Published online Feb 26, 2024. doi: 10.12998/wjcc.v12.i6.1120
Peer-review started: December 12, 2023
First decision: January 2, 2024
Revised: January 14, 2024
Accepted: January 27, 2024
Article in press: January 27, 2024
Published online: February 26, 2024
Processing time: 70 Days and 1.3 Hours
Remimazolam is a new benzodiazepine used for procedural sedation and general anesthesia. Several studies have used remimazolam for bendable bronchoscopy.
To assess the safety and efficacy of remimazolam for sedation in patients undergoing bendable bronchoscopy by performing a meta-analysis of rando
We searched the EMBASE, PubMed, Cochrane Library, and Web of Science data
Five studies with 1080 cases were included. Remimazolam had the same sedation success rate compared with CS [relative risk (RR): 1.35, 95%CI: 0.60-3.05, P = 0.474, I2 = 99.6%]. However, remimazolam was associated with a lower incidence of hypotension (RR: 0.61; 95%CI: 0.40-0.95, P = 0.027; I2 = 65.1%) and a lower incidence of respiratory depression (RR: 0.50, 95%CI: 0.33-0.77, P = 0.002, I2 = 42.3%). A subgroup analysis showed a higher success rate of sedation with remimazolam than midazolam (RR: 2.45, 95%CI: 1.76-3.42, P < 0.001). Compared with propofol, the incidence of hypotension (RR: 0.45, 95%CI: 0.32-0.64, P < 0.001, I2 = 0.0%), respiratory depression (RR: 0.48, 95%CI: 0.30-0.76, P = 0.002, I2 = 78.4%), hypoxemia (RR: 0.36, 95%CI: 0.15-0.87, P = 0.023), and injection pain (RR: 0.04, 95%CI: 0.01-0.28, P = 0.001) were lower.
Remimazolam is safe and effective during bronchoscopy. The sedation success rate was similar to that in the CS group. However, remimazolam has a higher safety profile, with fewer inhibitory effects on respiration and circulation.
Core Tip: We searched the databases of EMBASE, PubMed, Cochrane Library, and the Web of Science for randomized controlled trials of bendable bronchoscopic procedural sedation with remimazolam vs conventional sedatives (CS) from the time the database was created until August 2023. STATA 15.1 software was applied to data analyses. Five studies with 1080 cases were included. We finally came to the conclusion: Remimazolam is safe and effective for cases with bronchoscopy. Its sedation success rate is similar to CS. However, remimazolam has a higher safety profile with less inhibitory effects on respiration and circulation.
- Citation: Zhou Y, Zhao C, Tang YX, Liu JT. Efficacy and safety of remimazolam in bronchoscopic sedation: A meta-analysis. World J Clin Cases 2024; 12(6): 1120-1129
- URL: https://www.wjgnet.com/2307-8960/full/v12/i6/1120.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i6.1120
Bronchoscopy is an endoscopic tool for the diagnosis and treatment of respiratory disease, and plays a key role in the diagnosis and therapy of lung diseases[1]. However, bendable bronchoscopy is an invasive procedure, and patients often experience pain and anxiety as well as serious complications including respiratory depression, cardiac arrhythmias, and cerebrovascular accidents[2]. According to the American Thoracic Society, anesthesia is recommended for all patients undergoing bronchoscopic consultations in the absence of contraindications[3]. Procedural sedation involves the use of sedative drugs and analgesics in addition to routine consultation, which eliminates fear, improves comfort, increases tolerance, and reduces procedural complications while shortening the duration of the procedure[4].
Currently, conventional sedatives (CS) propofol, midazolam, and dexmedetomidine are widely used in painless bendable bronchoscopy practice. Propofol has a rapid onset of action and a short recovery time; however, it causes significant injection site pain, strong respiratory and circulatory depression, and has no antagonist[5]. Midazolam is antagonized by flumazenil. However, the prolonged postoperative sedation affects the time to discharge. Dexme
Remimazolam is a new and effective benzodiazepine whose metabolites are not pharmacologically active, resulting in a faster recovery of cognitive function[8]. Owing to its unique pharmacological properties, remimazolam has been widely used in endoscopy, particularly in gastroenteroscopy[9]. In recent years, with the development of painless diagnostic techniques, the use of remimazolam for bendable bronchoscopy has received much attention. However, there has been no relevant systematic review. Therefore, we conducted a meta-analysis of randomized controlled trials (RCTs) on remi
We searched the EMBASE, PubMed, Cochrane Library, and Web of Science databases from the origin to August 2023. The search terms include "Remimazolam" or "CNS 7056,” search scope was "Title and Abstract.” The search was limited to human studies in English. Relevant studies were independently obtained by two investigators.
Our inclusion criteria were as follows: (1) RCT study design; (2) patients underwent bendable bronchoscopy; (3) the interventional treatment was either Remimazolam or CS; (4) papers published from establishment to August 1, 2023; and (5) studies that were not in Chinese or English, duplicated, or had incomplete data were excluded.
The data were independently analyzed to extract relevant information: (1) Authors; (2) publication time; (3) country of publication; (4) type of study design; (5) American Society of Anesthesiologists classification (ASA classification); (6) number of participants in each study; (7) age range; (8) sex composition; and (9) specific interventions received by the participants, including the name of the medication, dosage, and dosing program. Disagreements in the extracted data were recorded and discussed with a 3rd researcher until a consensus was reached.
