Published online Dec 16, 2024. doi: 10.12998/wjcc.v12.i35.6826
Revised: July 2, 2024
Accepted: July 23, 2024
Published online: December 16, 2024
Processing time: 295 Days and 18 Hours
Tuberculosis is a chronic infectious disease and an important public health pro
A previously healthy 28-year-old woman presented to our hospital with a 1-mo
We speculate that the pks1 virulence gene in BLM-A21 may be the key virulence gene responsible for the wide
Core Tip: Tuberculosis is an important public health problem that threatens human health that primarily infects the lungs. We report a case of invasive pulmonary tuberculosis in a young woman with normal immune function. Comparison of the genetic characteristics of the patient’s strain with those of other disease-causing strains suggests that its virulence and wide dissemination was attributable to the presence of the pks1 gene, a genotype that can cause meningitis.
- Citation: Wu F, Yang B, Xiao Y, Ren LL, Chen HY, Hu XL, Pan YY, Chen YS, Li HR. psk1 virulence gene-induced pulmonary and systemic tuberculosis in a young woman with normal immune function: A case report. World J Clin Cases 2024; 12(35): 6826-6833
- URL: https://www.wjgnet.com/2307-8960/full/v12/i35/6826.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i35.6826
Tuberculosis is a chronic infectious disease caused by Mycobacterium tuberculosis (M. tuberculosis) and is an important public health problem. In 1979, China carried out the first national tuberculosis epidemiological survey, which showed that the prevalence of active tuberculosis was 717/100000[1]. Under the modern tuberculosis control and tuberculosis containment strategy implemented by China in 1992, the prevalence was reduced to 59/100000 by 2020[2]. This was mainly due to active prevention, including vaccination with the bacillus Calmette–Guérin (BCG) vaccine. Despite these achievements, China still has one of the highest burdens of tuberculosis worldwide, with 895000 new cases annually[2].
We report a rare case of disseminated pulmonary tuberculosis with secondary systemic hematogenous dissemination in a young woman with normal immune function and no underlying diseases and discuss the possible underlying causes.
A 28-year-old woman presented with a 1-month history of recurrent fever and lower back pain and a 1-week history of dyspnoea.
The patient had been healthy until the onset of the illness 1 month previously. She was admitted to Fujian Provincial Hospital in August 2021.
None.
She had no known underlying diseases or family history of hereditary diseases. Her parents and sister did not have similar symptoms. She had not been vaccinated with BCG, although her younger sister had received a BCG vaccination.
On admission her vital signs were as follows: Body temperature, 38.8 ℃; pulse rate, 117 beats/minute; respiratory rate, 40 breaths/minute; blood pressure, 127/64 mmHg; and peripheral oxygen saturation, 80% with an inhaled oxygen concentration of 29%. She had shallow, rapid breathing. Chest auscultation revealed bilateral diffuse moist rales. A lump measuring approximately 3.5 cm × 5.0 cm was present in her lumbosacral region, with slight tenderness, poor mobility, and no redness, swelling, or ulceration of the overlying skin.
Her arterial blood gas results with 29% oxygen supplementation were as follows: PH, 7.483; PCO2, 34.6 mmHg; PO2, 44.2 mmHg; and the oxygenation index was 152 mmHg. Haematology revealed a white blood cell count of 5100 cells/μL, with 63.6% segmented neutrophils; a haemoglobin level of 131 g/L; and a platelet count of 273000/μL. Blood biochemistry and immunology revealed the following: Serum albumin, 38 g/L; aspartate aminotransferase, 42 U/L; alkaline phosphatase, 110.6 U/L; lactate dehydrogenase, 548 U/L; procalcitonin, 2.4 ng/mL; C-reactive protein, 67.1 mg/L; and erythrocyte sedimentation rate, 13 mm/h. Immune function tests revealed the following: CD3 cell count, 106 cells/μL; CD4 cell count, 58 cells/μL; CD8 cell count, 43 cells/μL; NK cell count, 35 cells/μL; CD19 cell count, 86 cells/μL; CD45 cell count, 227 cells/μL; serum immunoglobulin G, 9.91 g/L; immunoglobulin A, 2.02 g/L; immunoglobulin M, 0.48 g/L; immunoglobulin E, 165 g/L; complement C3, 0.997 g/L; and complement C4, 0.125 g/L. The antinuclear antibody profile (full set of autoimmunity), antineutrophil cytoplasmic antibody, rheumatoid factor, and anticyclic citrulline polypeptide antibody tests were negative. Hepatitis B antibody, human immunodeficiency virus antibody, and syphilis-specific antibody tests were also negative. Sputum bacterial and fungal cultures were negative.
