Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1272
Peer-review started: December 22, 2023
First decision: January 9, 2024
Revised: January 21, 2024
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: March 6, 2024
Processing time: 70 Days and 1.2 Hours
Remimazolam is characterized by rapid action and inactive metabolites. It is used as the general anesthetic for many clinical surgeries. In this study, we performed a meta-analysis to evaluate whether remimazolam is superior to propofol for gastroenteroscopy in older patients.
To compare the adverse events and efficacy of remimazolam and propofol during gastroenteroscopy in older adults.
The PubMed, Web of Science, the Cochrane Library databases were queried for the relevant key words "remimazolam,” "and propofol,” "and gastrointestinal endoscopy or gastroscopy.” The search scope was "Title and Abstract,” and the search was limited to human studies and publications in English. Seven studies wherein remimazolam and propofol were compared were included for the meta-analysis.
We selected seven randomized controlled trials involving 1445 cases for the analysis. Remimazolam reduced the hypotension (relative risk, RR = 0.44, 95%CI: 0.29-0.66, P = 0.000), respiratory depression (RR = 0.46, 95%CI: 0.30-0.70, P = 0.000), injection pain (RR = 0.12, 95%CI: 0.05-0.25, P = 0.000), bradycardia (RR = 0.37, 95%CI: 0.24-0.58, P = 0.000), and time to discharge [weighted mean difference (WMD) = -0.58, 95%CI: -0.97 to -0.18, P = 0.005], compared to those after propofol administration. No obvious differences were observed for postoperative nausea and vomiting (RR = 1.09, 95%CI: 0.97-1.24, P = 0.151), dizziness (RR = 0.77, 95%CI: 0.43-1.36, P = 0.361), successful sedation rate (RR = 0.96, 95%CI: 0.93-1.00, P = 0.083), or the time to become fully alert (WMD = 0.00, 95%CI: -1.08-1.08, P = 0.998).
Remimazolam appears to be safer than propofol for gastroenteroscopy in older adults. However, further studies are required to confirm these findings.
Core Tip: We searched the databases of PubMed, Web of Science, and the Cochrane Library spanning from its establishment until October 2023. After carefully screening, 7 randomized controlled trials encompassing 1445 cases were included in our study. The Cochrane tool was utilized to evaluate the potential for bias. Ultimately, our findings indicate that using remimazolam in painless gastroenteroscopy for older patients offers greater hemodynamic stability and fewer negative side effects compared to propofol. Thus, remimazolam seemed like a safer option than propofol for gastroenteroscopy for older patients.
- Citation: Li FZ, Zhao C, Tang YX, Liu JT. Safety and efficacy comparison of remimazolam and propofol for intravenous anesthesia during gastroenteroscopic surgery of older patients: A meta-analysis. World J Clin Cases 2024; 12(7): 1272-1283
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1272.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1272
Gastroenteroscopy is the gold standard for the diagnosis of gastrointestinal diseases; however, patients often feel unwell during the examination, and may find it difficult to complete the process. Currently, painless gastroscopy, wherein sleep is induced through anesthesia to ensure safe and comfortable completion of the process is the standard model[1]. Consequently, various sedatives such as benzodiazepines, opioids (pethidine and fentanyl), propofol, ketamine, and haloperidol are used during gastroscopy[2].
With the aging of China's society, the demand for painless gastroenteroscopy for older patients is increasing[3]; most older patients have a combination of chronic diseases, and with aging, organ functions decrease, degenerative changes occur in tissues and cells, and the older patients who are on a variety of anesthetic drugs are less tolerant. Propofol is a typical clinical anesthetic used for painless gastroenteroscopy in China[4]. However, its usage is accompanied by various adverse reactions. Because propofol is an emulsion injection, patients are prone to strong pain during injection, and simultaneously, the drug has different degrees of inhibitory effects on the respiration of patients. Respiratory depression and hypotension are common during the operation, and dizziness and vomiting are common during the postoperative period, along with various other adverse reactions. The main effects of benzodiazepines include sedation, hypnosis, anxiety reduction, and anticonvulsant activity[5]. Remimazolam benzenesulfonate is a new short-acting benzodiazepine, which exerts sedative and anesthetic effects by facilitating GABA binding to the receptor benzodiazepine binding site[6-8]. Remimazolam was first used in Japan and has since been used under the supervision of other countries[9]. Currently, it is widely used clinically and has been highly effective during Phase III clinical trials in patients requiring endoscopy[10]. It has been subjected to various stages of clinical trials and relevant studies and can be used safely and effectively for procedural sedation (e.g., gastrointestinal endoscopy and bronchoscopy) as well as general anesthesia[11-14]. However, a review or meta-analysis of randomized controlled trials (RCTs) of remimazolam vs propofol for painless gastroenteroscopy in older adults has not been reported. Therefore, we determined the risk of adverse effects of remimazolam and propofol in the older population through RCTs and further explored the sedative effects of both.
A meta-analysis to analyze the performance of remimazolam was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)[15], and the Cochrane manual was used to assess the quality of the included studies to ensure reliable and valuable results.
The PubMed, Web of Science, and the Cochrane Library databases were thoroughly searched by two researchers. Data from their inception to October 2023 were included. The search keywords were as follows: “remimazolam,” “and propofol,” and “and gastrointestinal endoscopy or gastroscopy”. The reference lists were also checked to determine relevant potentially eligible trials.
The eligibility criteria included patients: (1) Aged 60-95 years; (2) of BMI within 18-30 kg/m2; (3) who underwent gastrointestinal endoscopy in outpatient operating rooms; (4) with an ASA-PS score of no more than grade II; and (5) who provided informed consent.
The exclusion criteria were those: (1) With cardiac dysfunction; (2) with abnormal results in routine blood tests before endoscopy; (3) who were hospitalized after endoscopic surgery; (4) those who took benzodiazepines or opioids every day within 1 month; and (5) with allergies to flumazenil or naloxone.
The inclusion criterion was endoscopic surgery using remimazolam or propofol for sedation.
The inclusion criteria were: (1) Published RCT for clinical trials; (2) at least 80% follow-up rate for the study with one primary outcome; (3) complete treatment outcome; and (4) a report of either the amount of remimazolam or propofol administered during surgery, with incidence of adverse reactions.
The exclusion criteria included: Review articles, protocols, animal studies, case studies irrelevant to the question, and non-extractable studies. The authors independently evaluated all eligible studies and resolved any differences between them through discussions with the first co-author.
The following data were extracted and analyzed: name of the first author, publication date, number of cases, and adverse reactions. The following data were extracted but not discussed: age, sex, anesthesia classification, and BMI of patients. We analyzed and integrated only the data in RCTs and did not analyze missing research data. Two authors (Fang-Zhuo Li, Cheng Zhao) independently extracted the data of all eligible studies and resolved any differences between them through discussions with the other authors.
The pooled data were analyzed using Stata/SE 17.0. After the chi-square test, heterogeneity was assessed using I2 and P values; results with I2 < 50% and P = 0.1 were regarded as having no substantial heterogeneity[16]. When the heterogeneity was high, a random effects model was selected; otherwise, a fixed effects model was applied[17,18], For continuous variables, weighted mean difference (WMD) and 95%CI were used to describe the results[16]. The RR and 95%CI were computed for dichotomous variables[19].
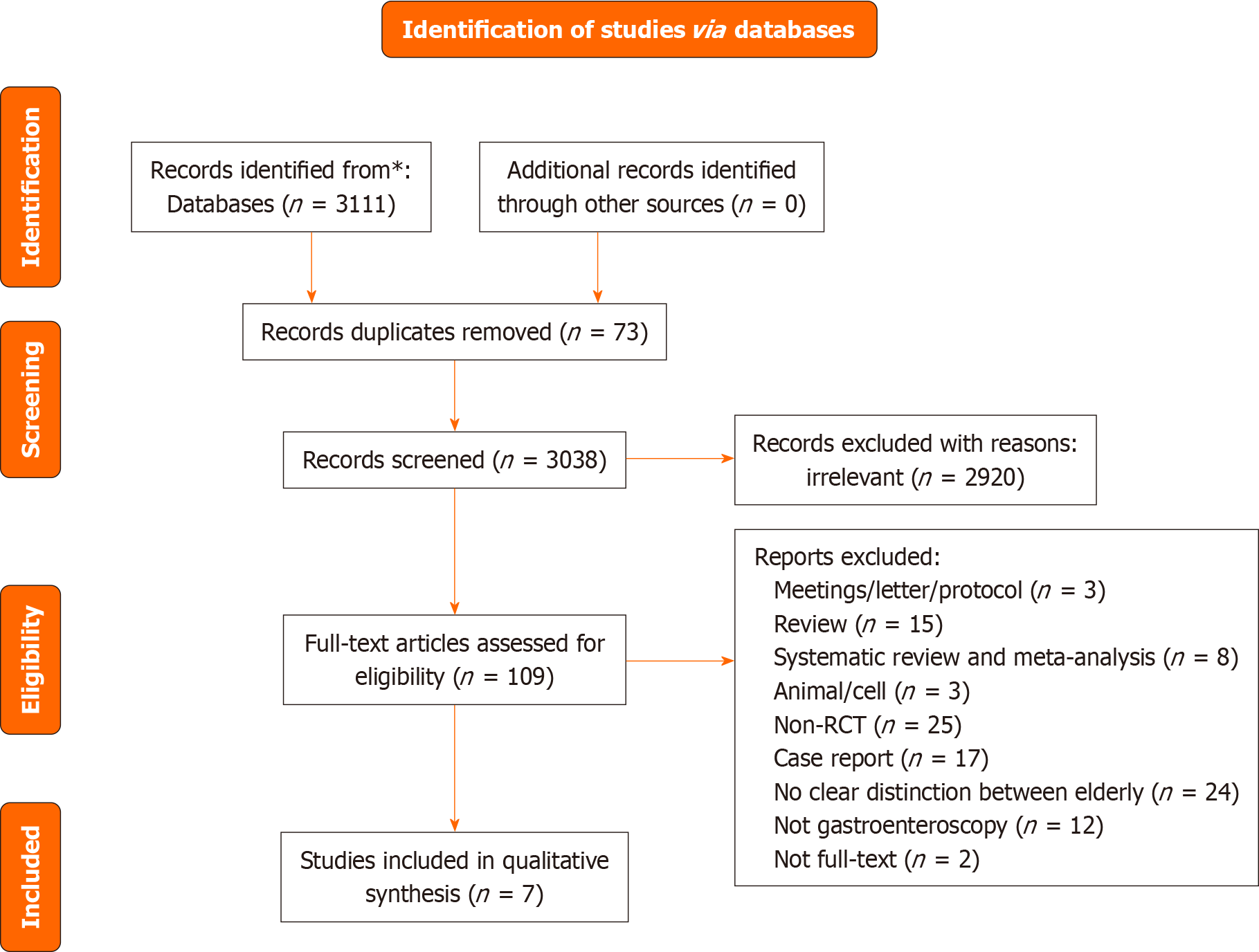
A flowchart of the search process is shown in Figure 1. A total of 3111 publications were searched. In total, 73 duplicates and 3038 papers were excluded. Ultimately, seven RCTs were included (Table 1), with a total of 1445 patients were included[20-26]. Among these, a grouping study on the dose of remimazolam was performed in one trial[21]; among the two conditions “Tan 2022 (0.1 mg/kg)” and “Tan 2022 (0.2 mg/kg),” we selected “Tan 2022 (0.1 mg/kg)” for our meta-analysis, as it was more close to the dose used in other studies.
| Ref. | Age (yr) | Gender (M/F) | ASA (I/II) | BMI (kg/m2) | No. of patients | |
| Ye et al[20], 2023 | Remimazolam | 68. 67 ± 4.55 | 39/25 | 44/64 | NM | 64 |
| Propofol | 68. 67 ± 4.55 | 36/29 | 48/65 | NM | 65 | |
| Tan et al[21], 2021 | Remimazolam | 66.4 ± 4.8 | 19/14 | 18/15 | 22.7 ± 3.0 | 33 |
| Propofol | 66.2 ± 5.0 | 21/12 | 18/15 | 23.2 ± 3.0 | 33 | |
| Hu et al[22], 2022 | Remimazolam | 70.11 ± 7.37 | 69/104 | 20/144 | 22.75 ± 3.15 | 173 |
| Propofol | 69.92 ± 7.57 | 72/101 | 26/140 | 22.73 ± 3.23 | 173 | |
| Liu et al[23], 2023 | Remimazolam | 67.5 ± 4.9 | 51/58 | 11/96 | 23.7 ± 3.0 | 107 |
| Propofol | 67.5 ± 5.7 | 51/56 | 10/99 | 24.0 ± 2.6 | 109 | |
| Guo et al[24], 2022 | Remimazolam | 70.4 ± 3.9 | 25/14 | 7/32 | 23.0 ± 3.0 | 38 |
| Propofol | 69.1 ± 4.0 | 22/16 | 7/31 | 23.0 ± 3.4 | 38 | |
| Lu et al[25], 2022 | Remimazolam | 70.6 ± 4.7 | 78/122 | 6/192 | 22.2 ± 2.5 | 200 |
| Propofol | 70.1 ± 4.5 | 83/117 | 17/181 | 22.2 ± 2.3 | 200 | |
| Liu et al[26], 2021 | Remimazolam | 68.87 ± 2.58 | 54/61 | 37/78 | 25.35 ± 2.07 | 115 |
| Propofol | 69.12 ± 2.75 | 58/59 | 46/71 | 24.75 ± 2.16 | 117 |
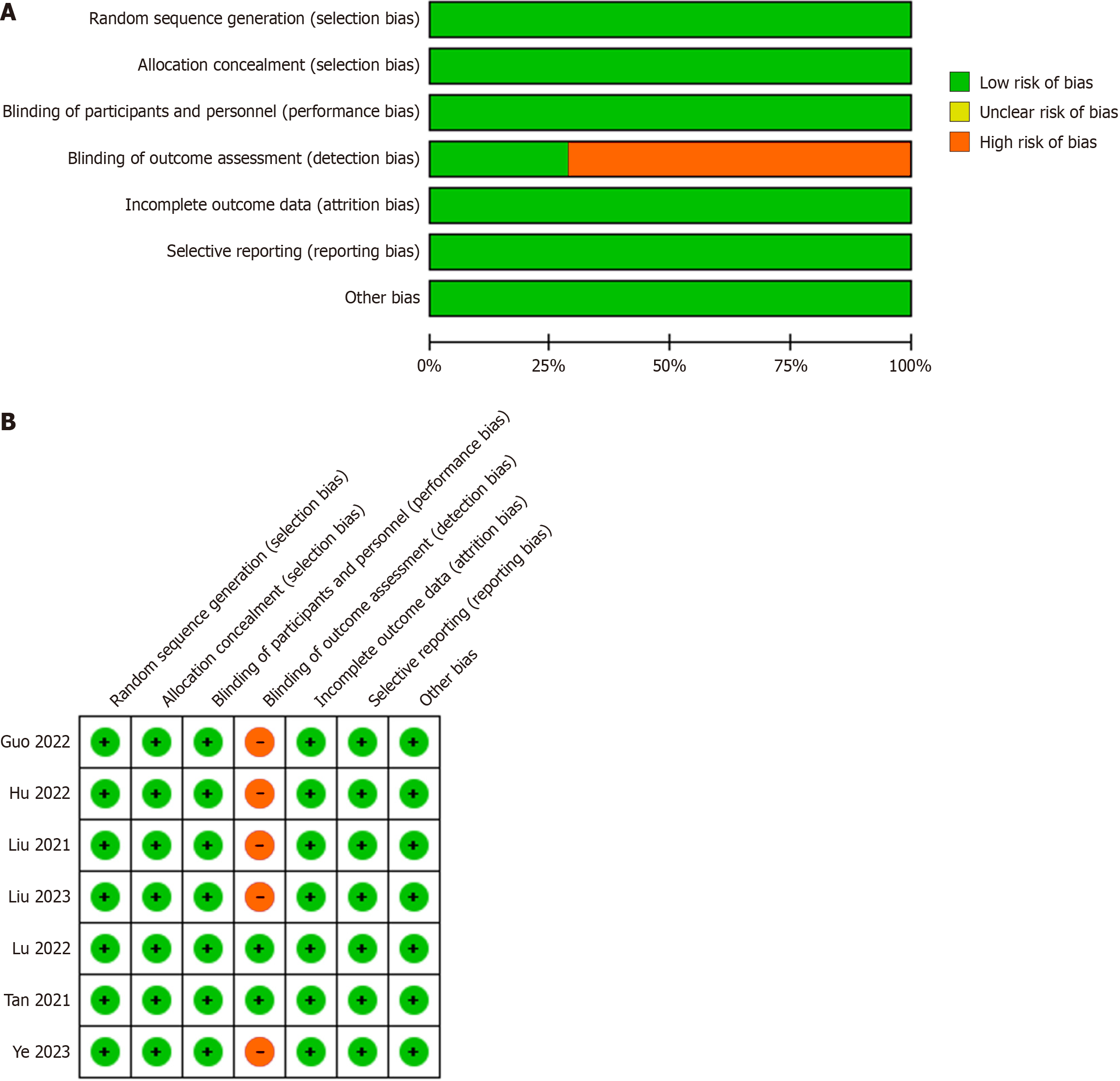
The risk of bias was evaluated using the Cochrane tool, and the results, shown in Figure 2, indicated that one study exhibited a high risk of selection bias because the study did not describe any randomized plan; however, all RCTs were of high quality.
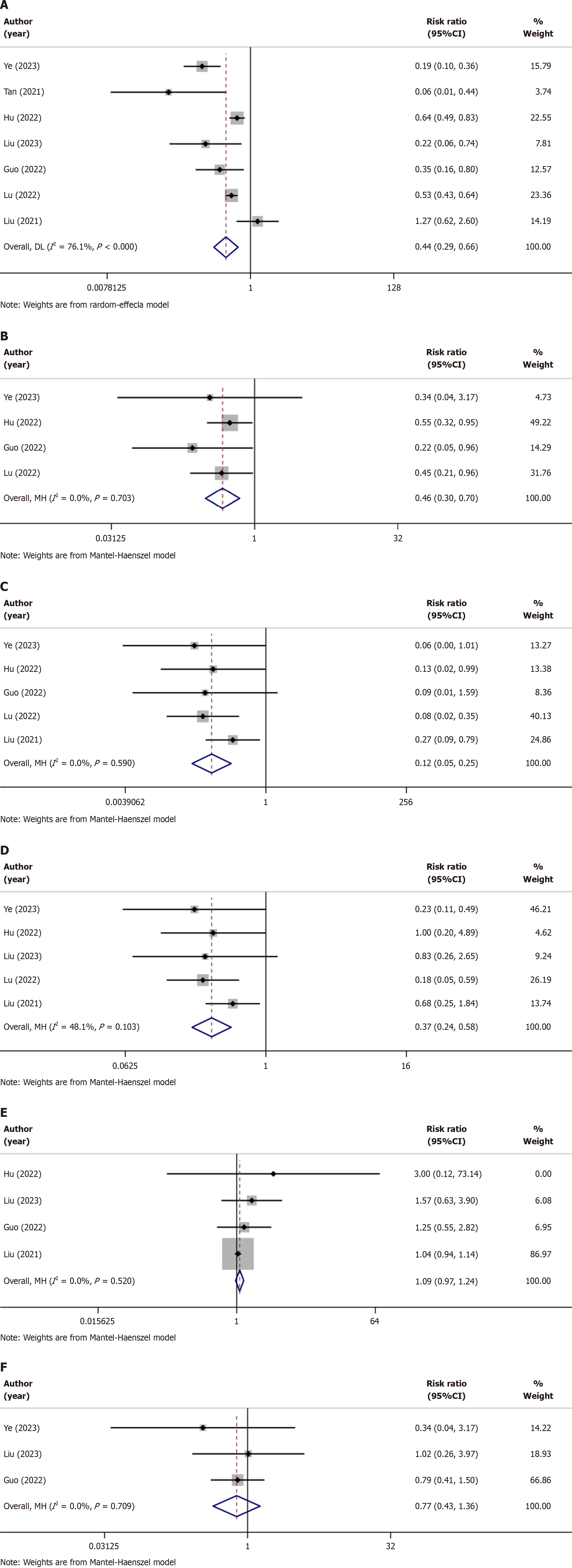
Hypotension: Details on hypotension caused by remimazolam and propofol use in patients during and after gastrointestinal endoscopic surgery were reported in 7 trials(Table 2)[20-26]. The hypotension of the patients less during and after gastrointestinal endoscopic surgery when sedated with remimazolam (RR = 0.44, P = 0.000) was lower than that of the patients of the propofol group. These studies had slightly higher heterogeneity; therefore, the random effects model was applied (I2 = 76.1%, P < 0.000; Figure 3A).
| Ref. | No. of patients | Hypotension | Respiratory depression | Injection pain | Bradycardia | PONV | Dizziness | Successful sedation rate | Time to discharge | Time to total alert | |
| Ye et al[20], 2023 | Remimazolam | 64 | 9 | 1 | 0 | 7 | NM | 1 | 64 | NM | 9 ± 1.51 |
| Propofol | 65 | 47 | 3 | 8 | 30 | NM | 3 | 65 | NM | 7.67 ± 2.27 | |
| Tan et al[21], 2021 | Remimazolam | 33 | 1 | NM | NM | NM | NM | NM | NM | NM | 3.82 ± 2.49 |
| Propofol | 33 | 16 | NM | NM | NM | NM | NM | NM | NM | 4.33 ± 2.97 | |
| Hu et al[22], 2022 | Remimazolam | 173 | 56 | 17 | 1 | 3 | 1 | NM | 172 | 19.92 ± 6.34 | 15.09 ± 4.06 |
| Propofol | 173 | 88 | 31 | 8 | 3 | 0 | NM | 173 | 19.55 ± 7.6 | 13.75 ± 4.22 | |
| Liu et al[23], 2023 | Remimazolam | 107 | 3 | NM | NM | 5 | 11 | 4 | 39 | NM | NM |
| Propofol | 109 | 14 | NM | NM | 6 | 7 | 4 | 107 | NM | NM | |
| Guo et al[24], 2022 | Remimazolam | 38 | 6 | 2 | 0 | NM | 10 | 11 | 35 | NM | NM |
| Propofol | 38 | 17 | 9 | 5 | NM | 8 | 14 | 36 | NM | NM | |
| Lu et al[25], 2022 | Remimazolam | 200 | 73 | 9 | 2 | 3 | NM | NM | 200 | NM | 9.8 ± 3.7 |
| Propofol | 200 | 139 | 20 | 24 | 17 | NM | NM | 200 | NM | 9.3 ± 3.7 | |
| Liu et al[26], 2021 | Remimazolam | 115 | 15 | 0 | 4 | 6 | 103 | NM | 111 | NM | NM |
| Propofol | 117 | 12 | 0 | 15 | 9 | 101 | NM | 117 | NM | NM |
Respiratory depression: Five trials reported the risk of respiratory depression in older patients after using the two sedatives during gastrointestinal endoscopic surgery (Table 2)[20,22,24-26]. The patients sedated with remimazolam experienced lower respiratory depression during gastrointestinal endoscopic surgery (RR = 0.46, P = 0.000) than that of the patients of the propofol group. There was no obvious heterogeneity among these studies, and the fixed effects model was applied (I2 = 0.0%, P = 0.703; Figure 3B).
Injection pain: Five trials reported the occurrence of injection pain in older patients after using the two sedatives (Table 2)[20,22,24-26]. Among them, remimazolam was shown to have a lower risk of causing injection pain (RR = 0.12, P = 0.000). A random-effects model was selected for the test (I2 = 0.00%, P = 0.590; Figure 3C).
Bradycardia: Bradycardia caused by the two sedatives was also reported in five trails (Table 2)[20,22,24-26]. From these trials, we concluded that bradycardia caused by the sedative effects of remimazolam in older patients (RR = 0.37, P = 0.000) was lower than that caused by propofol. These studies exhibited no heterogeneity; therefore, a heterogeneity-fixed effects model was selected (I2 = 48.1%, P = 0.103; Figure 3D).
Postoperative nausea and vomiting: Postoperative nausea and vomiting (PONV) is a common adverse event, particularly in older patients. Four trials reported PONV caused by the two sedatives (Table 2)[22-24,26]. From these limited number of trials, we found no obvious difference in PONV caused by the sedative effects of remimazolam or propofol during endoscopic surgery (RR = 1.09, P = 0.151), There was no difference in heterogeneity among the trials; hence, fixed effects model was selected (I2 = 0.00%, P = 0.520; Figure 3E).
Dizziness: Three trials reported the occurrence of dizziness in older patients after the use of two sedatives (Table 2)[20,23,24], which showed no obvious differences (RR = 0.77, P = 0.361), and these three trials had low heterogeneity; hence, the fixed-effects model was selected for the test (I2 = 0.0%, P = 0.709; Figure 3F).
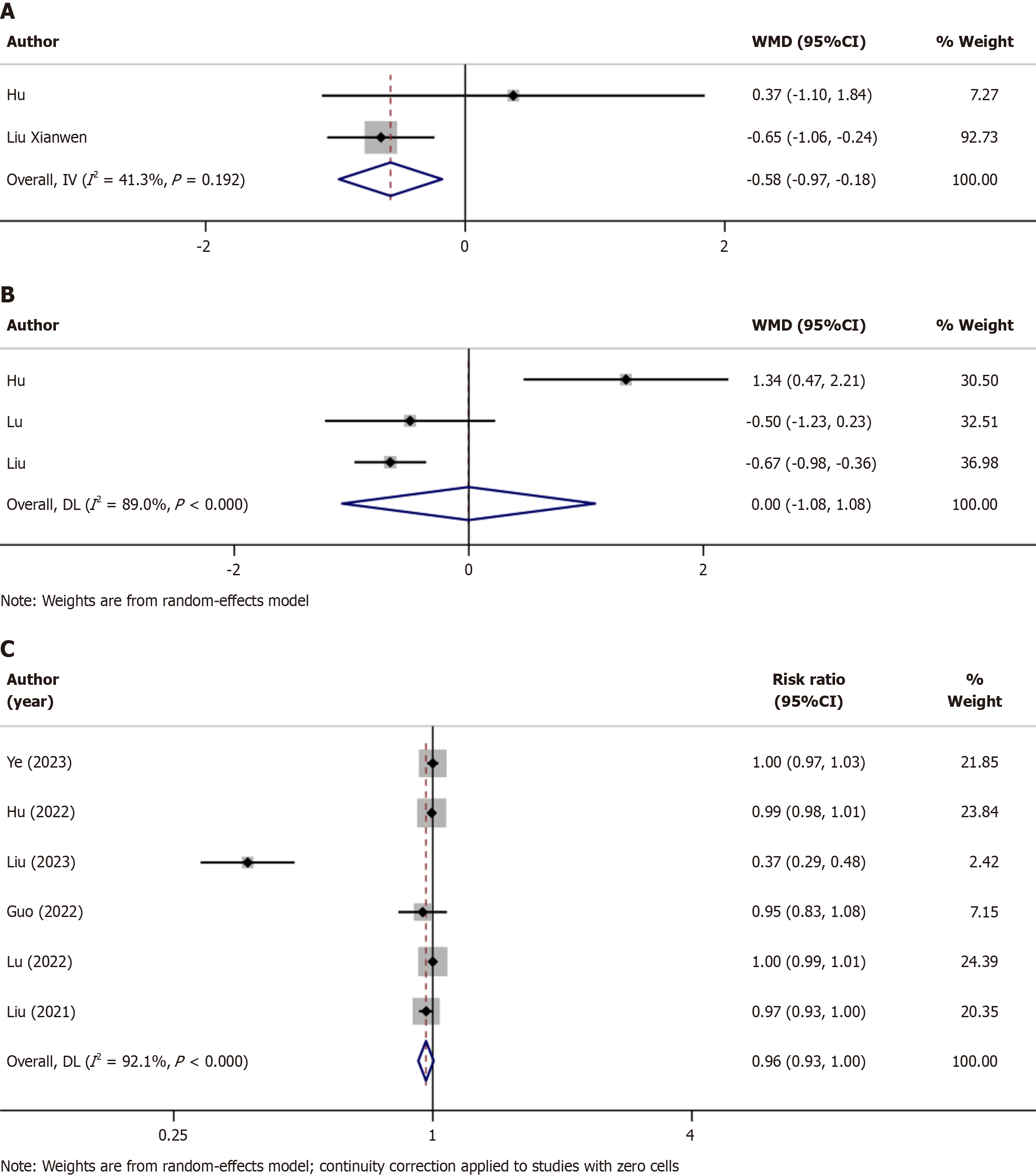
Time to discharge: Two trials recorded the time to discharge (Table 2)[22,26], and these studies were assessed based on the WMD to identify which sedative could reduce the time to discharge. Older patients injected with remimazolam had shorter discharge times (WMD = -0.58, P = 0.005); however, the abovementioned trials had obvious heterogeneity; hence, we selected a fixed-effects model for the analysis (I2 = 41.3%, P = 0.192; Figure 4A).
Time to become fully alert: The time taken by the patients to become completely alert after the operation was recorded in three trials (Table 2)[22,25,26]. We used the WMD for evaluation, which revealed that the time for patients to awake after remimazolam or propofol sedation were not statistically different (WMD = 0.00, 95%CI: -1.08-1.08, P = 0.998). Because the heterogeneity of the studies was quite high, the random effects model model was used in the test (I2 = 89.0%, P < 0.000; Figure 4B).
Successful sedation rate: Successful sedation rates were noted in seven trials (Table 2)[20-26]. The studies showed that both the sedatives could accomplish their respective functions during endoscopic surgery. Apparently, both anesthetics exhibited similar rates of sedation completion (RR = 0.96, P = 0.083). Owing to the lack of a statistical significance, we used a fixed-effects model for the analysis (I2 = 92.1%, P < 0.000); Figure 4C).
