Published online Mar 6, 2024. doi: 10.12998/wjcc.v12.i7.1284
Peer-review started: August 30, 2023
First decision: December 2, 2023
Revised: December 25, 2023
Accepted: February 5, 2024
Article in press: February 5, 2024
Published online: March 6, 2024
Processing time: 184 Days and 0.7 Hours
Gastrinoma is characterized by an excessive release of gastrin, leading to hypersecretion of gastric acid, subsequently resulting in recurrent peptic ulcers, chronic diarrhea, and even esophageal strictures. This case report aims to improve awareness and facilitate early diagnosis and treatment of gastrinoma by pre
This case demonstrates a patient with gastrinoma who developed RBES and complete esophageal obstruction despite management with maximal acid sup
It is essential to diagnose gastrinoma as early as possible, as inadequately controlled acid secretion over an extended period increases the risk of developing severe esophageal strictures. In patients with esophageal strictures causing complete luminal obstruction, blind reopening EIT presents challenges and carries a high risk of perforation.
Core Tip: We report a rare case of sporadic gastrinoma in a young woman who presented with chronic diarrhea, multiple peptic ulcers, and a refractory benign esophageal stricture. The case indicates that inadequately controlled acid secretion over an extended period increases the risk of developing severe esophageal strictures. It also highlights that in patients with complete esophageal strictures, blind reopening endoscopic incisional therapy presents challenges and carries a high risk of perforation.
- Citation: Chen QN, Bai BQ, Xu Y, Mei Q, Liu XC. Sporadic gastrinoma with refractory benign esophageal stricture: A case report. World J Clin Cases 2024; 12(7): 1284-1289
- URL: https://www.wjgnet.com/2307-8960/full/v12/i7/1284.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i7.1284
Gastrinoma, also known as Zollinger-Ellison syndrome (ZES), is a rare pancreatic neuroendocrine neoplasm characterized by excessive gastrin release leading to hypersecretion of gastric acid and subsequent manifestations of recurrent peptic ulcers, vomiting, abdominal pain, and chronic diarrhea[1]. Many of these symptoms can be significantly alleviated through treatment with acid inhibitors, particularly proton pump inhibitors.
The diagnosis of ZES is challenging, as its symptoms are nonspecific and often overlap with those of other gastrointestinal disorders, leading to an initial correct diagnosis by the referring physician in only 3% of patients. Moreover, its rarity, with an incidence of 0.5 to 15 cases per million population, makes it extremely difficult to diagnose accurately in an acute setting[2]. Consequently, patients are typically diagnosed only after years of presenting symptoms.
In this report, we present a rare case of sporadic ZES in a young woman who presented with chronic diarrhea, abdominal pain, and vomiting and later developed dysphagia because of severe reflux esophagitis with esophageal stricture. We will discuss in detail the diagnostic process and the treatments administered for her refractory benign esophageal stricture (RBES). This case report aims to improve awareness and facilitate early diagnosis and treatment of gastrinoma by presenting a rare case of gastrinoma with RBES. Additionally, it highlights that blind reopening endoscopic incisional therapy (EIT) presents challenges and carries a high risk of perforation.
A 33-year-old female patient was admitted with intermittent chronic watery diarrhea, abdominal pain, vomiting, and progressive dysphagia.
Over the past 2 years, the patient sought medical attention at a local hospital and was initially diagnosed with chronic idiopathic diarrhea. Laboratory and radiological investigations including stool for culture, parasites, fat analysis, a computed tomography (CT) scan of the abdomen, colonoscopy, and enteroscopy showed no abnormal findings. How
The patient also had a history of multiple peptic ulcers and had undergone an exploratory laparotomy with gastric perforation repair 3 months ago.
The patient denied a family history of hereditary diseases or cancer.
Upon physical examination, mild tenderness in the epigastrium was noted.