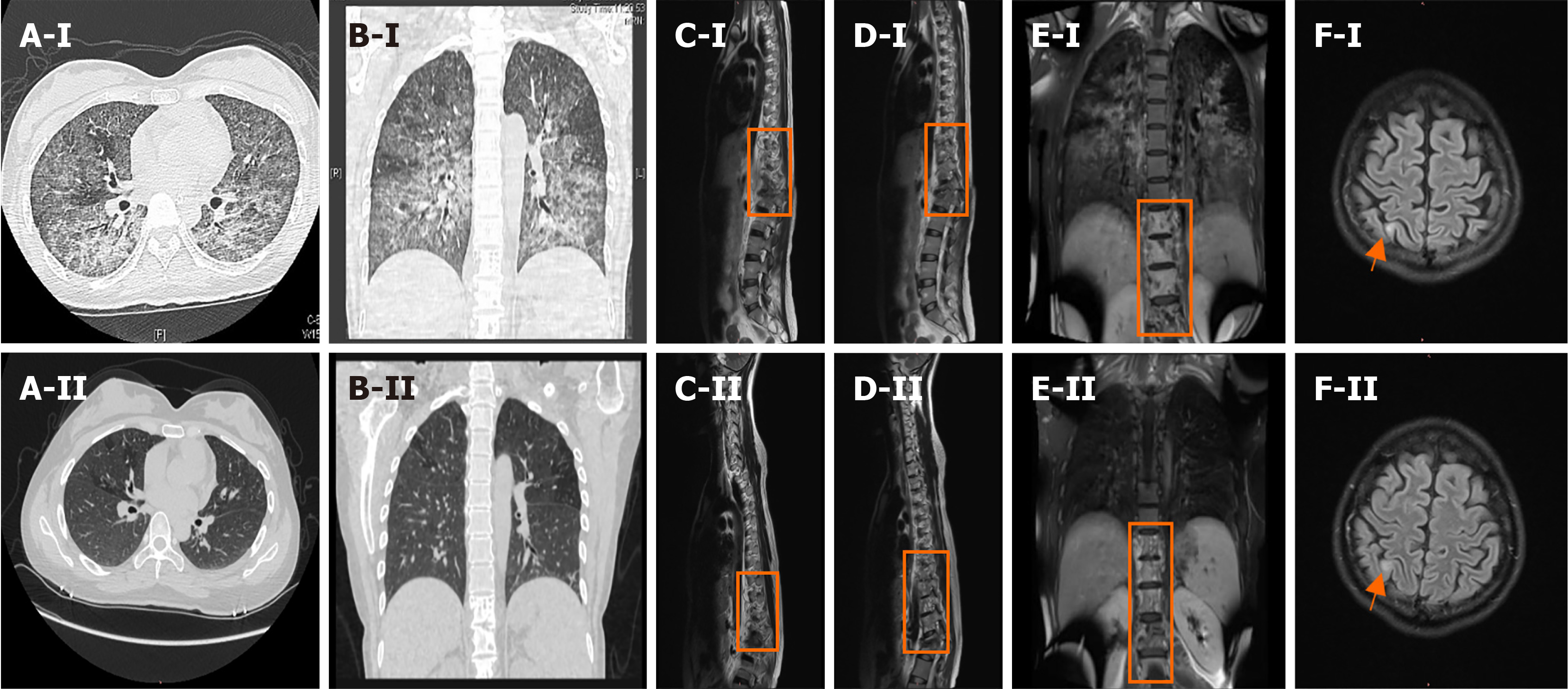
Chest computed tomography showed diffuse lesions in both lungs, bone destruction from the eighth thoracic vertebra to the first lumbar vertebra, and a paravertebral soft tissue mass (Figure 1A and B). Enhanced magnetic resonance imaging (MRI) of the thoracolumbar spine showed abnormal signal shadows from the ninth thoracic vertebral body to the 1st lumbar vertebral body and the surrounding soft tissue (Figure 1C-E). Brain enhanced MRI showed abnormal signals in the right parietal lobe (Figure 1F).