Two researchers evaluated the quality of the research papers. The Cochrane tool[10] was applied to calculate the risk of bias. Under the study conditions, items related to high or unclear bias risk were regarded as high risk[11]. Disagreements in quality evaluation were documented and discussed with a third researcher until a consensus was reached.
All statistical analyses were conducted using STATA15.1 (Stata Statistical Software: Release 18. College Station, TX: StataCorp). I2 and Q tests were used to test the heterogeneity between studies. If heterogeneity between studies existed (I2 ≤ 50% and P > 0.10), the data was analyzed via a fixed-effects model; otherwise, a random-effects model was used[12]. Subgroup analyses were conducted to compare the effects of propofol, midazolam, and dexmedetomidine; P < 0.05 was regarded as statistically significant.
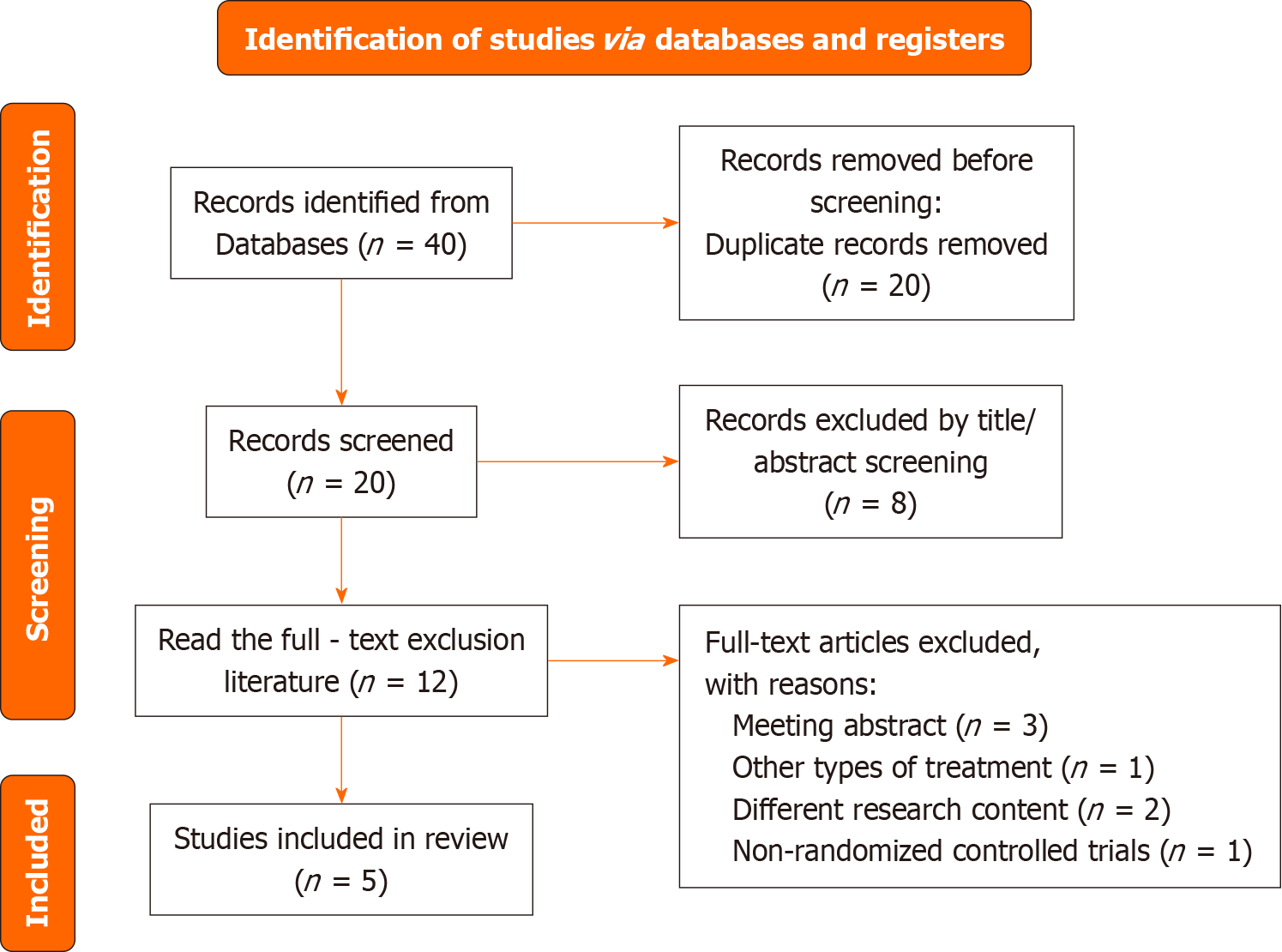
As shown in Figure 1, 40 studies were identified after a systematic literature search. After removing 20 duplicate studies, the 20 remaining studies were screened. Eight inappropriate studies were eliminated by screening titles and abstracts. Therefore, 12 articles were left for full-text reading. After careful reading of the full text, seven studies were excluded based on the inclusion and exclusion criteria. Finally, five studies were included.