General and antidiarrheal treatments had shown no effect, but somatostatin analog infusion with a dose of 3 mg was effective in alleviating her symptoms. Consequently, fasting serum gastrin and chromogranin A levels were measured, showing elevated values of fasting serum gastrin (845.00 pg/mL, normal range: 30-100 pg/mL) and chromogranin A (500 ng/mL, normal range: 10-110 ng/mL). These findings immediately raised suspicion of a neuroendocrine neoplasm in the form of gastrinoma. The gastrinoma was considered sporadic owing to the absence of a pituitary tumor, normal serum calcium, and parathyroid hormone levels.
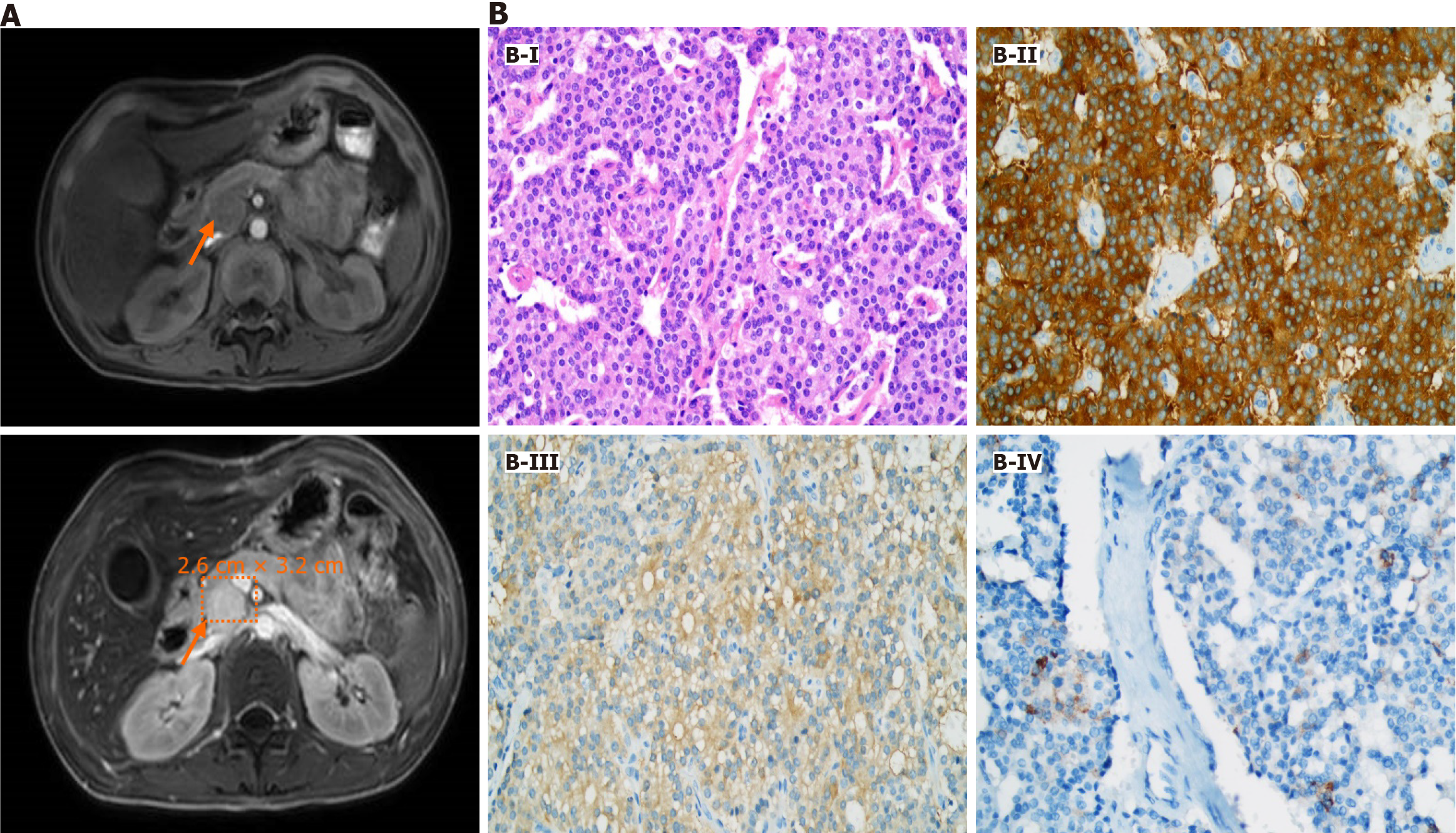
Upon admission to our unit, the esophagus-gastro-duodenoscopy revealed severe reflux esophagitis (grade D), esophageal ulcers, and strictures. Due to the negative findings obtained from the previous CT scan, we opted for abdominal contrast-enhanced magnetic resonance imaging (MRI) as the preferred diagnostic modality. This approach unveiled a mass of approximately 2.6 cm × 3.2 cm at the head of the pancreas without liver metastasis (Figure 1A).