The patient underwent immediate endotracheal intubation and bronchoscopy. A bronchoalveolar lavage fluid (BALF) smear was positive for acid-fast bacilli, and a Gene X-pert MTB/RIF assay was positive for M. tuberculosis DNA. Next-generation sequencing of blood and BALF, and BALF culture confirmed M. tuberculosis infection.
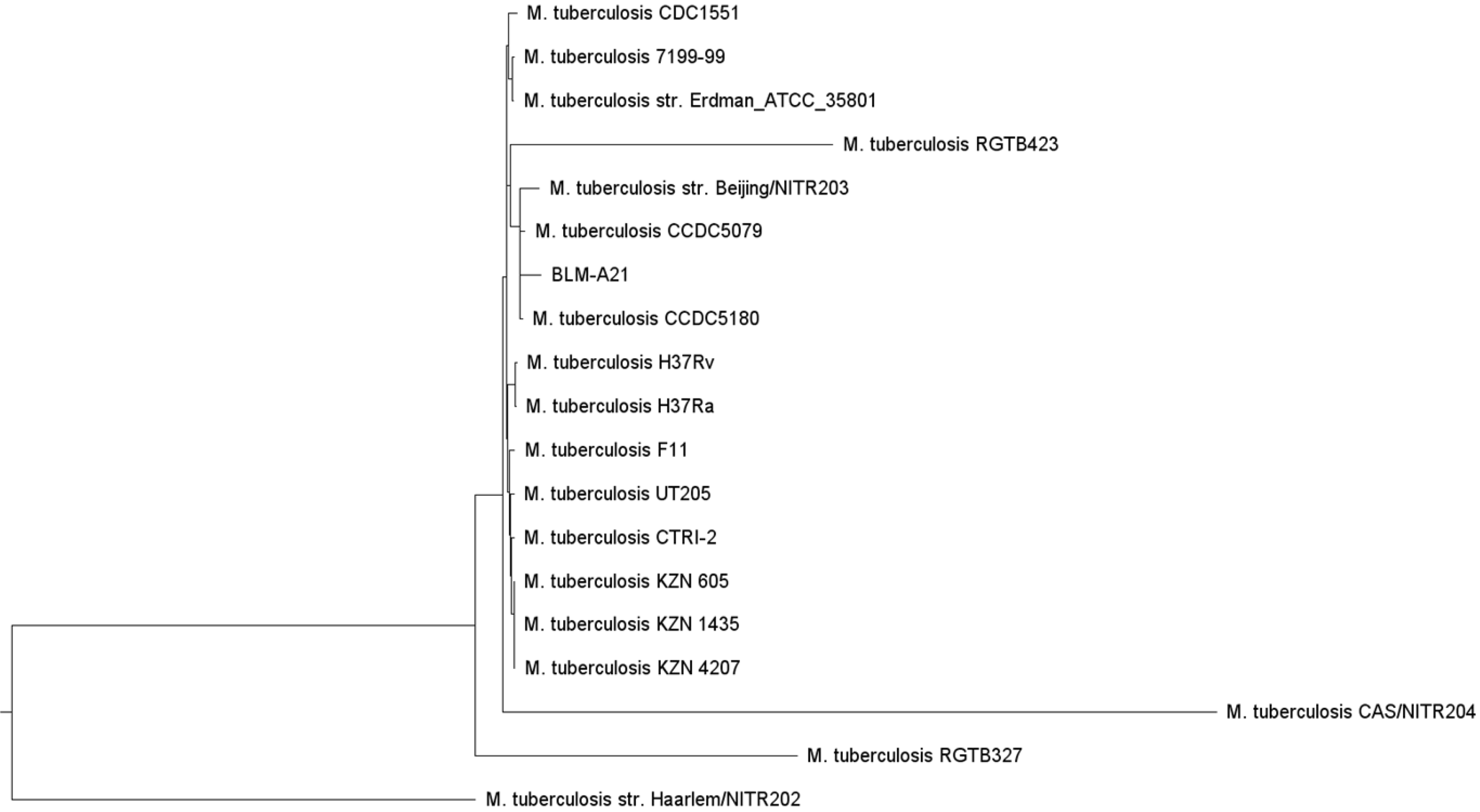
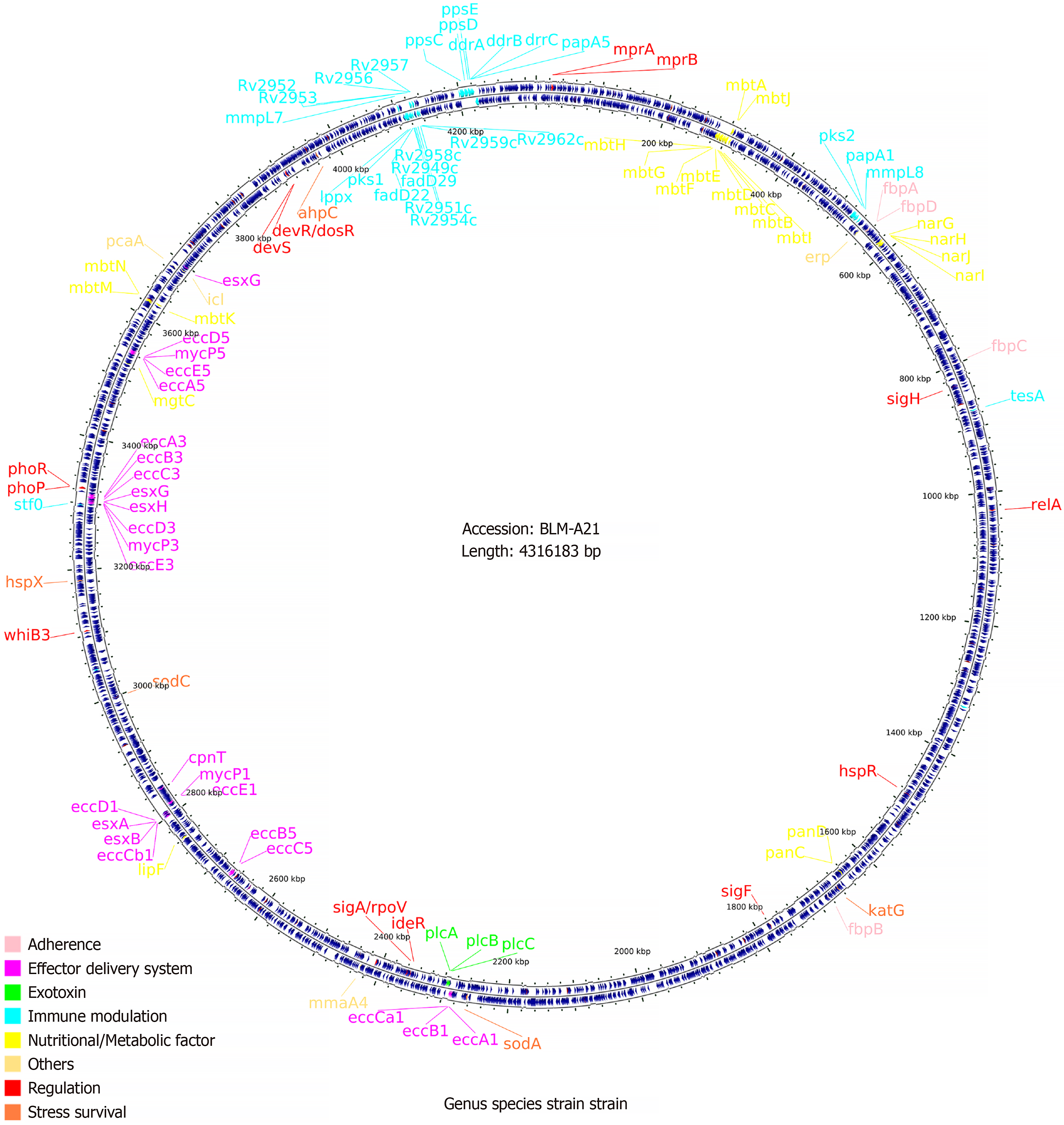
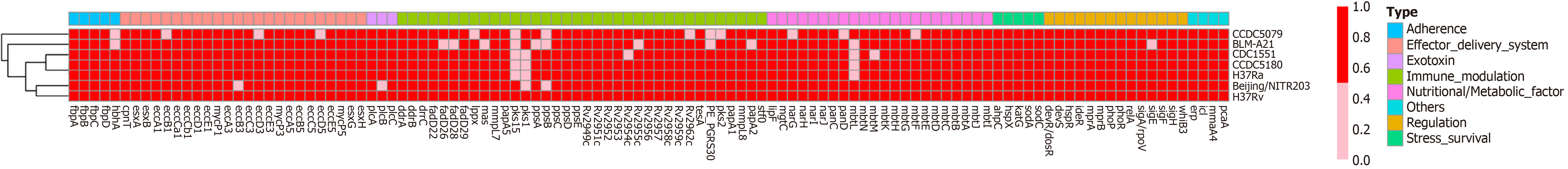
To ascertain why this M. tuberculosis strain had caused such a severe infection in a young adult with normal immune function, we analysed the strain using whole-genome sequencing. We designated the strain, which had a total of 4155 genes, BLM-A21. We selected other M. tuberculosis genomes from the virulence factor database (VFDB) (http://www.mgc.ac.cn/cgi-bin/VFs/genus.cgi?Genus=Mycobacterium) [3], developed by the bioinformatics research team of the institute of Pathogenic Biology, Chinese Academy of Medical Science. We then performed phylogenetic analysis using PhyML maximum likelihood software[4] to build an evolutionary tree (Figure 2). We found that BLM-A21 had a close evolutionary relationship to CCDC5079, CCDC5180, and Beijing NTR203. Therefore, we analysed the whole genomes of several strains, including CCDC5079, CCDC5180, Beijing NTR203, CDC1551, and classic H37Rv and H37Ra, for subsequent genome comparison, as these strains have previously shown strong dissemination ability[5,6]. We used the BLASTP performance comparison algorithm[7] and the virulence factor protein reference sequence of the VFDB to an