The meta-analysis results of the present study indicate that remimazolam is more suitable than propofol in terms of reduced adverse reactions such as hypotension, respiratory depression, injection pain, and bradycardia, after or during anesthesia; it could also reduce the patients’ discharge time. Owing to the lack of adequate clinical trials, the current data showed no obvious difference in the successful sedation rates of remimazolam and propofol and the time to complete alertness after surgery.
In this study, the remimazolam group experienced fewer postoperative adverse reactions than did the propofol group. The use of remimazolam for induction sedation was found safe and valuable for anesthesia sedation and, to some extent, reduced the incidence of adverse events during surgery. Propofol emulsions are characterized by a hydrated and lipid-encapsulated states, and injection pain is mainly caused by high concentrations of propofol in the hydrated state[27]. The prevalence of this problem in foreign countries is reportedly 28%-90% in adults and 28%-85% in children[28,29]; however, this could be an underestimation. In a questionnaire survey of patients and anesthesiologists, this problem ranked seventh[30]. This can undoubtedly increase the psychological burden on the older adults and children, by aggravating tension and anxiety, thus affecting the prognosis of the patients to a certain extent or reducing their quality of life, leading to a negative cycle.
Remimazolam is an ester-based drug whose metabolism is independent of organ functioning. The total dose of remimazolam has no effect on postoperative awakening or extubation times, and age is not a factor for extubation time or the infusion rate required to ensure adequate sedation[31]. This potential role offers tremendous advantages and clinical promise for anesthesia in older patients. Next, the effect of remimazolam could be reversed by flumazenil antagonism, terminating anesthesia and the patient's vital signs can be rapidly restored to baseline levels. These features provide unlimited prospects for promoting remimazolam use in patients who are critically ill. In addition, there are fewer clinical studies on remimazolam for intubation under general anesthesia than for painless endoscopy.
A recent meta-analysis[32] comparing the performance of remimazolam and propofol for painless endoscopy showed that remimazolam is a promising sedative for endoscopic cases, and the resultant respiratory and circulatory depression rates were less than those of the other drugs. In our study, we focused on the safety of older patients because the combi
(1) The included literature was limited, and more clinical studies are needed; (2) further high-quality studies are needed for analyzing the effects of sedation and clinical application of anesthesia in patients who are critically ill; (3) the dosages of remimazolam and propofol as well as their combination of are slightly different, and the final results could have been biased; (4) the quality of some studies was on the lower side, and some degree of heterogeneity was present; and (5) because of the lack of relevant international studies there were only seven articles in English.
The findings suggest that remimazolam may offer a safer alternative to propofol for gastroenteroscopy in elderly patients. The reduced incidence of hypotension, respiratory depression, injection pain, and bradycardia, along with a shorter time to discharge, support the favorable profile of remimazolam. While there were no significant differences in postoperative nausea and vomiting, dizziness, successful sedation rate, or time to full alertness, further research is warranted to validate and refine these conclusions.
Remimazolam is a new benzodiazepine with the advantages of rapid response, low metabolite activity, and no injection pain. An increasing number of clinical surgeries use remimazolam as the general anesthetic.
To the best of our knowledge, this is the first systematic review of the safety and efficacy of remimazolam as an intravenous anesthetic for gastroenteroscopy in older patients.
This study aimed to assess the safety and efficacy of remimazolam for sedation in older patients undergoing gastroenteroscopy.
We searched databases of PubMed, Cochrane Library, and the Web of Science, from the original to Oct 2023. The search terms include "remimazolam", "and propofol", "and gastrointestinal endoscopy or gastroscopy", search scope was "Title and Abstract". The search was limited to human studies and literature in English.
According to a meta-analysis, remimazolam surpasses propofol in managing negative effects such as hypotension, respiratory depression, injection pain, and bradycardia and shortens patients’ discharge time. However, the absence of sufficient clinical studies indicates that there is no clear variance in the successful sadation rate and time to full alertness after surgery.
In older patients undergoing endoscopy, remimazolam may be a safer option than propofol. However, further studies are required to confirm these findings.
With the increasing age of China’s population, the demand for painless gastroenteroscopy in older patients is increasing. The administration of remimazolam ensures sedation during endoscopy and simultaneously reduces the occurrence of complications and adverse events during surgery.
| 1. | Stogiannou D, Protopapas A, Tziomalos K. Is propofol the optimal sedative in gastrointestinal endoscopy? Acta Gastroenterol Belg. 2018;81:520-524. [PubMed] |
| 2. | Triantafillidis JK, Merikas E, Nikolakis D, Papalois AE. Sedation in gastrointestinal endoscopy: current issues. World J Gastroenterol. 2013;19:463-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 172] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (3)] |
| 3. | Travis AC, Pievsky D, Saltzman JR. Endoscopy in the elderly. Am J Gastroenterol. 2012;107:1495-501; quiz 1494, 1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Zhou S, Zhu Z, Dai W, Qi S, Tian W, Zhang Y, Zhang X, Huang L, Tian J, Yu W, Su D. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. 2021;127:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handb Exp Pharmacol. 2008;335-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 6. | Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 7. | Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, Wiard RP, Feldman PL, Collins H, Waszczak BL, Tilbrook GS. CNS 7056: a novel ultra-short-acting Benzodiazepine. Anesthesiology. 2007;107:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Rogers WK, McDowell TS. Remimazolam, a short-acting GABA(A) receptor agonist for intravenous sedation and/or anesthesia in day-case surgical and non-surgical procedures. IDrugs. 2010;13:929-937. [PubMed] |
| 9. | Keam SJ. Remimazolam: First Approval. Drugs. 2020;80:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 10. | Li S, Wu B, Peng B, Zhang Q, Zhao H, Hou K, An L. The Choice of Anesthetic Drugs in Outpatient Hysteroscopic Surgery: A Systematic Review and Network Meta-Analysis. Dis Markers. 2022;2022:2408685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115:274-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Worthington MT, Antonik LJ, Goldwater DR, Lees JP, Wilhelm-Ogunbiyi K, Borkett KM, Mitchell MC. A phase Ib, dose-finding study of multiple doses of remimazolam (CNS 7056) in volunteers undergoing colonoscopy. Anesth Analg. 2013;117:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 13. | Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, Wilhelm-Ogunbiyi K. A Phase IIa, randomized, double-blind study of remimazolam (CNS 7056) vs midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 14. | Lee A, Shirley M. Remimazolam: A Review in Procedural Sedation. Drugs. 2021;81: 1193-1201. [PubMed] |
| 15. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51341] [Article Influence: 10268.2] [Reference Citation Analysis (2)] |
| 16. | McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1642] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 17. | Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719-748. [PubMed] |
| 18. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 31184] [Article Influence: 779.6] [Reference Citation Analysis (1)] |
| 19. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48560] [Article Influence: 2111.3] [Reference Citation Analysis (4)] |
| 20. | Ye E, Wu K, Ye H, Zhang W, Chu L, Zhang K, Xie G, Jin Y, Fang X. Comparison of 95% effective dose of remimazolam besylate and propofol for gastroscopy sedation on older patients: A single-centre randomized controlled trial. Br J Clin Pharmacol. 2023;89:3401-3410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 21. | Tan Y, Ouyang W, Tang Y, Fang N, Fang C, Quan C. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J Gastroenterol Hepatol. 2022;37:576-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 22. | Hu B, Jiang K, Shi W, Xiao S, Zhang S, Zhang Y, Zhou Y, Tan C, Tan S, Zou X. Effect of Remimazolam Tosilate on Respiratory Depression in Elderly Patients Undergoing Gastroscopy: A Multicentered, Prospective, and Randomized Study. Drug Des Devel Ther. 2022;16:4151-4159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 23. | Liu F, Cheng X, Wang Y, Li K, Peng T, Fang N, Pasunooti KK, Jun S, Yang X, Wu J. Effect of remimazolam tosilate on the incidence of hypoxemia in elderly patients undergoing gastrointestinal endoscopy: A bi-center, prospective, randomized controlled study. Front Pharmacol. 2023;14:1131391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 24. | Guo J, Qian Y, Zhang X, Han S, Shi Q, Xu J. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. 2022;22:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 25. | Lu K, Wei S, Ling W, Wei Y, Ran X, Huang H, Wang M, Wei N, Liao Y, Qin Z, Pan M, Wei Q, Fu L, Xiong B, Ma C, Jiang J, Huang Y. Remimazolam versus propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: A multicenter, randomized controlled trial. J Clin Pharm Ther. 2022;47:2230-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 26. | Liu X, Ding B, Shi F, Zhang Y, Liu L, Sha Y, Zhao T. The Efficacy and Safety of Remimazolam Tosilate versus Etomidate-Propofol in Elderly Outpatients Undergoing Colonoscopy: A Prospective, Randomized, Single-Blind, Non-Inferiority Trial. Drug Des Devel Ther. 2021;15:4675-4685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 27. | Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J. Reducing pain during propofol injection: the role of the solvent. Anesth Analg. 1996;82:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Nathanson MH, Gajraj NM, Russell JA. Prevention of pain on injection of propofol: a comparison of lidocaine with alfentanil. Anesth Analg. 1996;82:469-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Rahman Al-Refai A, Al-Mujadi H, Petrova Ivanova M, Marzouk HM, Batra YK, Al-Qattan AR. Prevention of pain on injection of propofol: a comparison of remifentanil with alfentanil in children. Minerva Anestesiol. 2007;73:219-223. [PubMed] |
| 30. | Kim E, Kim CH, Kim HK, Kwon JY, Lee DW, Kim HY. Effect of nitrous oxide inhalation on pain after propofol and rocuronium injection. J Anesth. 2013;27:868-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Zhou J, Leonowens C, Ivaturi VD, Lohmer LL, Curd L, Ossig J, Schippers F, Petersen KU, Stoehr T, Schmith V. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Chen J, Zhou H, Liu K. A commentary on "The safety and efficacy between remimazolam and propofol in intravenous anaesthesia of endoscopy operation: A systematic review and meta-analysis". Int J Surg. 2023;109:4371-4372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Schneider-Stock R, Germany S-Editor: Gong ZM L-Editor: A P-Editor: Zheng XM