The patient successfully underwent the operation, and the diagnosis of gastrinoma was confirmed through postoperative pathology and immunohistochemistry (Figure 1B). The tumor cells, arranged into solid nests, exhibited abundant cytoplasm, fine and granulated chromatin, and inconspicuous nucleoli. Tumor cells showed positivity for synaptophysin, neuron-specific enolase, and gastrin.
Sporadic gastrinoma; RBES.
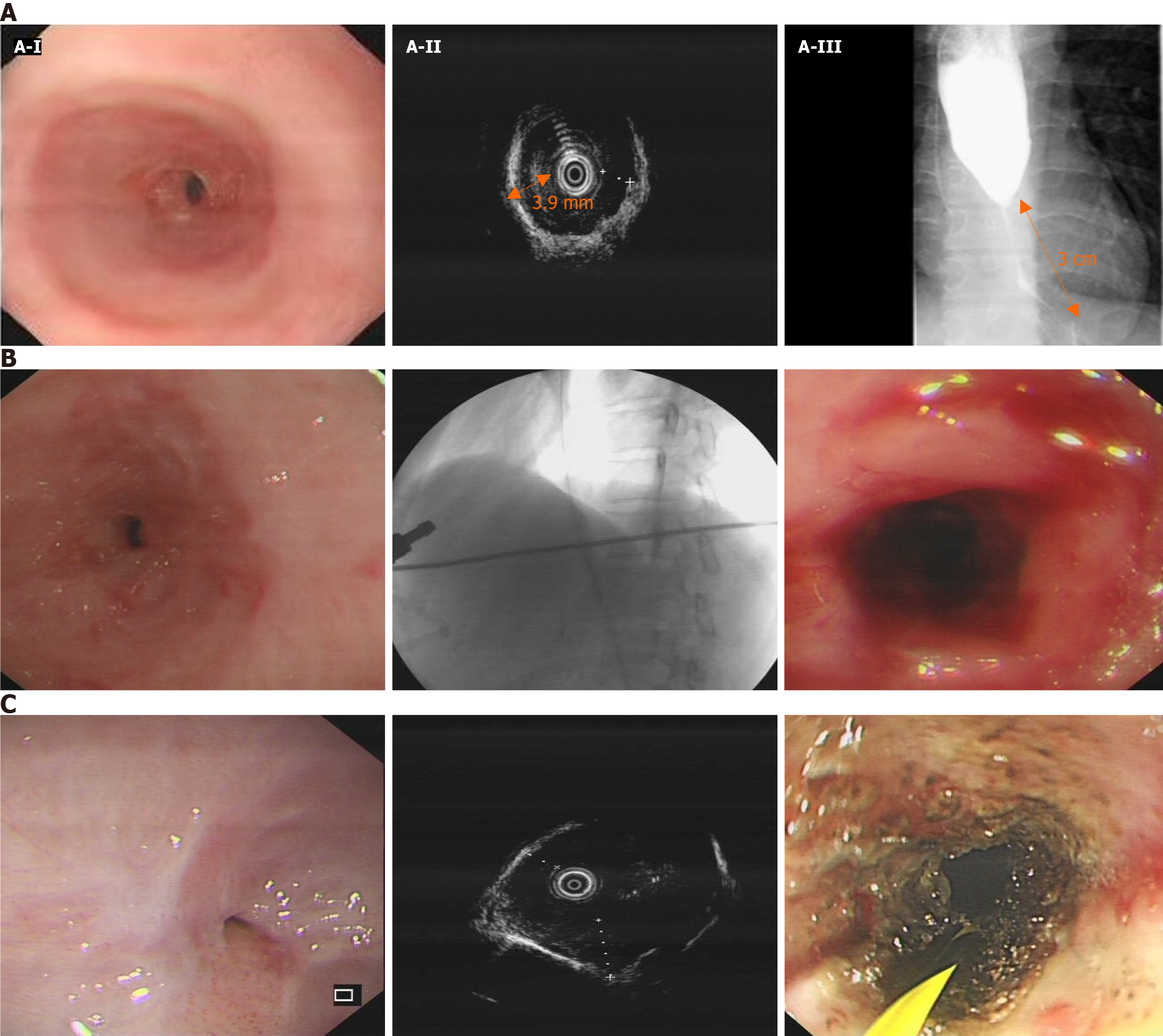
According to the consensus of Chinese experts in pancreatic neuroendocrine tumors, radical pancreatic resection surgery should be performed for pancreatic neuroendocrine tumors with a diameter greater than 2 cm, regardless of their functionality. For neuroendocrine tumors in the head of the pancreas, it is recommended to undergo pancreaticoduodenectomy[3]. Therefore, our team opted for pancreaticoduodenectomy. Although the patient's watery diarrhea resolved after surgery, she continued to experience severe dysphagia due to an esophageal stricture during the follow-up period. Comprehensive evaluations of the esophageal stricture by esophagus-gastro-duodenoscopy, endoscopic ultrasonography, and esophagography showed severe esophageal stricture (Figure 2A). Her esophageal stricture led to the decision to improve her symptoms through periodic wire-guided endoscopic bougie dilations (EBD). However, despite five sessions of EBD (Figure 2B), it was not possible to maintain a satisfactory luminal diameter, indicating the presence of RBES. According to the guidelines, we performed ultrasonography-guided and wire-guided EIT combined with triamcinolone acetonide injection (Figure 2C).
During the 2-year follow-up, the patient recovered from watery diarrhea after surgery. Unfortunately, during the patient's fourth EIT session, when the esophageal lumen was completely obstructed, she unexpectedly developed esophageal perforation. She was managed with conservative treatment because she declined further surgery. As a result, she could only receive long-term enteral nutrition through a nasogastric tube, significantly reducing her quality of life.