Table 1 shows all the studies included, all five studies[13-17] were RCTs, four[13-15,17] were from China, and one[16] was from the United States. The five studies[13-17] were classified as ASA classes I-III. In studies published between 2018 and 2023, 1080 patients aged from 18 to 75 years, and 52.50% male underwent bendable bronchoscopy; 657 patients were sedated with remimazolam and 423 patients were sedated with CS, of which 281 were sedated with propofol[13-15], 69 with midazolam[16], and 73 with dexmedetomidine[17].
| Ref. | Country | Study design | ASA status | Number of patients | Age | Gender (M/F) | Remimazolam | Control |
| Gao et al[13], 2023 | China | RCT | I-III | 60 | 18-70 | 39/21 | Initial dose: 6 mg/kg/h; Maintenance dose: 0.6-2 mg/kg/h | Propofol: Initial dose: 2 mg/kg; Maintenance dose: 4-6 mg/kg/h |
| Zhang et al[14], 2023 | China | RCT | I-III | 192 | 18-64 | 92/100 | Initial dose: 0.2 mg/kg; Top-up dose: 0.05 mg/kg | Propofol: Initial dose: 1.5 mg/kg; Top-up dose: 0.5-1.0 mg/kg |
| Zhou et al[15], 2022 | China | RCT | I-III | 310 | 18-75 | 154/156 | Initial dose: 0.2 mg/kg; Top-up dose: 0.1 mg/kg | Propofol: Initial dose: 2 mg/kg; Top-up dose: 0.75 mg/kg |
| Pastis et al[16], 2019 | USA | RCT | I-III | 372 | 50-74 | 174/198 | Initial dose: 5 mg; Top-up dose: 2.5 mg | Midazolam: Initial dose: 1-1.75 mg; Top-up dose: 0.5-1 mg |
| Chen et al[17], 2022 | China | RCT | I-III | 146 | 45-65 | 108/38 | Initial dose: 12 mg/kg/h; Maintenance dose: 1-2 mg/kg/h | Dexmedetomidine: Initial dose: 0.5 μg/kg; Maintenance dose: 0.2-0.7 μg/kg/h |
The same standard was applied to evaluate sedation in 3 studies[15-17]. These studies divided patients into two groups according to the type of sedation used. The percentages of successfully sedated patients were 473/538 using remimazolam and 245/301 in the CS group (propofol 154/155, midazolam 24/73, and dexmedetomidine 67/73) (Table 2). The frequencies of intraoperative adverse events and complications, including hypotension, respiratory depression, and hypoxemia, are shown in Table 3.
| Ref. | Patients in each group (n) | Hypotension (n) | Hypertension (n) | Respiratory depression (n) | Hypoxemia (n) | Bradycardia (n) | Tachycardia (n) | Injection pain (n) | ||||||||
| Remimazolam | Control | Remimazolam | Control | Remimazolam | Control | Remimazolam | Control | Remimazolam | Control | Remimazolam | Control | Remimazolam | Control | Remimazolam | Control | |
| Gao et al[13], 2023 | 30 | Propofol: 30 | 11 | Propofol: 22 | 1 | Propofol: 2 | NA | NA | 1 | Propofol: 2 | 2 | Propofol: 5 | 6 | Propofol: 9 | NA | NA |
| Zhang et al[14], 2023 | 96 | Propofol: 96 | 1 | Propofol: 8 | NA | NA | 13 | Propofol: 38 | NA | NA | 0 | Propofol: 22 | NA | NA | NA | NA |
| Zhou et al[15], 2022 | 155 | Propofol: 155 | 22 | Propofol: 49 | 13 | Propofol: 5 | 9 | Propofol: 8 | 13 | Propofol: 5 | NA | NA | NA | NA | 1 | Propofol: 26 |
| Pastis et al[16], 2019 | 303 | Midazolam: 69 | 127 | Midazolam: 34 | 186 | Midazolam: 41 | 7 | Midazolam: 3 | 186 | Midazolam: 41 | 13 | Midazolam: 3 | 4 | Midazolam: 0 | 2 | Midazolam: 0 |
| Chen et al[17], 2022 | 73 | Dexmedetomidine: 73 | 9 | Dexmedetomidine: 8 | 2 | Dexmedetomidine: 3 | 2 | Dexmedetomidine: 2 | 2 | Dexmedetomidine: 3 | 3 | Dexmedetomidine: 2 | NA | NA | NA | NA |
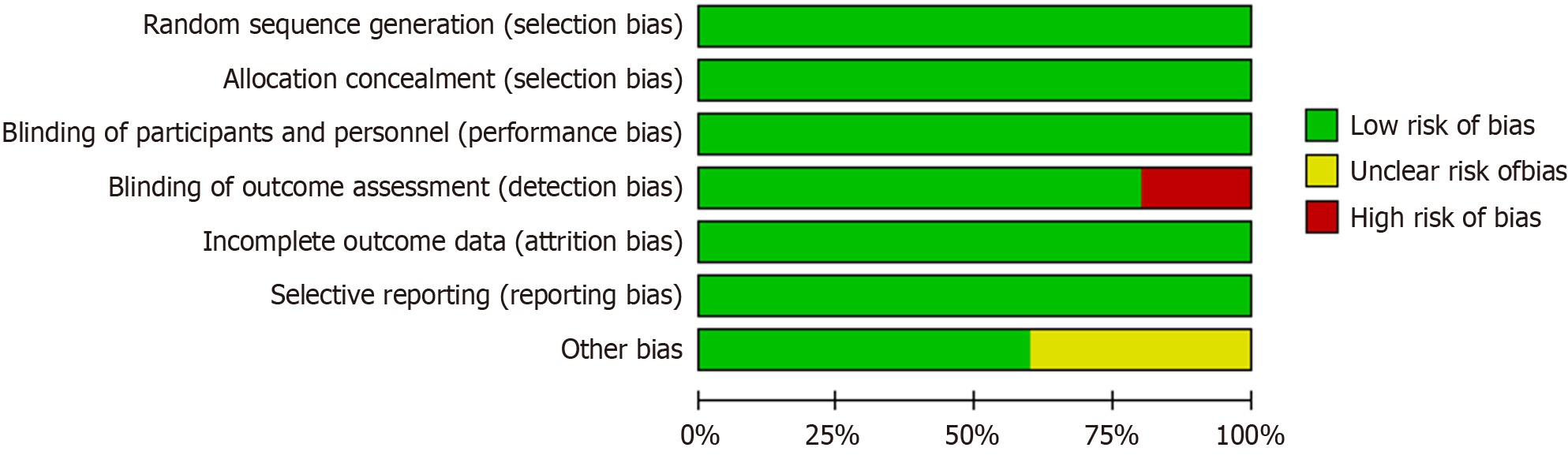
The Cochrane method was used to calculate the risk of bias in the RCTs, as shown in Figures 2 and 3. Five studies showed a low risk of bias for randomized sequence generation (100%), blinding of participants (100%), blinding of outcomes (80%), selective reporting (100%), and others (60%). Three of these exhibited high quality according to the assessment results (Figures 2 and 3).
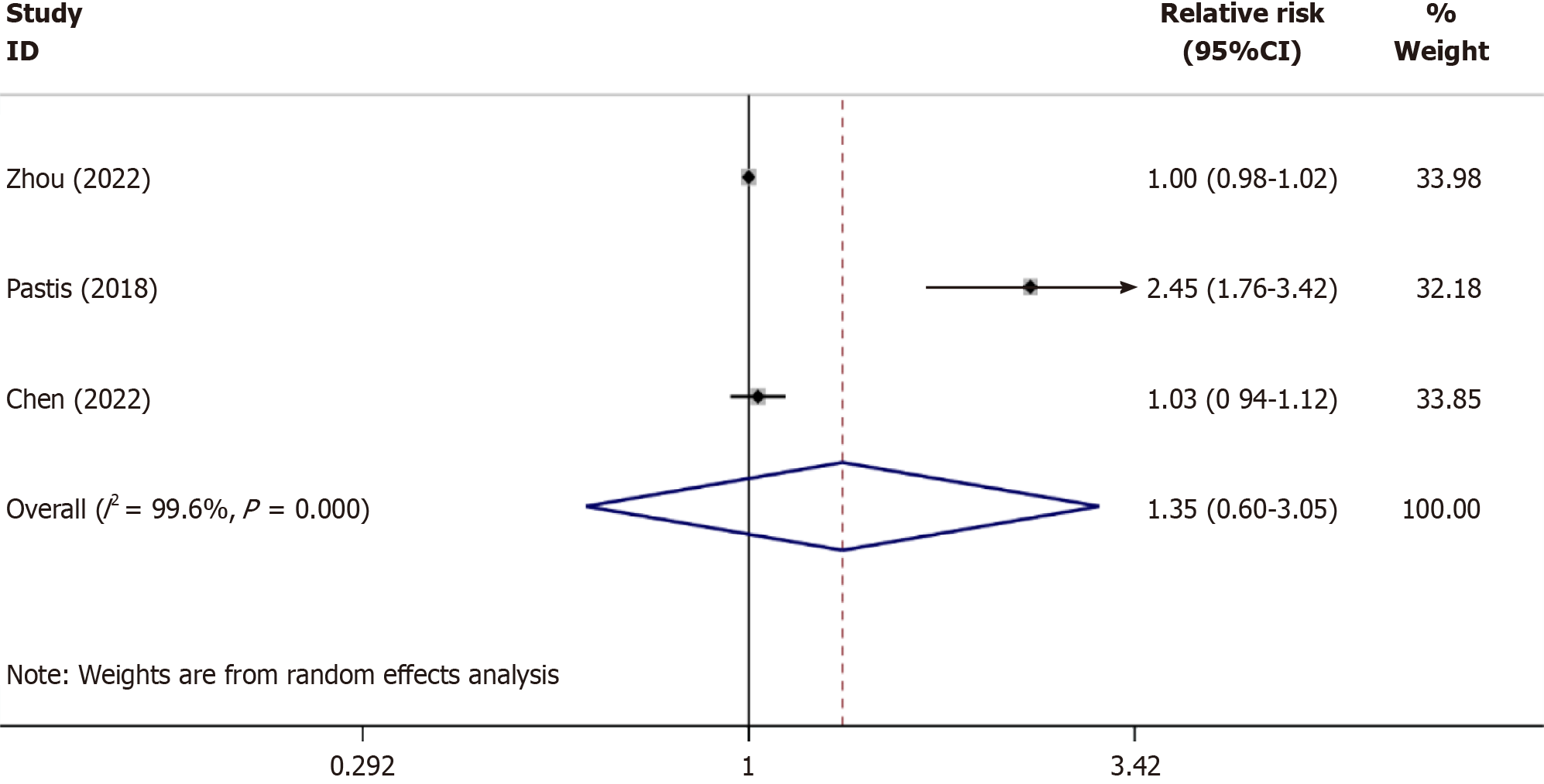
The sedative efficiency: Three studies[15-17] reported the success rates of sedation with remimazolam and CS, involving 1032 cases (research group, n = 538; CS group, n = 301). The heterogeneity test results, I2 = 99.6% and P < 0.001 in the Q-test, indicate statistically significant heterogeneity among different studies. Therefore, a random-effects model was used for subsequent tests. As shown in Figure 4, the relative risk (RR) value of the 3 studies pooled was 1.35, (95%CI: 0.60-3.05), P = 0.474, suggesting that the success rate of remimazolam for bronchoscopic sedation was similar to that of CS.
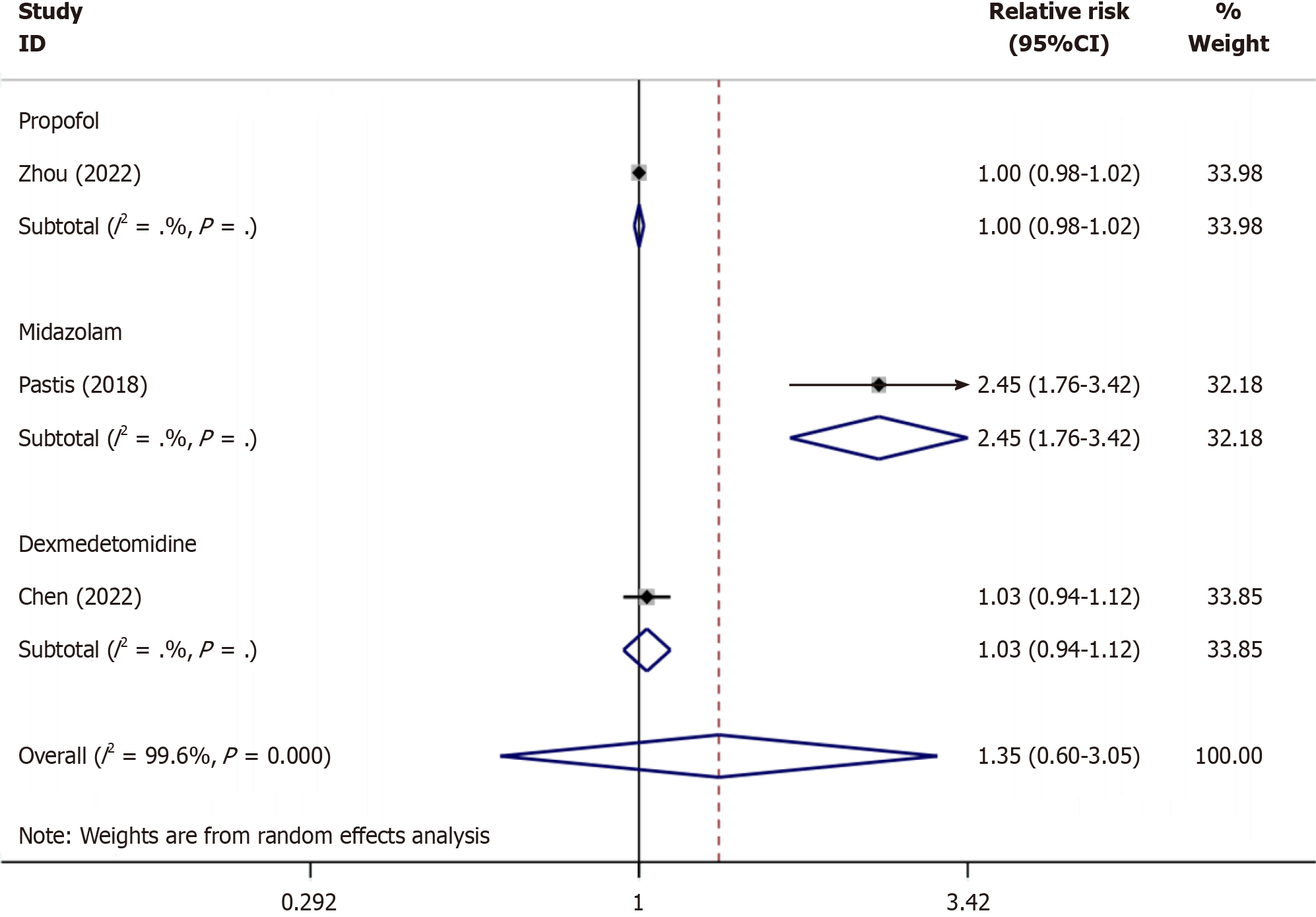
As shown in Figure 5, subgroup analysis showed that the success rate of remimazolam sedation was similar to that of propofol (RR: 1, P = 1.000), remimazolam sedation was more successful than midazolam sedation (RR: 2.45, P ≤ 0.001), and remimazolam and dexmedetomidine had similar sedation success rates (RR: 1.03, P = 0.513).
The incidence of adverse events: As shown in Table 4, there was a significant difference in the incidence of hypotension and respiratory depression between the remimazolam and CS groups (hypotension: RR = 0.61, I2 = 65.1%, P = 0.027; respiratory depression: RR = 0.50, I2 = 42.3%, P = 0.002). The incidence of hypertension, hypoxemia, bradycardia, tachycardia, and injection pain was similar between the two groups.
| Control | Complications | Relative risk | 95%CI | I2 value (%) | P value for effect |
| Conventional sedatives | Hypotension | 0.61 | (0.40, 0.95) | 65.1 | 0.027 |
| Hypertension | 1.11 | (0.89, 1.38) | 23.5 | 0.359 | |
| Respiratory depression | 0.50 | (0.33, 0.77) | 42.3 | 0.002 | |
| Hypoxemia | 0.74 | (0.37, 1.47) | 59.7 | 0.387 | |
| Bradycardia | 0.72 | (0.33, 1.56) | 0.0 | 0.403 | |
| Tachycardia | 0.78 | (0.33, 1.85) | 0.0 | 0.576 | |
| Injection pain | 0.17 | (0.01, 5.30) | 72.3 | 0.316 |
As shown in Table 5, subgroup analyses revealed obvious differences between the two groups in the incidence of hypotension, respiratory depression, hypoxemia, and injection pain (hypotension: RR = 0.42, I2 = 0.0%, P < 0.001; respiratory depression: RR = 0.48, I2 = 78.4%, P = 0.002; hypoxemia: RR = 0.4, I2 = 0.0%, P < 0.001; and injection pain: RR = 0.04, I2 = 0.0%, P < 0.001). There was no obvious heterogeneity in the incidence of hypertension, bradycardia, or tachy
| Control | Complications | Relative risk | 95%CI | I2 value (%) | P value for effect |
| Propofol | Hypotension | 0.45 | (0.32, 0.64) | 0.0 | 0.000 |
| Hypertension | 2.00 | (0.82, 4.85) | 37.6 | 0.125 | |
| Respiratory depression | 0.48 | (0.30, 0.76) | 78.4 | 0.002 | |
| Hypoxemia | 0.36 | (0.15, 0.87) | - | 0.023 | |
| Bradycardia | 0.33 | (0.08, 1.33) | 0.0 | 0.119 | |
| Tachycardia | 0.67 | (0.27, 1.64) | - | 0.378 | |
| Injection pain | 0.04 | (0.01, 0.28) | - | 0.001 | |
| Midazolam | Hypotension | 0.85 | (0.65, 1.12) | - | 0.247 |
| Hypertension | 1.03 | (0.83, 1.28) | - | 0.766 | |
| Respiratory depression | 0.53 | (0.14, 2.00) | - | 0.350 | |
| Hypoxemia | 1.16 | (0.68, 1.97) | - | 0.595 | |
| Bradycardia | 0.99 | (0.29, 3.37) | - | 0.983 | |
| Tachycardia | 2.07 | (0.11, 38.05) | - | 0.624 | |
| Injection pain | 1.15 | (0.06, 23.72) | - | 0.927 | |
| Dexmedetomidine | Hypotension | 0.61 | (0.40, 0.95) | - | 0.797 |
| Hypertension | 0.67 | (0.11, 3.87) | - | 0.652 | |
| Respiratory depression | 1.00 | (0.14, 6.91) | - | 1.000 | |
| Hypoxemia | 0.80 | (0.33, 1.91) | - | 0.616 | |
| Bradycardia | 1.50 | (0.26, 8.71) | - | 0.652 |
This study aimed to explore the efficacy and safety of remimazolam during bronchoscopy. Based on these results, remimazolam had a sedation success rate similar to that of CS. However, remimazolam was associated with a lower risk of hypotension and respiratory depression than was CS. It can be concluded that remimazolam for bronchoscopy provides satisfactory sedation and a favorable safety profile. We compared the efficacy and safety of that with CS (propofol, midazolam, and dexmedetomidine) in bronchoscopic sedation, analyzing a total of 5 studies on the application of remimazolam for bronchoscopy. Of these, three papers compared remimazolam vs propofol, one used midazolam, and one used dexmedetomidine. Due to the heterogeneity among the three sedative drugs, this study conducted a meta-analysis and found that remimazolam showed a higher success rate of sedation than midazolam. Compared with propofol, remimazolam has a lower risk of hypotension, respiratory depression, and injection pain.
Remimazolam is a novel benzodiazepine analog[18]. It can be quickly metabolized in vivo by esterases independent of renal metabolism, and its metabolites are inactive[19]. The effects of this drug can be reversed by flumazenil, with a rapid onset of action and safe sedation[20]. In addition, the use of remimazolam reduces patient healthcare costs compared with midazolam during bronchoscopy[21]. Therefore, it is a promising drug for bronchoscopic diagnosis and therapy[22]. The number of endoscopic procedures is increasing, and anesthesia is beneficial for endoscopic procedures[9,23]. Anesthetic drug selection for bronchoscopic surgery should improve the safety of the procedure without compromising the success rate[24,25]. This meta-analysis showed that remimazolam reduced intraoperative adverse events and complications while maintaining the sedation success rate.
When writing this article, we identified two similar systematic reviews and meta-analyses[26,27] that compared the reliability and safety of other sedatives in endoscopy, however, we incorporated a wider range of adverse events and complications which included hypotension, hypertension, respiratory depression, hypoxemia, bradycardia, tachycardia, and injection pain, to evaluate the safety of remimazolam more comprehensively. Our study showed that remimazolam exhibited the same success rate as CS for bronchoscopy, which is in contrast to existing studies[27] that stated that remimazolam had a higher procedural success rate than CS. This may be related to the diverse types of endoscopies included in that report, including upper gastrointestinal endoscopy, colonoscopy, hysteroscopy, and bronchoscopy, whereas only 1 bronchoscopy was included which was clinically heterogeneous. Furthermore, bronchoscopy is generally more stimulating than gastrointestinal endoscopy and hysteroscopy and requires deeper intraoperative sedation[28]. Further studies are warranted to investigate the success of remimazolam vs other sedatives at different sedation depths. The occurrence of hypotension and injection pain was lower in patients for whom remimazolam was used for sedation compared with propofol, which is consistent with two previous reports[26,29]. This suggests that remimazolam offers significant advantages in terms of respiration, circulation, and pain during injection.
Our study is the first to explore the efficacy of remimazolam vs CS in bronchoscopic procedures using subgroup analysis, providing evidence for the selection of bronchoscopic sedation drugs that remimazolam is safe and effective for bronchoscopic sedation. In clinical practice, patients undergoing bronchoscopy are predominantly elderly and chronically ill[30], and remimazolam facilitates intraoperative safety and postoperative recovery by significantly reducing respiratory and circulatory depression compared to CS. However, our study has some limitations. First, the definitions of different types of surgical operations, sedation drugs, sedation doses, and outcome metrics varied, which may have influenced the results. Second, most of the patients in the included studies were from China, and there may be racial differences between the populations. Third, different types and uses of opioids in the included studies may have affected the results. Fourth, only a few studies were included because there is limited research on anesthesia during bronchoscopic surgery. There were fewer within-group studies in which we performed subgroup analyses. The reliability of the outcome metrics in a single study was examined, and more studies are needed for future analyses.
Remimazolam is safe and effective during bronchoscopy. The sedation success rate was similar to that of the traditional sedatives (propofol, midazolam, and dexmedetomidine). However, it exhibits a weaker inhibitory effect on respiration. Some scholars have reported the sedation efficacy and incidence of adverse events of remimazolam during bronchoscopy, and RCTs with more samples are needed to validate our findings.
Remimazolam is a new ultra-short-acting benzodiazepine sedative that is currently used for procedural sedation and general anesthesia. Several studies have used remimazolam for bendable bronchoscopes.
This is the first systematic review on the safety and efficacy of remimazolam during bronchoscopy.
This study aimed to assess the safety and efficacy of remimazolam for the sedation of patients undergoing bendable bronchoscopy.
We searched databases of EMBASE, PubMed, Cochrane Library, and the Web of Science, from the original to August 2023. The search terms include "Remimazolam" or "CNS 7056", search scope was "Title and Abstract". The search was limited to human studies and literature in English.
This meta-analysis included five studies. The sedation success rate of remimazolam was similar to that of conventional sedatives (CS). However, remimazolam is associated with a lower incidence of hypotension and respiratory depression. The subgroup analysis showed a higher success rate for sedation with remimazolam than with midazolam. The incidences of hypotension, respiratory depression, hypoxemia, and injection pain were lower with remimazolam than with propofol.
Remimazolam is safe and effective for bronchoscopic sedation. The success rate was similar to that of CS. However, remimazolam has a higher safety profile, with fewer inhibitory effects on respiration and circulation.
Endoscopic surgery outside the operating room is currently increasing, and anesthesia provides strong support for the development of endoscopic surgery. The use of remimazolam can fulfill sedation requirements during bronchoscopic procedures while reducing the incidence of intraoperative adverse events and complications.
| 1. | Criner GJ, Eberhardt R, Fernandez-Bussy S, Gompelmann D, Maldonado F, Patel N, Shah PL, Slebos DJ, Valipour A, Wahidi MM, Weir M, Herth FJ. Interventional Bronchoscopy. Am J Respir Crit Care Med. 2020;202:29-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Kamel T, Helms J, Janssen-Langenstein R, Kouatchet A, Guillon A, Bourenne J, Contou D, Guervilly C, Coudroy R, Hoppe MA, Lascarrou JB, Quenot JP, Colin G, Meng P, Roustan J, Cracco C, Nay MA, Boulain T; Clinical Research in Intensive Care Sepsis Group (CRICS-TRIGGERSEP). Benefit-to-risk balance of bronchoalveolar lavage in the critically ill. A prospective, multicenter cohort study. Intensive Care Med. 2020;46:463-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Wahidi MM, Jain P, Jantz M, Lee P, Mackensen GB, Barbour SY, Lamb C, Silvestri GA. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 2011;140:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Hong KS, Choi EY, Park DA, Park J. Safety and Efficacy of the Moderate Sedation During Flexible Bronchoscopic Procedure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2015;94:e1459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Pertzov B, Krasulya B, Azem K, Shostak Y, Izhakian S, Rosengarten D, Kharchenko S, Kramer MR. Dexmedetomidine versus propofol sedation in flexible bronchoscopy: a randomized controlled trial. BMC Pulm Med. 2022;22:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Keating GM. Dexmedetomidine: A Review of Its Use for Sedation in the Intensive Care Setting. Drugs. 2015;75:1119-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 280] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 7. | McCambridge AJ, Boesch RP, Mullon JJ. Sedation in Bronchoscopy: A Review. Clin Chest Med. 2018;39:65-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Hu Q, Liu X, Wen C, Li D, Lei X. Remimazolam: An Updated Review of a New Sedative and Anaesthetic. Drug Des Devel Ther. 2022;16:3957-3974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 9. | Zhao MJ, Hu HF, Li XL, Li XM, Wang DC, Kuang MJ. The safety and efficacy between remimazolam and propofol in intravenous anesthesia of endoscopy operation: a systematic review and meta-analysis. Int J Surg. 2023;109:3566-3577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 10. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 26226] [Article Influence: 1748.4] [Reference Citation Analysis (4)] |
| 11. | Koster G, Wetterslev J, Gluud C, Zijlstra JG, Scheeren TW, van der Horst IC, Keus F. Effects of levosimendan for low cardiac output syndrome in critically ill patients: systematic review with meta-analysis and trial sequential analysis. Intensive Care Med. 2015;41:203-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Wang H, Luo Q, Li Y, Zhang L, Wu X, Yan F. Effect of Prophylactic Levosimendan on All-Cause Mortality in Pediatric Patients Undergoing Cardiac Surgery-An Updated Systematic Review and Meta-Analysis. Front Pediatr. 2020;8:456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Gao S, Wang T, Cao L, Li L, Yang S. Clinical effects of remimazolam alone or in combination with dexmedetomidine in patients receiving bronchoscopy and influences on postoperative cognitive function: a randomized-controlled trial. Int J Clin Pharm. 2023;45:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 14. | Zhang L, Yu L, Xu L, Wang JF, Li JY, Chen ZJ. Effectiveness of remimazolam besylate combined with alfentanil for fiberoptic bronchoscopy with preserved spontaneous breathing: a prospective, randomized, controlled clinical trial. Eur Rev Med Pharmacol Sci. 2023;27:6071-6080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 15. | Zhou YY, Yang ST, Duan KM, Bai ZH, Feng YF, Guo QL, Cheng ZG, Wu H, Shangguan WN, Wu XM, Wang CH, Chai XQ, Xu GH, Liu CM, Zhao GF, Chen C, Gao BA, Li LE, Zhang M, Ouyang W, Wang SY. Efficacy and safety of remimazolam besylate in bronchoscopy for adults: A multicenter, randomized, double-blind, positive-controlled clinical study. Front Pharmacol. 2022;13:1005367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 16. | Pastis NJ, Yarmus LB, Schippers F, Ostroff R, Chen A, Akulian J, Wahidi M, Shojaee S, Tanner NT, Callahan SP, Feldman G, Lorch DG Jr, Ndukwu I, Pritchett MA, Silvestri GA; PAION Investigators. Safety and Efficacy of Remimazolam Compared With Placebo and Midazolam for Moderate Sedation During Bronchoscopy. Chest. 2019;155:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | Chen X, Xin D, Xu G, Zhao J, Lv Q. The Efficacy and Safety of Remimazolam Tosilate Versus Dexmedetomidine in Outpatients Undergoing Flexible Bronchoscopy: A Prospective, Randomized, Blind, Non-Inferiority Trial. Front Pharmacol. 2022;13:902065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Kilpatrick GJ. Remimazolam: Non-Clinical and Clinical Profile of a New Sedative/Anesthetic Agent. Front Pharmacol. 2021;12:690875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 19. | Choi JY, Lee HS, Kim JY, Han DW, Yang JY, Kim MJ, Song Y. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: A randomized non-inferiority trial. J Clin Anesth. 2022;82:110955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 94] [Reference Citation Analysis (0)] |
| 20. | Lee A, Shirley M. Remimazolam: A Review in Procedural Sedation. Drugs. 2021;81:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Pedersen MH, Danø A, Englev E, Kattenhøj L, Munk E. Economic benefits of remimazolam compared to midazolam and propofol for procedural sedation in colonoscopies and bronchoscopies. Curr Med Res Opin. 2023;39:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 22. | Wesolowski AM, Zaccagnino MP, Malapero RJ, Kaye AD, Urman RD. Remimazolam: Pharmacologic Considerations and Clinical Role in Anesthesiology. Pharmacotherapy. 2016;36:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, Barish CF, Pruitt R, Cash BD, Quirk D, Tiongco F, Sullivan S, Bernstein D. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88:427-437.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 24. | Pastis NJ, Hill NT, Yarmus LB, Schippers F, Imre M, Sohngen W, Randall O, Callahan SP, Silvestri GA. Correlation of Vital Signs and Depth of Sedation by Modified Observer's Assessment of Alertness and Sedation (MOAA/S) Scale in Bronchoscopy. J Bronchology Interv Pulmonol. 2022;29:54-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | José RJ, Shaefi S, Navani N. Sedation for flexible bronchoscopy: current and emerging evidence. Eur Respir Rev. 2013;22:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Zhu X, Wang H, Yuan S, Li Y, Jia Y, Zhang Z, Yan F, Wang Z. Efficacy and Safety of Remimazolam in Endoscopic Sedation-A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2021;8:655042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 27. | Tang Y, Yang X, Yu Y, Shu H, Xu J, Li R, Zou X, Yuan S, Shang Y. Remimazolam versus traditional sedatives for procedural sedation: a systematic review and meta-analysis of efficacy and safety outcomes. Minerva Anestesiol. 2022;88:939-949. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Nelson ME. Moderate Sedation Changes for Bronchoscopy in 2017. Chest. 2017;152:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Zhang J, Cairen Z, Shi L, Pang S, Shao Y, Wang Y, Lu Z. Remimazolam versus propofol for procedural sedation and anesthesia: a systemic review and meta-analysis. Minerva Anestesiol. 2022;88:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Mondoni M, Radovanovic D, Sotgiu G, Di Marco F, Carlucci P, Centanni S, Santus P. Interventional pulmonology techniques in elderly patients with comorbidities. Eur J Intern Med. 2019;59:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rotenberg O, United States S-Editor: Gong ZM L-Editor: A P-Editor: Zheng XM