. [PubMed] [DOI] [Full Text] |
| 3. | Gonzaga MI, Grant L, Curtin C, Gootee J, Silberstein P, Voth E. The epidemiology and survivorship of clear cell sarcoma: a National Cancer Database (NCDB) review. J Cancer Res Clin Oncol. 2018;144:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Kawai A, Hosono A, Nakayama R, Matsumine A, Matsumoto S, Ueda T, Tsuchiya H, Beppu Y, Morioka H, Yabe H; Japanese Musculoskeletal Oncology Group. Clear cell sarcoma of tendons and aponeuroses: a study of 75 patients. Cancer. 2007;109:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Basile G, Mattei JC, Alshaygy I, Griffin AM, Catton CN, Chung PW, Shultz DB, Razak ARA, Demicco EG, Ferguson PC, Wunder JS. Curability of patients with lymph node metastases from extremity soft-tissue sarcoma. Cancer. 2020;126:5098-5108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Sara AS, Evans HL, Benjamin RS. Malignant melanoma of soft parts (clear cell sarcoma). A study of 17 cases, with emphasis on prognostic factors. Cancer. 1990;65:367-374. [PubMed] [DOI] [Full Text] |
| 7. | Hocar O, Le Cesne A, Berissi S, Terrier P, Bonvalot S, Vanel D, Auperin A, Le Pechoux C, Bui B, Coindre JM, Robert C. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. 2012;2012:984096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Li AB, Jiang BJ, Wang HH, Yang YS, Zhang XB, Lan GH, Shu WB. Prognostic Factors for Survival in Patients with Clear Cell Sarcoma: An Analysis of the Surveillance, Epidemiology, and End Results (SEER) Database. Med Sci Monit. 2019;25:6950-6956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Huang J, Luo RK, Du M, Zeng HY, Chen LL, Ji Y. Clear cell sarcoma of the pancreas: a case report and review of literature. Int J Clin Exp Pathol. 2015;8:2171-2175. [PubMed] |
| 10. | Xiang H, Xiang W, Wang L. Primary clear cell-sarcoma of the pancreas: A case report. Asian J Surg. 2023;46:4842-4843. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Mavrogenis A, Bianchi G, Stavropoulos N, Papagelopoulos P, Ruggieri P. Clinicopathological features, diagnosis and treatment of clear cell sarcoma/melanoma of soft parts. Hippokratia. 2013;17:298-302. [PubMed] |
| 12. | Zambrano E, Reyes-Mugica M, Franchi A, Rosai J. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Yegen G, Güllüoğlu M, Mete Ö, Önder S, Kapran Y. Clear cell sarcoma-like tumor of the gastrointestinal tract: a case report and review of the literature. Int J Surg Pathol. 2015;23:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Huang W, Zhang X, Li D, Chen J, Meng K, Wang Y, Lu Z, Zhou X. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch. 2006;448:200-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Lucas DR, Nascimento AG, Sim FH. Clear cell sarcoma of soft tissues. Mayo Clinic experience with 35 cases. Am J Surg Pathol. 1992;16:1197-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 157] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Kuiper DR, Hoekstra HJ, Veth RP, Wobbes T. The management of clear cell sarcoma. Eur J Surg Oncol. 2003;29:568-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Cheney MD, Giraud C, Goldberg SI, Rosenthal DI, Hornicek FJ, Choy E, Mullen JT, Chen YL, Delaney TF. MRI surveillance following treatment of extremity soft tissue sarcoma. J Surg Oncol. 2014;109:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Liburd CG, Gibson TN, Hanchard B, Waugh N, McNaughton D. Cutaneous Malignant Melanoma in Jamaica, 1958 to 2007. West Indian Med J. 2014;63:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 19. | Jones RL, Constantinidou A, Thway K, Ashley S, Scurr M, Al-Muderis O, Fisher C, Antonescu CR, D'Adamo DR, Keohan ML, Maki RG, Judson IR. Chemotherapy in clear cell sarcoma. Med Oncol. 2011;28:859-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Stacchiotti S, Palassini E, Negri T, Orsenigo M, Bertulli R, Morosi C, Pilotti S, Fiore M, Gronchi A, Casali PG. Clear cell sarcoma (CCR): Clinical behavior and response to chemotherapy. J Clin Oncol. 2010;28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Clark MA, Johnson MB, Thway K, Fisher C, Thomas JM, Hayes AJ. Clear cell sarcoma (melanoma of soft parts): The Royal Marsden Hospital experience. Eur J Surg Oncol. 2008;34:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Deenik W, Mooi WJ, Rutgers EJ, Peterse JL, Hart AA, Kroon BB. Clear cell sarcoma (malignant melanoma) of soft parts: A clinicopathologic study of 30 cases. Cancer. 1999;86:969-975. [PubMed] |
| 23. | Kataria B, Sharma A, Biswas B, Bakhshi S, Pushpam D. Pazopanib in rare histologies of metastatic soft tissue sarcoma. Ecancermedicalscience. 2021;15:1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, Du F, Sun Y, Wu Q, Qu G, Wang S, Song J, Yu J, Lu Y, Zhu X, Niu X, He Z, Wang J, Yu H, Cai J. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin Cancer Res. 2018;24:5233-5238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 250] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 25. | Smrke A, Frezza AM, Giani C, Somaiah N, Brahmi M, Czarnecka AM, Rutkowski P, Van der Graaf W, Baldi GG, Connolly E, Duffaud F, Huang PH, Gelderblom H, Bhadri V, Grimison P, Mahar A, Stacchiotti S, Jones RL. Systemic treatment of advanced clear cell sarcoma: results from a retrospective international series from the World Sarcoma Network. ESMO Open. 2022;7:100522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Wang J, Gao S, Yang Y, Liu X, Zhang P, Dong S, Wang X, Yao W. Clinical Experience with Apatinib and Camrelizumab in Advance Clear Cell Sarcoma: A Retrospective Study. Cancer Manag Res. 2021;13:8999-9005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Protsenko SA, Semionova AI, Komarov YI, Aleksakhina SN, Ivantsov AO, Iyevleva AG, Imyanitov EN. BRAF-mutated clear cell sarcoma is sensitive to vemurafenib treatment. Invest New Drugs. 2015;33:1136-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Bianchi G, Charoenlap C, Cocchi S, Rani N, Campagnoni S, Righi A, Frisoni T, Donati DM. Clear cell sarcoma of soft tissue: a retrospective review and analysis of 31 cases treated at Istituto Ortopedico Rizzoli. Eur J Surg Oncol. 2014;40:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Kim SY, South Korea; Lieto E, Italy; Vinh-Hung V, Martinique S-Editor: Liu JH L-Editor: Filipodia P-Editor: Yu HG