The reported case exhibits several distinctive features that warrant discussion. Firstly, the patient presented with chronic diarrhea and multiple peptic ulcers, which were initially misdiagnosed as chronic idiopathic diarrhea and sporadic peptic ulcers. However, it is important to note that ZES-related ulcers are usually multiple, located at unusual sites, and usually complicated by bleeding, perforation, or esophageal strictures, in contrast to sporadic peptic ulcers. Moreover, approximately 75% of patients with gastrinoma presenting with watery diarrhea, which can easily be misdiagnosed as chronic idiopathic diarrhea, as in this case[4].
Interestingly, the case presented with an uncommon symptom of refractory esophageal stricture. ZES is typically associated with minimal esophageal morbidity because of advancements in antisecretory medications. A prospective study investigating esophageal involvement and its complications in ZES found that sporadic ZES rarely presents with a benign esophageal stricture, with a frequency as low as 0.4%[5]. After excluding esophageal cancer and drug or chemical-induced esophageal stricture, we considered that the prolonged acid reflux, attributed to gastrinoma, led to the formation of esophageal ulcers and scars, consequently resulting in refractory esophageal stricture. It is also noteworthy that the abdominal CT of the patient did not reveal any abnormalities, while the contrast-enhanced MRI showed a mass in the head of the pancreas. A study from Peking Union Medical College Hospital indicates that, compared with conventional contrast-enhanced CT, MRI has higher sensitivity and specificity in diagnosing pancreatic gastrinoma[6]. Additionally, MRI aids in determining the proximity of the tumor to the pancreatic duct.
