Published online Dec 16, 2024. doi: 10.12998/wjcc.v12.i35.6775
Revised: August 29, 2024
Accepted: September 9, 2024
Published online: December 16, 2024
Processing time: 91 Days and 20.6 Hours
Chronic kidney disease (CKD) and chronic periodontitis (CP) are prevalent conditions which significantly impact public health worldwide. Both diseases share inflammatory and oxidative stress mechanisms, an indication of a likely bidirectional relationship. This editorial explored the association between CKD and CP by highlighting common inflammatory mechanisms and recent research findings that address this interrelationship. Through reviews of recent studies, we discussed how periodontal bacteria may activate systemic immune responses that affect both periodontal and renal tissues. Additionally, meta-analysis data indicated an increased risk of CKD development in patients with CP, and vice versa. The results suggest the need for more rigorous research in the future in order to address the confounding factors and evaluate specific periodontal health interventions and their direct effects on kidney function. We emphasized the importance of comprehensive and multidisciplinary care for the improvement of the overall health of patients affected by CP and CKD.
Core Tip: In this editorial, we reviewed the recent meta-analysis by Yang et al, which investigated the association between chronic periodontitis (CP) and chronic kidney disease (CKD). The analysis showed that CP patients have increased risk of CKD, and vice versa. This review also incorporated findings from other significant studies that support this link. We highlighted the need for more consistent definitions, rigorous adjustment for confounding factors, and well-designed prospective studies to ascertain the causal relationship between CP and CKD. This ongoing investigation is crucial for enhancing the management of periodontal health of CKD patients and for improving overall patient outcomes.
- Citation: Martínez Nieto M, De Leon Rodríguez ML, Anaya Macias RDC, Lomelí Martínez SM. Periodontitis and chronic kidney disease: A bidirectional relationship based on inflammation and oxidative stress. World J Clin Cases 2024; 12(35): 6775-6781
- URL: https://www.wjgnet.com/2307-8960/full/v12/i35/6775.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i35.6775
Chronic kidney disease (CKD) presents a global public health problem. The Clinical Practice Guideline for the evaluation and management of chronic kidney disease (KDIGO) has estimated that currently, approximately 9.1% of the global population has this condition in one clinical stage or the other[1,2]. The World Health Organization (WHO) has stated that CKD is the fourteenth leading cause of death worldwide[3,4]. Moreover, WHO has projected that CKD may become the fifth leading cause of death by 2040[3,5]. This is due to the high morbidity and mortality associated with cardio
Periodontitis is a highly prevalent disease which affects approximately 50% of the general population[1,9], and it is the sixth most prevalent dental disease worldwide[5,11,12]. A previous estimate indicated that at least 743 million people worldwide were affected by periodontitis. However, over the last 30 years, up to 99% increase in prevalence of periodontitis has been observed, especially in developing countries[1]. This makes it an epidemiologically relevant condition[13]. Chronic periodontitis (CP) is an inflammatory infection that affects the supporting tissues of the teeth, i.e., the gums, cementum, alveolar bone, and periodontal ligament. The development and maturation of dental biofilm consisting of bacterial colonies on the teeth, is the primary etiological factor that contributes to the pathogenesis of periodontal disease[1,14]. Some biomarkers associated with the inflammatory processes observed in CP are C-reactive protein, tumor necrosis factor-alpha, interleukin-6, and interleukin-1 beta[3,15].
Multiple studies have demonstrated the relationship amongst periodontitis, various systemic conditions such as diabetes mellitus, pregnancy and CKD[7]. In all cases, it was determined that the association is governed by systemic immunoinflammatory reactions in patients with periodontitis[3,11,14], especially in those with severe stages of the disease. This suggests a direct relationship between the severity of CP and the progression of CKD, with the worsening of one disease potentially exacerbating the other[7,10,16].
The objective of this editorial was to study the association between CP and CKD, thereby highlighting common inflammatory mechanisms and recent research findings that address this interrelationship. In doing so, we hoped to emphasize the importance of comprehensive and multidisciplinary care in improving the overall health of patients affected by the two diseases.
Although CP and CKD have various causes, recent studies have demonstrated a bidirectional association between the two conditions[2,5,11,17]. Clinical trials suggest higher incidence and severity of periodontal problems in CKD patients, with figures ranging from 75% to 90% in different studies[3]. Cross-sectional studies have shown that advanced CP increases the risk of CKD in stages 4 and 5 up to 3.9 folds[7,9]. A cohort study on a large number of CKD patients demon
In another comparative study on 66 periodontal disease patients, 33 of whom had pre-dialysis CKD, while 33 had no renal disease, all subjects received non-surgical periodontal treatment. Serum inflammatory markers were measured before and after periodontal treatment. It was found that patients with periodontitis and CKD had significantly higher levels of these parameters than patients without CKD before receiving non-surgical treatment (P < 0.05). However, six weeks after non-surgical management, there were significant reductions in levels of inflammatory markers (P < 0.05), thereby demonstrating the importance of maintaining adequate periodontal health in these patients[3,19].
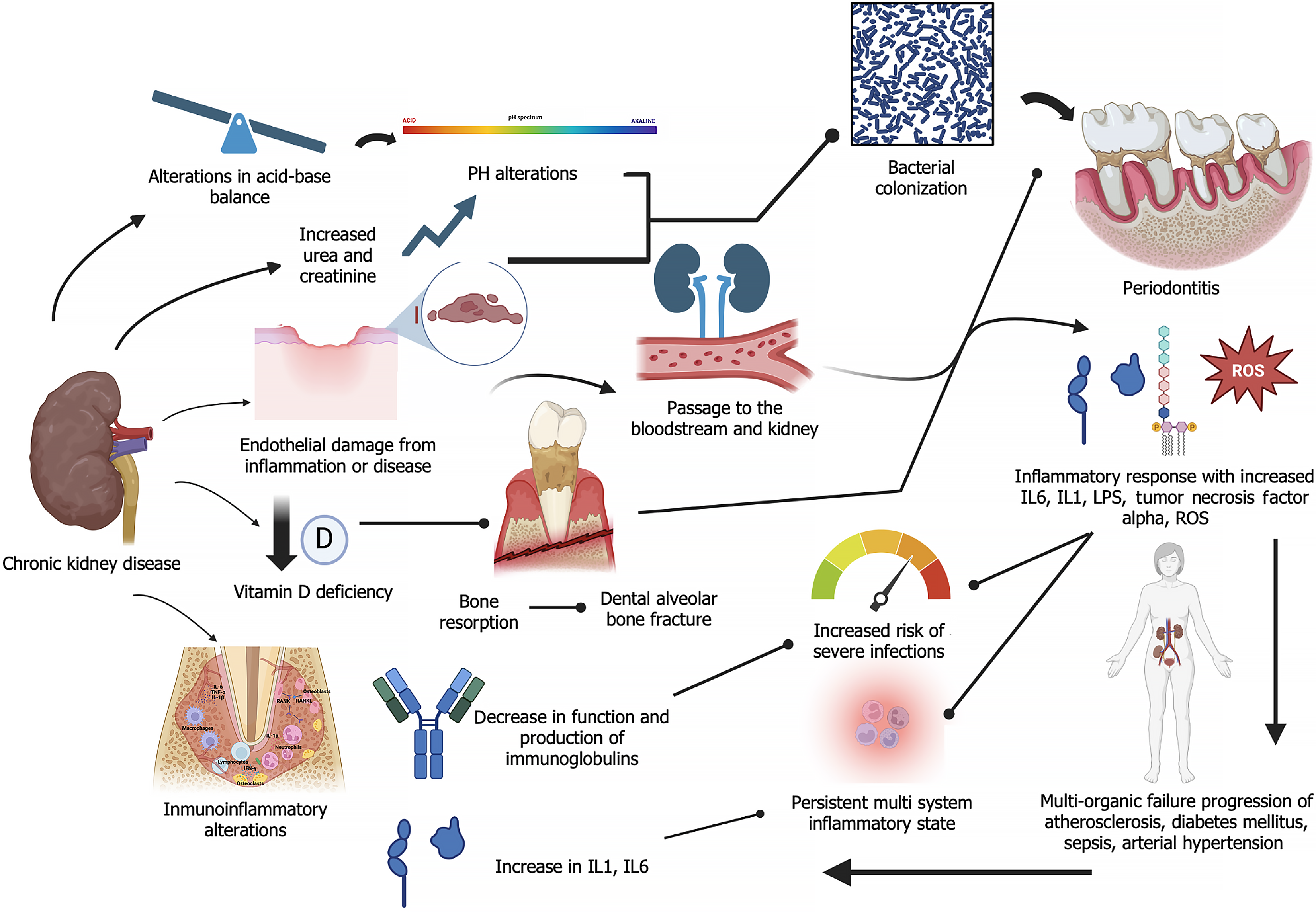
Various mechanisms have been described in the association of these conditions. These mechanisms include the migration of bacteria from periodontal pockets along with cytokines and pro-inflammatory factors and lipopolysaccharides that cause endothelial damage, resulting in a persistent systemic inflammatory state. This favors the development of hypertension and cardiovascular diseases which are significant risk factors for CKD and renal endothelial damage[5,20]. Additionally, the systemic inflammatory state promotes insulin resistance which leads to the onset or worsening of diabetes mellitus[9,21], another disease that may cause CKD. Changes in CKD, such as increased serum urea and changes in salivary pH, modify the oral microbiota and increase the risk of pathogenic bacterial colonization[5,10,17].
The exacerbated inflammatory state caused by both diseases leads to a significant imbalance in oxidative stress response at the systemic level, with increased generation of reactive oxygen species (ROS)[15,21], and a decrease in glutathione peroxidase, a key antioxidant and a potent enzyme involved in regulating oxidative stress. This enzyme is produced mainly in the kidney, but it is also found in other structures, including periodontal tissues. A comparative study amongst four groups (healthy, periodontitis, CKD without periodontitis, and CKD with periodontitis) measured serum glutathione peroxidase levels, and it was observed that patients with CP had the highest levels of this enzyme, while those with CKD and CP had reduced levels, which may be associated with multiple causes[22] (Figure 1). However, the study is inconclusive.
Several studies on the connection between PD and CKD have been carried out by focusing on inflammation and oxidative stress as key mechanisms. These connections are particularly relevant in patients with underlying conditions like diabetes and hypertension.
Shinjo et al[23] reported that hyperglycemia, hyperlipidemia, chronic inflammation, and impaired insulin function are crucial factors in the progression of periodontitis in individuals with diabetes. The relationship between hyperglycemia and oxidative stress is particularly significant, as elevated glucose levels in people with diabetes may damage pancreatic β-cells, leading to insulin deficiency and chronic hyperglycemia, which in turn, trigger oxidative stress through inflammation, leading to diabetes-related complications. Additionally, hyperglycemia-related oxidative stress may cause macrophages to adopt an M1 polarization, leading to excessive production of inflammatory cytokines. Furthermore, hyperlipidemia, often linked to obesity-induced insulin resistance, contributes to chronic inflammation which further exacerbates periodontitis in diabetic patients[23].
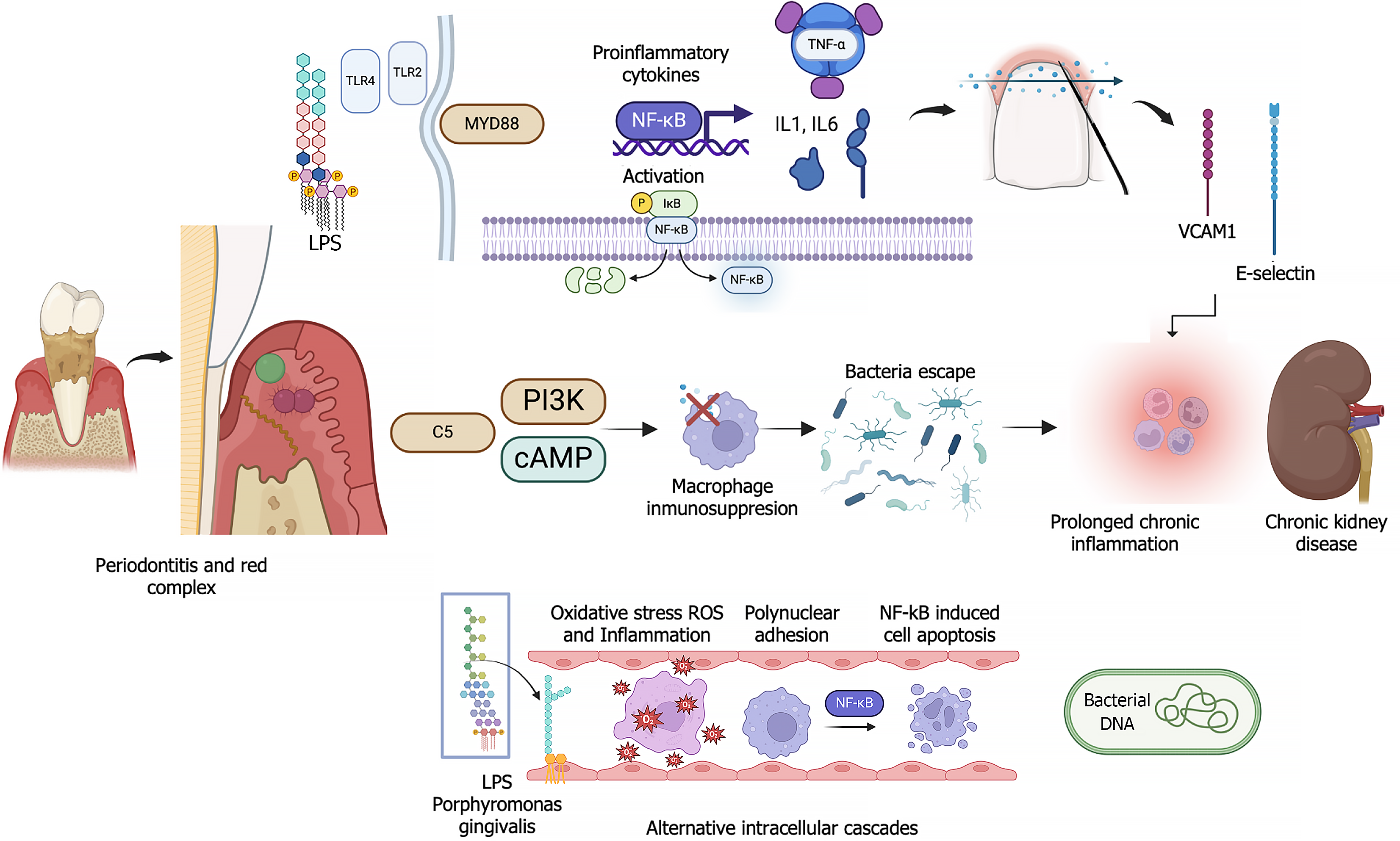
The link between periodontitis and hypertension is driven mainly by systemic inflammation and immune system activation. The inflammation leads to endothelial dysfunction, a critical factor in the etiology of hypertension. Immune cells such as T cells, and cytokines, e.g., interferon-gamma, which are involved in periodontitis and hypertension, damage blood vessels and increase sodium retention in the kidneys, thereby raising blood pressure. Additionally, chronic oral bacterial infections, particularly infections with Porphyromonas gingivalis which often occur in periodontitis, intensify systemic inflammation, thereby further contributing to hypertension and increasing the cardiovascular burden[24] (Figure 2).
Yang et al[25] published an intriguing paper, which was focused on the correlation between CP and CKD. Data from 22 studies on the clinical attachment level (CAL) and pocket probing depth (PPD) of CKD and non-CKD individuals were integrated. The results demonstrated that patients with CP were 1.54 times more likely to develop CKD than non-CP subjects (relative risk, RR: 1.54, 95%CI: 1.40-1.70). The incidence of CP in CKD patients was 1.98 times higher than that in healthy individuals (OR: 1.98, 95%CI: 1.53-2.57). Patients with CKD presented higher levels of CAL [standardized mean difference (SMD): 0.65, 95%CI: 0.29-1.01] and PPD (SMD: 0.33, 95%CI: 0.02-0.63), when compared to healthy controls. The study established a bidirectional association between CP and CKD through a meta-analysis of observational studies. Additionally, the risk of CKD was higher in patients with CP[25]. These findings are similar to those reported by Deschamps-Lenhardt et al[5]. In the latter study, a total of 37 articles from observational investigations were subjected to systematic review, out of which only 17 were used for the meta-analysis. The primary objective was to investigate the association between CP and CKD through analyses of related studies and studies on the effect of CP on renal health. The meta-analysis showed a positive association between CKD and PD, and the strength of the association increased when severe PD was considered [OR = 2.39 (1.70-3.36)]. This association was identified even after adjusting for major CKD risk factors or after using precise diagnostic criteria [OR = 2.26 for severe PD (1.69-3.01)][5]. In each of these studies[5,25], it was concluded that there was a strong correlation between CP and CKD. However, Yang et al[25] provided a more detailed analysis of how specific clinical parameters, e.g., CAL and PPD are affected in patients with CKD, thereby highlighting the importance of oral health in managing systemic diseases. However, in contrast, Nanayakkara et al[26] analyzed the possible association between CP and CKD through a systematic review and meta-analysis of observational studies reported in 47 articles. They concluded that participants with CP were 3.54 times more likely to have CKD than subjects without periodontitis, although significant heterogeneity was observed amongst the studies (I² = 88.3%, P < 0.001). However, the findings were inconclusive on directional association: The random effects model showed an incidence rate ratio (IRR) of 2.10, while the fixed effects model resulted in an IRR of 1.76, with significant heterogeneity (I² = 78.3%, P = 0.031)[26]. Therefore, the results indicated that there was a non-directional association between CP and CKD, although evidence for a causal association was limited. Thus, there is need for adequately designed prospective studies and longer follow-up periods in order to establish the causal relationship more clearly.
Although comparative studies provide valuable insights into the association between CP and CKD, it is essential to consider methodological limitations and potential biases in order to accurately interpret the results. The heterogeneities in the measurement methods, definitions and diagnostic criteria for CKD and CP, as well as the variabilities in the study populations presented in the study by Yang et al[25], may affect the validity of the findings. The research by Deschamps-Lenhardt et al[5] highlighted variabilities in the inclusion and exclusion criteria used in the integrated studies, which may compromise the representativeness of the results. Additionally, the absence of uniform adjustments for critical risk factors such as diabetes, smoking, and hypertension, may have introduced bias in the results, since these factors are associated with both CP and CKD. Furthermore, Nanayakkara et al[26] reported high heterogeneity (I² = 88.3%) amongst the integrated studies, indicating significant variabilities in study design, population, and outcome measures. These variabilities made it difficult for the researchers to conclusively establish the association between CP and CKD. Future studies should consider standardizing methods and definitions, rigorous adjustments for confounding factors, and employment of more robust designs, so as to enhance the quality and reliability of the findings.
Given the bidirectional relationship between CP and CKD, it is crucial for periodontists and nephrologists to collaborate closely in developing and implementing treatment strategies aimed at improving the management and outcomes of patients affected by the two concurrent diseases. Systemic inflammation and oxidative stress are underlying mechanisms that link both diseases. Thus, it is very likely that comprehensive management will significantly benefit patients’ overall health.
It is essential for periodontists and nephrologists to work together to design, develop, and implement comprehensive treatment plans that address both periodontal and renal health. Effective communication and teamwork between these professionals are vital for early detection and timely intervention. In dental and nephrology clinics, it would be highly beneficial to design early detection programs for identifying patients at risk of developing CP and CKD. Early detection allows for preventive interventions that may slow down the progression of these conditions, while periodic evaluations of periodontal and renal health enable the early identification of changes and relevant treatment adjustments.
Specific periodontal therapeutic interventions and systemic inflammation control should be successfully implemented. Non-surgical and surgical periodontal therapies should be tailored in order to control the inflammatory process and reduce the bacterial load in CKD patients. Treatments should be personalized to meet each patient’s specific needs. Regarding systemic inflammation control, interventions aimed at managing inflammation and oxidative stress such as the use of anti-inflammatory and antioxidant drugs, should be considered in order to improve both periodontal and renal health. It is crucial for healthcare professionals to be trained to recognize the signs and symptoms of CP and CKD, and it is vital to understand the importance of simultaneous management for the two conditions. Continuing education may enhance knowledge and cooperation among specialists.
In clinical practice, integrating these recommendations will significantly improve the management of patients with CP and CKD, thereby enhancing their quality of life and reducing the progression of the conditions. A multidisciplinary and comprehensive approach is essential for effectively addressing this bidirectional relationship and its implications for overall health.
The findings presented in the meta-analysis by Yang et al[25] on the association between CP and CKD open several important directions for future research for enhancement of the understanding of this relationship.
Although the current study has established an association between CP and CKD, future research must ensure uniformity in the definition and classification of CP in order to guarantee more accurate comparisons and dose-response analyses. It would be valuable to conduct randomized controlled trials to assess whether immune suppression induced by CKD increases susceptibility to CP. Additionally, it would be beneficial to investigate whether the systemic inflammatory response caused by CP leads to chronic pathological changes in renal function. Furthermore, more rigorous and consistent adjustment for confounding factors is required to reduce bias and obtain more reliable results. Research on the bidirectional relationship between CP and CKD would provide insights into how each condition may influence the other, and help develop comprehensive, multidisciplinary treatment strategies.
There is need for studies on the efficacy of specific periodontal health intervention such as non-surgical periodontal therapy, in improving renal outcomes. Randomized clinical trials aimed at investigating how periodontal treatment may influence CKD progression would be particularly valuable. Additionally, well-designed longitudinal cohort studies would be beneficial in assessing the long-term impact of periodontal interventions on renal health.
In summary, prioritizing these future research directions will not only deepen the understanding of the association between CP and CKD but also unravel the underlying mechanisms and yield more robust and precise conclusions on their relationship.
Although several studies have established an association between CP and CKD, the causal relationship between these two conditions remains uncertain due to the presence of multiple uncontrolled confounding factors in the analyzed studies. Additionally, the significant heterogeneity amongst studies suggests that the evidence is not yet conclusive enough to allow for proposal of a definitive association. Therefore, prospective research with adequate control and design are needed for the identification of the specific impact of CP on the progression of CKD, as well as studies on the specific interventions in periodontal health, in order to determine their direct effect on renal function. Until then, managing periodontal health in patients with CKD should be considered a general preventive measure without attributing a decisive influence on the progression of chronic kidney disease. Fostering interdisciplinary collaboration between periodontists and nephrologists is essential for the design, development, and implementation of treatment plans that address both periodontal and renal health. Public health policies should also prioritize the early detection and preventive management of CP and CKD by developing comprehensive health programs that integrate oral and renal care. These programs should ensure that all patients have access to quality care and promote preventive interventions to reduce the progression of both conditions. Additionally, awareness campaigns should be designed to educate the public on the importance of maintaining good oral health to prevent renal complications.
| 1. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105:S117-S314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2166] [Article Influence: 1083.0] [Reference Citation Analysis (0)] |
| 2. | Sundström J, Bodegard J, Bollmann A, Vervloet MG, Mark PB, Karasik A, Taveira-Gomes T, Botana M, Birkeland KI, Thuresson M, Jäger L, Sood MM, VanPottelbergh G, Tangri N; CaReMe CKD Investigators. Prevalence, outcomes, and cost of chronic kidney disease in a contemporary population of 2·4 million patients from 11 countries: The CaReMe CKD study. Lancet Reg Health Eur. 2022;20:100438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 187] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 3. | Chaudhry A, Kassim NK, Zainuddin SLA, Taib H, Ibrahim HA, Ahmad B, Hanafi MH, Adnan AS. Potential Effects of Non-Surgical Periodontal Therapy on Periodontal Parameters, Inflammatory Markers, and Kidney Function Indicators in Chronic Kidney Disease Patients with Chronic Periodontitis. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Wu H, Wang S, Wei Z. Periodontitis and risk of mortality in patients with chronic kidney disease: A systematic review with meta-analysis. J Periodontal Res. 2024;59:868-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Deschamps-Lenhardt S, Martin-Cabezas R, Hannedouche T, Huck O. Association between periodontitis and chronic kidney disease: Systematic review and meta-analysis. Oral Dis. 2019;25:385-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Mahendra J, Palathingal P, Mahendra L, Alzahrani KJ, Banjer HJ, Alsharif KF, Halawani IF, Muralidharan J, Annamalai PT, Verma SS, Sharma V, Varadarajan S, Bhandi S, Patil S. Impact of Red Complex Bacteria and TNF-α Levels on the Diabetic and Renal Status of Chronic Kidney Disease Patients in the Presence and Absence of Periodontitis. Biology (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Schütz JDS, de Azambuja CB, Cunha GR, Cavagni J, Rösing CK, Haas AN, Thomé FS, Fiorini T. Association between severe periodontitis and chronic kidney disease severity in predialytic patients: A cross-sectional study. Oral Dis. 2020;26:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Kapellas K, Singh A, Bertotti M, Nascimento GG, Jamieson LM; Perio-CKD collaboration. Periodontal and chronic kidney disease association: A systematic review and meta-analysis. Nephrology (Carlton). 2019;24:202-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Chang JF, Yeh JC, Chiu YL, Liou JC, Hsiung JR, Tung TH. Periodontal Pocket Depth, Hyperglycemia, and Progression of Chronic Kidney Disease: A Population-Based Longitudinal Study. Am J Med. 2017;130:61-69.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Hickey NA, Shalamanova L, Whitehead KA, Dempsey-Hibbert N, van der Gast C, Taylor RL. Exploring the putative interactions between chronic kidney disease and chronic periodontitis. Crit Rev Microbiol. 2020;46:61-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Chatzopoulos GS, Jiang Z, Marka N, Wolff LF. Periodontal Disease, Tooth Loss, and Systemic Conditions: An Exploratory Study. Int Dent J. 2024;74:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Hajishengallis G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol 2000. 2022;89:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 259] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 13. | Gamonal J, Bravo J, Malheiros Z, Stewart B, Morales A, Cavalla F, Gomez M. Periodontal disease and its impact on general health in Latin America. Section I: Introduction part I. Braz Oral Res. 2020;34:e024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Fischer RG, Lira Junior R, Retamal-Valdes B, Figueiredo LC, Malheiros Z, Stewart B, Feres M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz Oral Res. 2020;34:e026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 15. | Li L, Zhang YL, Liu XY, Meng X, Zhao RQ, Ou LL, Li BZ, Xing T. Periodontitis Exacerbates and Promotes the Progression of Chronic Kidney Disease Through Oral Flora, Cytokines, and Oxidative Stress. Front Microbiol. 2021;12:656372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Loos BG, Van Dyke TE. The role of inflammation and genetics in periodontal disease. Periodontol 2000. 2020;83:26-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 391] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 17. | Hajishengallis G, Chavakis T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21:426-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 1046] [Article Influence: 209.2] [Reference Citation Analysis (0)] |
| 18. | Tai YH, Chen JT, Kuo HC, Chang WJ, Wu MY, Dai YX, Liu WC, Chen TJ, Wu HL, Cherng YG. Periodontal disease and risk of mortality and kidney function decline in advanced chronic kidney disease: a nationwide population-based cohort study. Clin Oral Investig. 2021;25:6259-6268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Vachhani KS, Bhavsar NV. Effects of non-surgical periodontal therapy on serum inflammatory factor high-sensitive C-reactive protein, periodontal parameters and renal biomarkers in patients with chronic periodontitis and chronic kidney disease. Dent Med Probl. 2021;58:489-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Lertpimonchai A, Rattanasiri S, Tamsailom S, Champaiboon C, Ingsathit A, Kitiyakara C, Limpianunchai A, Attia J, Sritara P, Thakkinstian A. Periodontitis as the risk factor of chronic kidney disease: Mediation analysis. J Clin Periodontol. 2019;46:631-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Palathingal P, Mahendra J, Annamalai PT, Varma SS, Mahendra L, Thomas L, Baby D, Jose A, Srinivasan S, R A. A Cross-Sectional Study of Serum Glutathione Peroxidase: An Antioxidative Marker in Chronic Periodontitis and Chronic Kidney Disease. Cureus. 2022;14:e22016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Kanzaki H, Wada S, Narimiya T, Yamaguchi Y, Katsumata Y, Itohiya K, Fukaya S, Miyamoto Y, Nakamura Y. Pathways that Regulate ROS Scavenging Enzymes, and Their Role in Defense Against Tissue Destruction in Periodontitis. Front Physiol. 2017;8:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Shinjo T, Nishimura F. The bidirectional association between diabetes and periodontitis, from basic to clinical. Jpn Dent Sci Rev. 2024;60:15-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 24. | Szczepaniak P, Mikołajczyk TP, Cześnikiewicz-Guzik M, Guzik TJ. Periodontitis as an inflammatory trigger in hypertension: From basic immunology to clinical implications. Kardiol Pol. 2021;79:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Yang F, Shu CJ, Wang CJ, Chen K. Meta-analysis of the association between chronic periodontitis and chronic kidney disease. World J Clin Cases. 2024;12:5094-5107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 26. | Nanayakkara S, Zhou X. Periodontitis May Be Associated With Chronic Kidney Disease, but Evidence on Causal Association Is Limited. J Evid Based Dent Pract. 2019;19:192-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/