The patient was diagnosed with severe pulmonary tuberculosis and secondary systemic disseminated tuberculosis, including spinal tuberculosis with a paravertebral abscess, and tuberculous meningitis.
After receiving isoniazid, rifampicin, ethambutol, and pyrazinamide, the patient’s temperature dropped; her cough and shortness of breath improved, and the tracheal intubation was removed. Imaging of the patient's brain, chest, and vertebral body performed 6 months after starting treatment showed that her condition had greatly improved (Figure 1A-F). Eight months after starting antituberculous treatment, the patient underwent debridement, bone graft, and internal fixation surgery for spinal tuberculosis, and left psoas abscess debridement.
After the surgery the patient has recovered the ability to walk unaided. Pain was evaluated using a visual analogue scale (VAS), with pain intensity graded on a scale of 0 (no pain) to 10 (most severe pain). The Oswestry Disability Index (ODI), which consists of 10 questions with a total score of 100 points, was used to evaluate the degree of lumbar functional impairment. A higher score indicates more severe the functional impairment. Both the VAS and ODI improved markedly after treatment. The VAS and ODI results before, and 6 ½ months after, treatment are shown in Table 1.
| Before treatment | 6 ½ months after treatment | |
| VAS score | 6 | 1 |
| ODI score | 44 | 12 |
BCG is an attenuated form of Mycobacterium bovis that provides immune protection and has been the only vaccine available against tuberculosis in China since the 1930s. In 2000, the BCG vaccination coverage in newborns reached 90%, effectively preventing miliary tuberculosis and tuberculous meningitis in children, and reducing the risk of M. tuberculosis infection in adults[9]. The patient, who had not received BCG vaccination suffered from severe M. tuberculosis infection, whereas her sister, who had received BCG vaccination, did not become ill.
Previous studies have shown that most children with hematogenous disseminated pulmonary tuberculosis had not received BCG vaccination[10], suggesting that the risk of severe tuberculosis is higher in individuals without BCG vaccination.
Severe pulmonary tuberculosis is very rare, accounting for approximately 3%-7% of cases[11]. Most patients with severe tuberculosis have had previous contact with an individual with tuberculosis, have weakened cellular immune function, or have other conditions such as anaemia, malnutrition, and a delay in seeking medical treatment[12-15]. However, this patient had none of these predisposing factors. Therefore, we hypothesised that the pks1 virulence gene of BLM-A21 may have been the reason for the severity of the patient’s disease.
A previous study showed that, compared with strains with a high risk of dissemination, strains that lack pks1–15, a phenol glycolipid (PGL)-related synthesis gene, have weak ability to disseminate to the central nervous system[16]. We found that BLM-A21 carried the pks1 gene, but lacked the pks15 gene, whereas the number of other virulence genes was consistent with that of other low-dissemination strains such as CDC 1551 and H37Rv. Based on an in vitro live bacterial transcriptome experiment, pks1 has been reported to have a greater effect than pks15 on regulating fadD22, Rv2949c, lppX, fadD29, and other genes, thus promoting PGL synthesis. PGL is related to several cell functions, particularly the imper
We hypothesize that the pks1 virulence gene of the BLM-A21 M. tuberculosis strain, induced severe pulmonary tuberculosis and secondary systemic disseminated tuberculosis in this unvaccinated patient with normal immune function. The mechanism whereby the pks1 gene causes highly invasive tuberculosis needs further study.
We thank the patient for her permission to publish this report, cooperation in drafting the final manuscript, and permission to use images.
| 1. | Tu DH. [Tuberculosis control in China for 60 years]. Zhonghua Jiehe He Huxi Zazhi. 2013;36:886-887. [DOI] [Full Text] |
| 2. | World Health Organization. Global tuberculosis report 2017. Geneva: World Health Organization; 2017. Available from: https://www.who.int/publications/i/item/9789241565516. |
| 3. | Liu B, Zheng D, Jin Q, Chen L, Yang J. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019;47:D687-D692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 717] [Cited by in RCA: 1341] [Article Influence: 223.5] [Reference Citation Analysis (0)] |
| 4. | Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol. 2009;537:113-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 630] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 5. | Kohli S, Singh Y, Sharma K, Mittal A, Ehtesham NZ, Hasnain SE. Comparative genomic and proteomic analyses of PE/PPE multigene family of Mycobacterium tuberculosis H₃₇Rv and H₃₇Ra reveal novel and interesting differences with implications in virulence. Nucleic Acids Res. 2012;40:7113-7122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Bucsan AN, Rout N, Foreman TW, Khader SA, Rengarajan J, Kaushal D. Mucosal-activated invariant T cells do not exhibit significant lung recruitment and proliferation profiles in macaques in response to infection with Mycobacterium tuberculosis CDC1551. Tuberculosis (Edinb). 2019;116S:S11-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10130] [Cited by in RCA: 14445] [Article Influence: 849.7] [Reference Citation Analysis (0)] |
| 8. | Stothard P, Grant JR, Van Domselaar G. Visualizing and comparing circular genomes using the CGView family of tools. Brief Bioinform. 2019;20:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 9. | Zhu BD, Wang HH. [History and current status of tuberculosis vaccine research]. Zhonghua Jiehe He Huxi Zazhi. 2007;30:378-382. |
| 10. | Yang M, Yuan P, Wang Y, Chen L, Shi ZY, Luo DX, Huang XQ. [Analysis of clinical prevalence of 502 cases of hematogenous disseminated pulmonary tuberculosis in Sichuan]. Sichuan Yixue. 2018;39:977-982. |
| 11. | Liu TL. [Practical tuberculosis]. Shenyang: Liaoning Science and Technology Press; 1987; 284-289. |
| 12. | Yun J, Wang AM. [Clinical characteristics of 146 patients with hematogenous disseminated pulmonary tuberculosis and analysis of influencing factors of curative effect]. Zhongguo Bingan. 2016;17:70-73. |
| 13. | Liu XN, Li Y. [Analysis of clinical characteristics and risk factors of young patients with severe pulmonary tuberculosis]. Linchuang Feike Zazhi. 2020;25:1419-1423. |
| 14. | Ashenafi S, Bekele A, Aseffa G, Amogne W, Kassa E, Aderaye G, Worku A, Bergman P, Brighenti S. Anemia Is a Strong Predictor of Wasting, Disease Severity, and Progression, in Clinical Tuberculosis (TB). Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 15. | Tedla K, Medhin G, Berhe G, Mulugeta A, Berhe N. Delay in treatment initiation and its association with clinical severity and infectiousness among new adult pulmonary tuberculosis patients in Tigray, northern Ethiopia. BMC Infect Dis. 2020;20:456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Tsenova L, Ellison E, Harbacheuski R, Moreira AL, Kurepina N, Reed MB, Mathema B, Barry CE 3rd, Kaplan G. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis. 2005;192:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 186] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Ramos B, Gordon SV, Cunha MV. Revisiting the expression signature of pks15/1 unveils regulatory patterns controlling phenolphtiocerol and phenolglycolipid production in pathogenic mycobacteria. PLoS One. 2020;15:e0229700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/