The patient experienced a severe esophageal stricture and proton pump inhibitor therapy was not effective. Therefore, endoscopic treatment was considered to improve esophageal stricture. Fugazza et al[7] reported that endoscopic dilation employing bougies or a balloon was proven successful in the majority of cases. However, it was difficult to maintain a satisfactory luminal diameter despite five sessions of EBD in our case, indicating the presence of RBES. RBES is not only difficult to dilate but also tends to recur within weeks. In such instances, alternative endoscopic treatments like stent placement or EIT combined with steroid injections should be considered. Manfredi et al[8] conducted a study to evaluate the safety and efficacy of EIT in a pediatric population with refractory esophageal strictures and showed that in the refractory group, 61% of the patients met the criteria for treatment success and 2.3% with adverse events. The patient in our case undered ultrasonography-guided and wire-guided EIT combined with triamcinolone acetonide injection and showed great improvements initially. Unfortunately, the patient experienced esophageal perforation following the fourth session of EIT. Therefore, even though EIT shows promise as an adjunct treatment option for RBES and may be considered before surgical resection even in severe cases, the complication rate, albeit low, is significant. Consequently, EIT should only be considered by experienced endoscopists in close consultation with surgeons. Further prospective longitudinal studies are needed to validate this.
In conclusion, it is essential to diagnose gastrinoma as early as possible, as inadequately controlled acid secretion over an extended period increases the risk of developing severe esophageal strictures. In patients with esophageal strictures causing complete luminal obstruction, the blind reopening EIT presents challenges and carries a high risk of perforation. Additionally, it highlights the persistent challenges that gastroenterologists encounter in managing RBES despite the availability of various treatment options.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Edholm D, Sweden; Nazir Z, Pakistan S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhao S
| 1. | Binet Q, Borbath I. The Zollinger-Ellison Syndrome. N Engl J Med. 2022;387:1699. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Niederle B, Selberherr A, Bartsch DK, Brandi ML, Doherty GM, Falconi M, Goudet P, Halfdanarson TR, Ito T, Jensen RT, Larghi A, Lee L, Öberg K, Pavel M, Perren A, Sadowski SM, Tonelli F, Triponez F, Valk GD, O'Toole D, Scott-Coombes D, Thakker RV, Thompson GB, Treglia G, Wiedenmann B. Multiple Endocrine Neoplasia Type 1 and the Pancreas: Diagnosis and Treatment of Functioning and Non-Functioning Pancreatic and Duodenal Neuroendocrine Neoplasia within the MEN1 Syndrome - An International Consensus Statement. Neuroendocrinology. 2021;111:609-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Expert Committee on Neuroendocrine Neoplasms; Chinese Society of Clinical Oncology. [Chinese expert consensus on gastroenteropancreatic neuroendocrine neoplasms (2022 edition)]. Zhonghua Zhong Liu Za Zhi. 2022;44:1305-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Chatzipanagiotou O, Schizas D, Vailas M, Tsoli M, Sakarellos P, Sotiropoulou M, Papalambros A, Felekouras E. All you need to know about gastrinoma today | Gastrinoma and Zollinger-Ellison syndrome: A thorough update. J Neuroendocrinol. 2023;35:e13267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 5. | Hoffmann KM, Gibril F, Entsuah LK, Serrano J, Jensen RT. Patients with multiple endocrine neoplasia type 1 with gastrinomas have an increased risk of severe esophageal disease including stricture and the premalignant condition, Barrett's esophagus. J Clin Endocrinol Metab. 2006;91:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Zhu L, Xue H, Sun Z, Li P, Qian T, Xing X, Li N, Zhao Y, Wu W, Jin Z. Prospective comparison of biphasic contrast-enhanced CT, volume perfusion CT, and 3 Tesla MRI with diffusion-weighted imaging for insulinoma detection. J Magn Reson Imaging. 2017;46:1648-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Fugazza A, Repici A. Endoscopic Management of Refractory Benign Esophageal Strictures. Dysphagia. 2021;36:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Manfredi MA, Clark SJ, Medford S, Staffa SJ, Ngo PD, Hamilton TE, Smithers CJ, Jennings RW. Endoscopic Electrocautery Incisional Therapy as a Treatment for Refractory Benign Pediatric Esophageal Strictures. J Pediatr Gastroenterol Nutr. 2018;67:464-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |