Published online Nov 6, 2024. doi: 10.12998/wjcc.v12.i31.6506
Revised: August 13, 2024
Accepted: August 16, 2024
Published online: November 6, 2024
Processing time: 105 Days and 0.4 Hours
Mucosa-associated lymphoid tissue (MALT) lymphoma, a type of non-Hodgkin lymphoma, originates in the mucosal lining of body organs and internal cavities, including the nose, mouth, lungs, and digestive tract. The lymphoma develops when the body produces abnormal B lymphocytes. These lymphomas develop at the edge of the lymphoid tissue, called the marginal zone, and, hence, are classi
We report the case of a patient who presented with a mass on the frontal cheek. Magnetic resonance imaging revealed a tumor in the buccal fat pad, and histo
Consideration of MALT lymphoma is essential in the differential diagnosis of frontal cheek masses.
Core Tip: Mucosa-associated lymphoid tissue (MALT) lymphoma mostly originates from the stomach, although it can originate from other mucosal-lined organs and internal cavities as well. Many cases have suggested a strong association between a history of Helicobacter pylori (H. pylori) infection and the development of gastric MALT lymphoma. In the present study, a patient with a history of H. pylori infection developed a buccal fat pad mass. Upon investigation, it was determined to be a case of extragastric MALT lymphoma. Hence, the role of H. pylori infection in the development of extragastric MALT lymphoma should be considered.
- Citation: Miyake K, Hirasawa K, Nishimura H, Tsukahara K. Rare incidence of mucosa-associated lymphoid tissue lymphoma presenting as buccal fat pad tumor: A case report. World J Clin Cases 2024; 12(31): 6506-6512
- URL: https://www.wjgnet.com/2307-8960/full/v12/i31/6506.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i31.6506
Mucosa-associated lymphoid tissue (MALT) lymphoma predominantly originates in the stomach. However, it can also occur in other areas such as the digestive tract, lungs, head and neck region, salivary glands[1], and thyroid gland[2]. Occurrences of MALT lymphoma in the buccal fat pad are extremely rare. To the best of our knowledge, no cases of MALT lymphoma originating from the buccal fat pad site have been reported to date. This rarity underscores the unique nature of the present case report and highlights the importance of recognizing such atypical presentations of MALT lymphoma.
Extragastric MALT lymphoma often presents diagnostic challenges. In some cases, patients may undergo surgery for a suspected benign tumor, only to receive a final diagnosis of MALT lymphoma upon postoperative pathological examination. This difficulty in diagnosis accentuates the need for further research to establish more reliable diagnostic and treatment methods for these cases.
In this report, we describe a case of MALT lymphoma arising in the buccal fat pad and provide a review of the relevant literature. By detailing this rare case, we aim to contribute to a better understanding of the diagnostic and therapeutic challenges associated with MALT lymphoma in this unusual location, ultimately improving the clinical management of similar cases.
A 61-year-old man presented with a chief complaint of left cheek swelling.
The patient first noticed the swelling in his left cheek 3 months prior. The swelling gradually increased in size, prompting a referral to our hospital for further evaluation and treatment.
His medical history included infections with hepatitis C virus (HCV) and Helicobacter pylori (H. pylori).
On physical examination, a palpable, non-tender, elastic mass with good mobility was noted in the left buccal region. The mass was not palpable intraorally. Intraoral examination revealed multiple dental caries and mild oral hygiene issues, but no other abnormalities were observed in the oropharynx.
Laboratory tests revealed the following results: White blood cell count, 4950/μL; red blood cell count, 430 × 104/μL; platelet count, 17.8 × 104/μL; total protein, 6.7 g/dL; albumin, 4.4 g/dL; aspartate aminotransferase, 18 IU/L; alanine aminotransferase, 25 IU/L; lactate dehydrogenase, 165 IU/L; alkaline phosphatase, 58 IU/L; blood urea nitrate, 11.5 mg/dL; creatine, 0.82 mg/dL; C-reactive protein, 0.02 mg/dL; and soluble interleukin-2 receptor, 413 U/mL. Fine-needle aspiration cytology revealed reactive lymphadenopathy of class II.
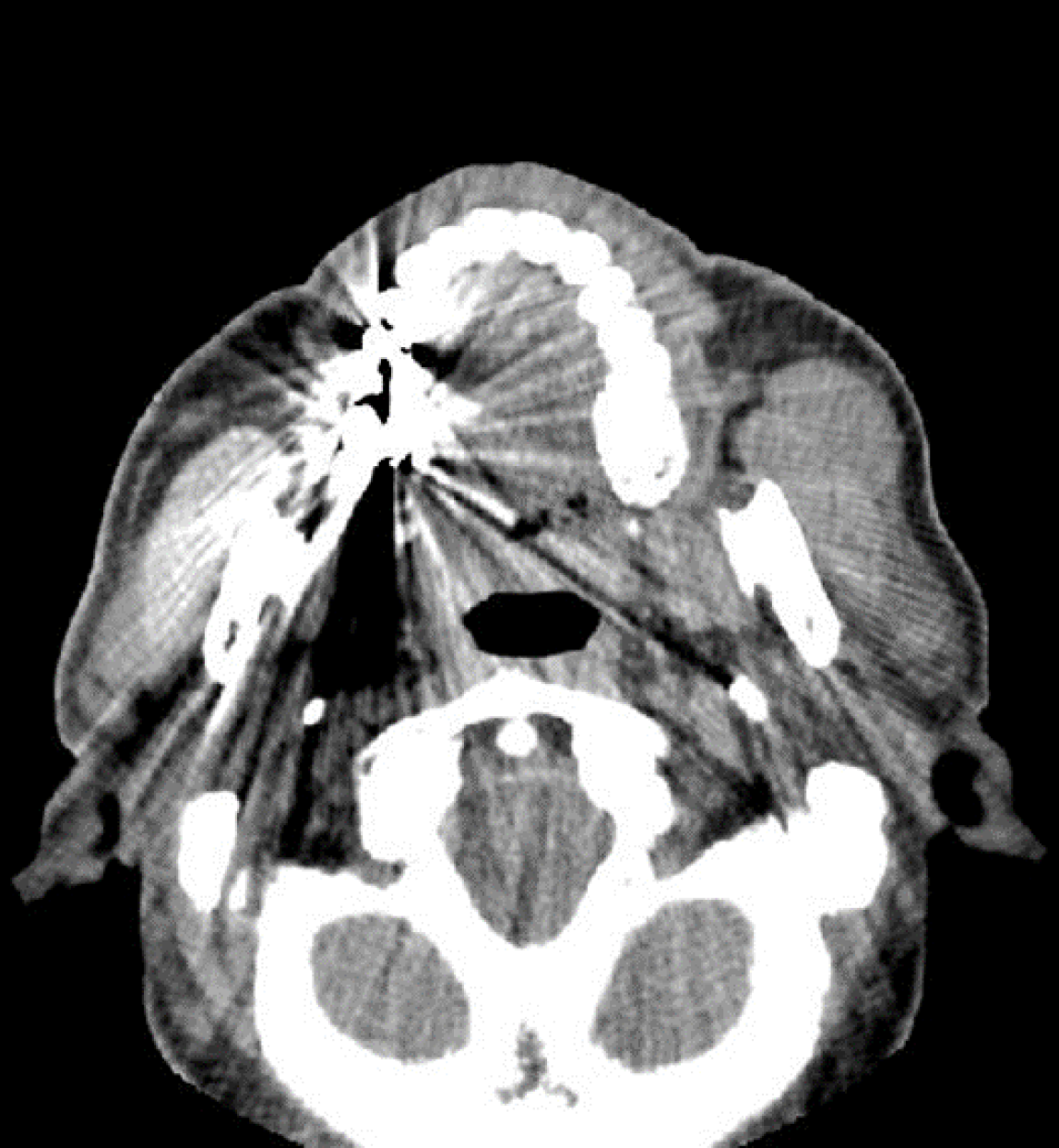
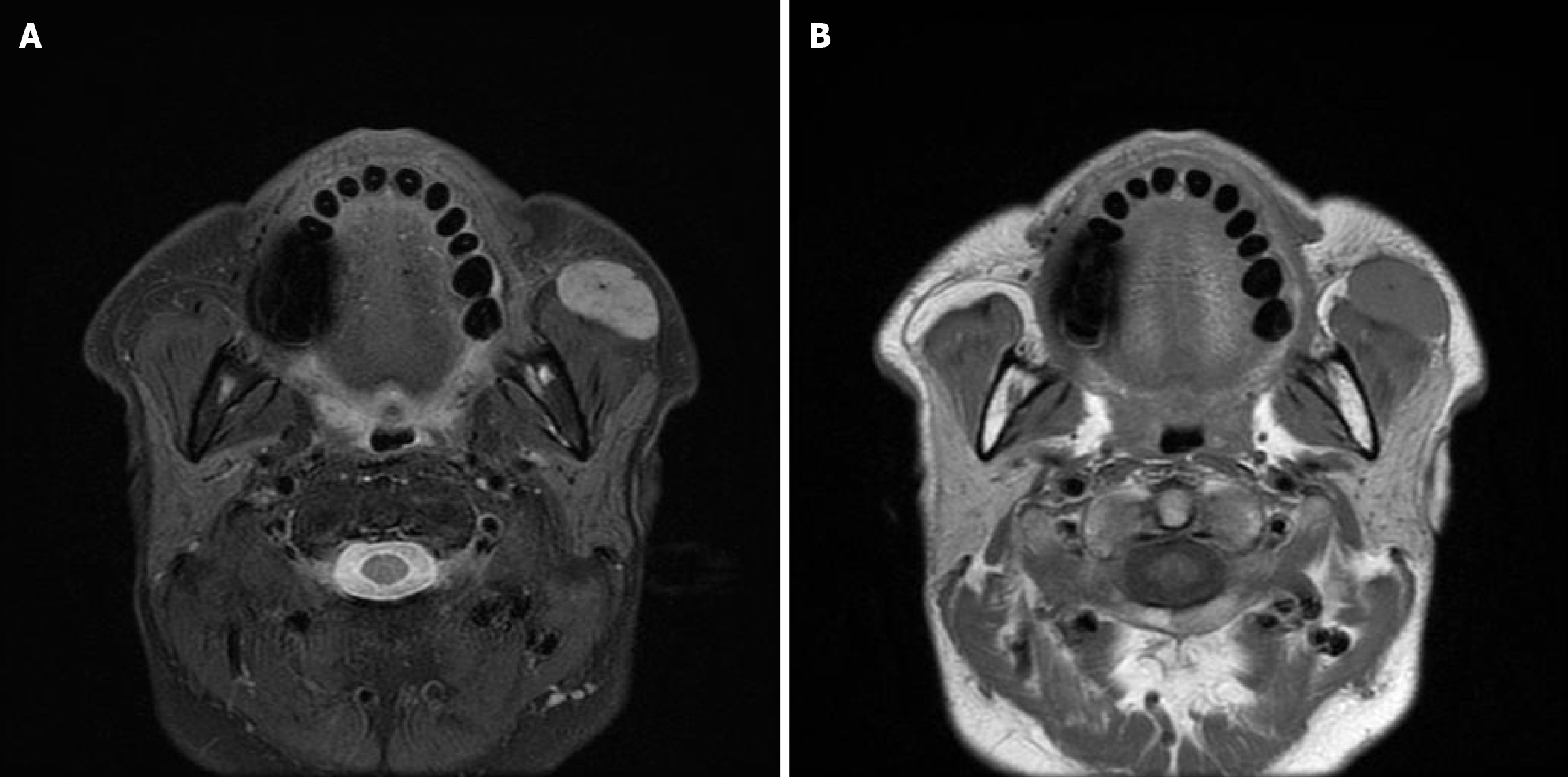
Plain computed tomography (CT) revealed a well-defined soft tissue shadow anterior to the left masseter muscle (Figure 1). Magnetic resonance imaging (MRI) indicated a well-defined mass with moderate T1 and T2 signal intensity, measuring 30 mm, in the anterior portion of the left masseter muscle. The mass had no continuity with the parotid glands (Figure 2).
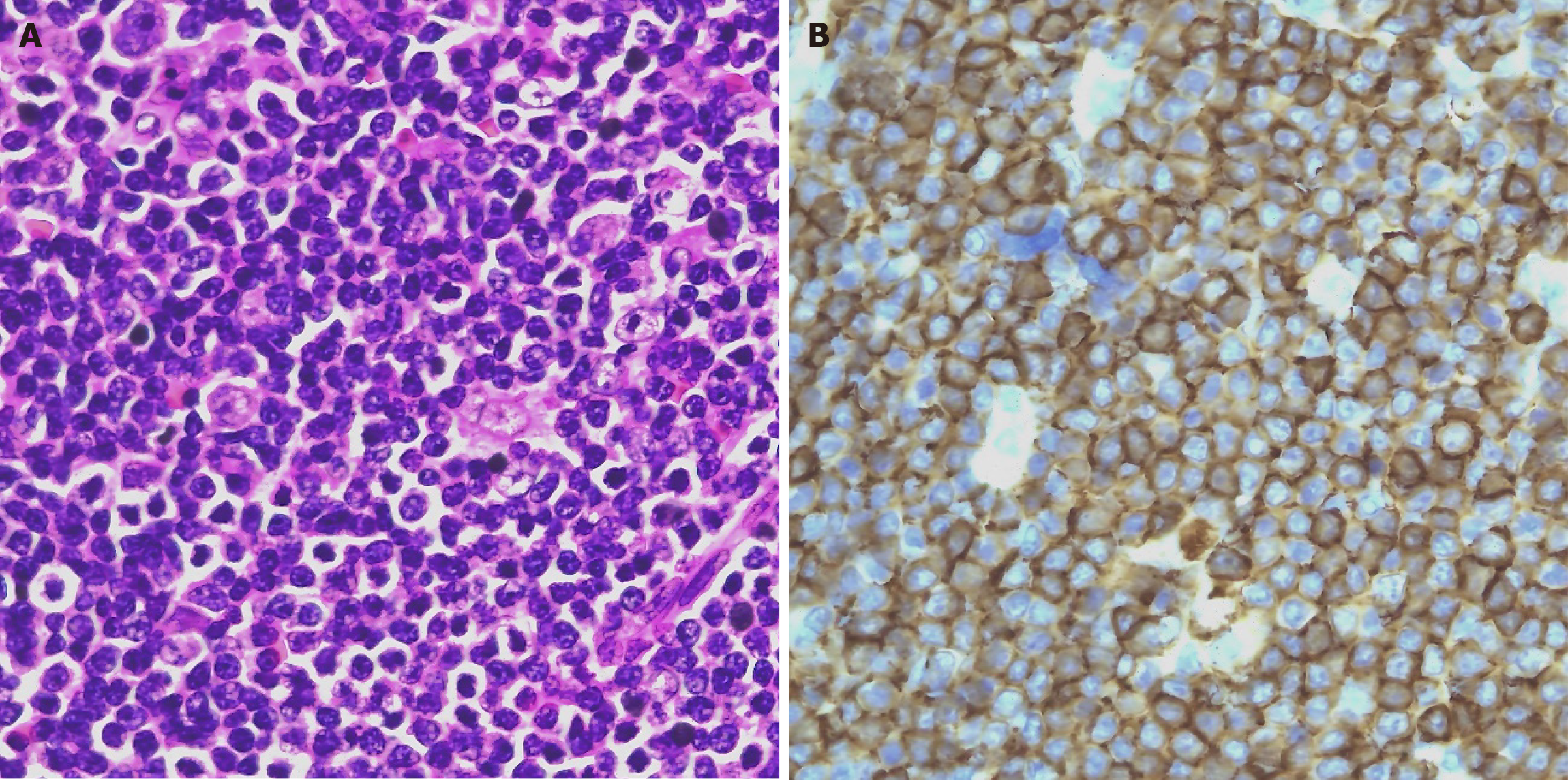
Histopathological examination revealed a proliferation of small- to medium-sized lymphocytes with indistinct follicular structures. Immunohistochemical staining indicated the following profile: CD3-negative, CD5-negative, CD10-negative, CD20-positive, BCL2-positive, BCL6-negative, MUM1-negative, CCND1-negative, and EBER-negative. Plasma cells were positive for κ and negative for λ light chains, indicating monoclonal B-cell proliferation. Based on the site of origin and plasma cell differentiation, a diagnosis of MALT lymphoma was confirmed (Figure 3).
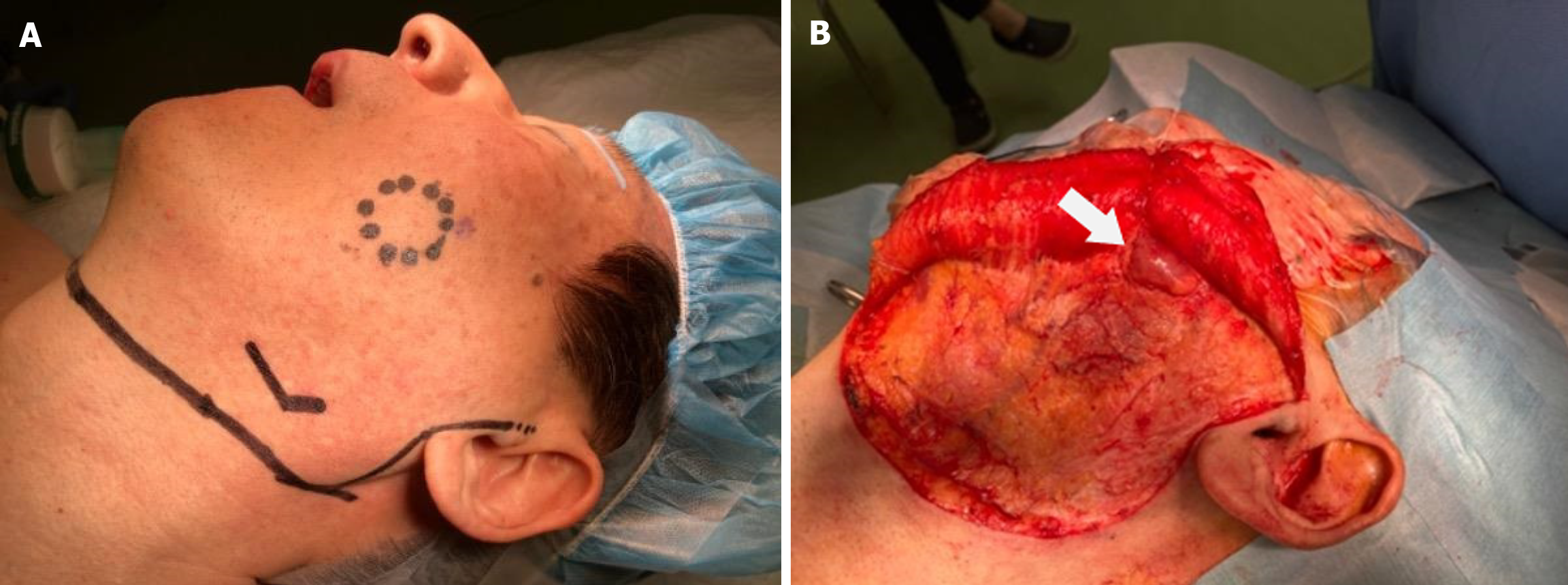
Surgical exploration was performed, involving an S-shaped incision from the preauricular area to the neck and elevation of the flap until the cheek tumor was exposed. The tumor was located anterior to the parotid gland but did not extend into it. The tumor was removed as one lump (Figure 4).
Postoperatively, positron-emission tomography (PET)/CT revealed no abnormal uptake throughout the body, confirming the diagnosis of MALT lymphoma originating from the buccal fat pad. One year after the surgery, there was no evidence of recurrence on MRI.
Unlike primary lymphoid organs, such as the lymph nodes, tonsils, and spleen, lymphoid cells undergo antigen-dependent changes. They are primarily found in areas exposed to the external environment, such as the digestive tract, salivary glands, respiratory tract, thymus, and urogenital tract. MALT lymphomas originating from these sites were first proposed by Isaacson et al[3] in 1983. They can occur at various sites, with the stomach being the most common (50%), followed by the salivary glands (13%), skin (6%), and conjunctiva (5%)[4]. In gastric MALT lymphoma, chronic gastritis caused by H. pylori infection is considered the etiology, and eradication therapy is effective[5]. Similarly, salivary gland and thyroid MALT lymphomas are often associated with Sjögren's syndrome and Hashimoto's thyroiditis, respectively[6]. In contrast, hepatic MALT lymphoma is associated with chronic hepatitis caused by HCV or hepatitis B virus[7].
In the present case, MALT lymphoma occurred in the buccal fat pad without any evidence of chronic inflammation of the oropharynx, thyroid gland, or salivary glands. Its occurrence at a site where MALT is not normally present, without any obvious precursor lesions, suggests an exceptionally rare case where the tumor originated from the buccal fat pad. Although cutaneous cases have been reported in the cheek region, the location of the tumor in this case, deeper than the facial subcutaneous tissue and facial muscles, without continuity with the skin, makes a primary cutaneous origin unlikely. Suzuki et al[8] reported a case originating in the subcutaneous tissue of the right cheek, which was considered secondary to the accessory parotid gland. The anatomical position of the tumor in the present case differed from the typical location of the accessory parotid gland. The possibility of development from ectopic salivary gland tissue was considered but could not be histologically confirmed.
H. pylori infection was confirmed in the present case. Although such an infection plays a role in the development of gastric MALT lymphoma, extragastric MALT lymphoma associated with H. pylori infection or concurrent gastric cancer is uncommon in the literature[9-11]. Furthermore, recent studies have revealed that H. pylori infection in the stomach can also lead to colonization in the oral cavity[12,13]. In our case, the patient had untreated dental caries and had not visited a dentist for over a decade. A previous study has linked H. pylori infection in the oral cavity to dental caries[12], suggesting that an unsanitary oral environment leads to chronic inflammation in the buccal lymph nodes.
The patient had an untreated HCV infection. HCV infection occurs in 35% of patients with extragastric MALT lymphoma[14]. Particularly, HCV infection is detected in 47% of cases of salivary gland-origin MALT lymphoma, which is higher than that in other extragastric MALT lymphomas, including those in the skin, orbit, lung, breast, liver, small intestine, and large intestine[14]. Although the primary site in this case was not the salivary gland, lymph nodes adjacent to the submandibular gland were the primary sites, suggesting a potential association with HCV infection.
H. pylori infection induces chronic gastritis via the activation of inflammatory pathways, leading to the proliferation of B cells and formation of lymphoid follicles. Additionally, HCV infection has been implicated in the pathogenesis of MALT lymphoma via direct and indirect mechanisms. Directly, HCV may infect B cells, leading to their transformation and clonal expansion[15]. Indirectly, the chronic immune response to HCV infection may result in continuous B cell activation, creating an environment conducive to the development of lymphoma. Considering the association of H. pylori and HCV with MALT lymphoma, it is plausible that co-infection or the sequential acquisition of these infections could increase the risk of developing MALT lymphoma.
Radiotherapy or surgical excision may be considered in patients with localized extragastric MALT lymphoma. When diagnosed postoperatively, determining whether the tumor has been completely excised or whether any residual disease remains is crucial. If residual disease is present, radiotherapy should be considered[16]. In the present case, because MALT lymphoma was diagnosed after surgical excision, postoperative PET/CT and MRI were performed to confirm the absence of residual lesions, and the patient was placed on surveillance. Although the therapeutic effect of H. pylori eradication therapy in extragastric MALT lymphoma is still under investigation, this approach has reportedly been efficacious in the early treatment of thyroid and duodenal MALT lymphomas[17,18]. Considering the implication of H. pylori infection in developing MALT lymphoma, even at extragastric sites, H. pylori eradication therapy was administered postoperatively.
Further research on such cases is necessary to determine the most appropriate treatment approach. This case illustrates that consideration of MALT lymphoma is essential in the differential diagnosis of frontal cheek masses.
Our study supports the association between a history of H. pylori infection with the dev elopement of not only gastric MALT lymphomas, as extensively reported, but also extragastric MALT lymphomas. We recommend eradication therapy as an effective approach for treating MALT lymphomas. As this case study reports a rare incidence of extragastric MALT lymphoma development in the buccal fat pad, reporting and following up on the development mass in MALT-associated organs in cases with a history of H. pylori infection will be crucial.
The authors are sincerely grateful to all participants of the study.
| 1. | Anacak Y, Miller RC, Constantinou N, Mamusa AM, Epelbaum R, Li Y, Calduch AL, Kowalczyk A, Weber DC, Kadish SP, Bese N, Poortmans P, Kamer S, Ozsahin M. Primary mucosa-associated lymphoid tissue lymphoma of the salivary glands: a multicenter Rare Cancer Network study. Int J Radiat Oncol Biol Phys. 2012;82:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Oh SY, Kim WS, Kim JS, Kim SJ, Lee S, Lee DH, Kang HJ, Song MK, Kim HJ, Kwon JH, Kwak JY, Park BB, Do YR, Jeong SH, Suh C. Primary thyroid marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type: clinical manifestation and outcome of a rare disease - consortium for improving survival of lymphoma study. Acta Haematol. 2012;127:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 4. | Zucca E, Conconi A, Pedrinis E, Cortelazzo S, Motta T, Gospodarowicz MK, Patterson BJ, Ferreri AJ, Ponzoni M, Devizzi L, Giardini R, Pinotti G, Capella C, Zinzani PL, Pileri S, López-Guillermo A, Campo E, Ambrosetti A, Baldini L, Cavalli F; International Extranodal Lymphoma Study Group. Nongastric marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Blood. 2003;101:2489-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 360] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 5. | Nakamura S, Matsumoto T, Ye H, Nakamura S, Suekane H, Matsumoto H, Yao T, Tsuneyoshi M, Du MQ, Iida M. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: a clinicopathologic and molecular study with reference to antibiotic treatment. Cancer. 2006;107:2770-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Thieblemont C, Bertoni F, Copie-Bergman C, Ferreri AJ, Ponzoni M. Chronic inflammation and extra-nodal marginal-zone lymphomas of MALT-type. Semin Cancer Biol. 2014;24:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Liu X, Cao X, Pang Y, Min F. Primary hepatic mucosa-associated lymphoid tissue lymphoma with HP and previous HBV infection: A case report and literature review. J Infect Chemother. 2022;28:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Suzuki T, Matsuzuka T, Kunii M, Saijoh S, Yanagawa A, Nakamura K, Omori K. A case report of malignant lymphoma in the accessory parotid gland. J J pn Soc Head Neck Surg. 2016;25:469-473. [DOI] [Full Text] |
| 9. | Doi H, Horiike N, Hiraoka A, Koizumi Y, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y, Ninomiya T, Ishimaru Y, Miyagawa M, Takamura K, Kawasaki H, Kozuka T, Maeda T, Yoshino T. Primary hepatic marginal zone B cell lymphoma of mucosa-associated lymphoid tissue type: case report and review of the literature. Int J Hematol. 2008;88:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Murakami J, Fukushima N, Ueno H, Saito T, Watanabe T, Tanosaki R, Kobayashi Y, Matsuno Y, Tobinai K. Primary hepatic low-grade B-cell lymphoma of the mucosa-associated lymphoid tissue type: a case report and review of the literature. Int J Hematol. 2002;75:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Takada K, Yoshihara N, Komiyama E, Ikeda S. A case of primary cutaneous extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue. J JOCD. 2021;38:624-628. [DOI] [Full Text] |
| 12. | Iwai K, Azuma T, Yonenaga T, Watanabe K, Obora A, Deguchi F, Kojima T, Tomofuji T. Association between dental caries and Helicobacter pylori infection in Japanese adults: A cross-sectional study. PLoS One. 2022;17:e0271459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 13. | Abdul NS, Khalid Alkhelaiwi A, Awadh Alenazi A, Fehaid Alrashidi R, Ghaleb Salma R. The Association of Helicobacter pylori in the Oral Cavity With Dental Caries in Patients With and Without Gastric Infection: A Systematic Review. Cureus. 2023;15:e38398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 14. | Arcaini L, Burcheri S, Rossi A, Paulli M, Bruno R, Passamonti F, Brusamolino E, Molteni A, Pulsoni A, Cox MC, Orsucci L, Fabbri A, Frezzato M, Voso MT, Zaja F, Montanari F, Merli M, Pascutto C, Morra E, Cortelazzo S, Lazzarino M. Prevalence of HCV infection in nongastric marginal zone B-cell lymphoma of MALT. Ann Oncol. 2007;18:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 860] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 16. | Ghesquières H, Berger F, Felman P, Callet-Bauchu E, Bryon PA, Traverse-Glehen A, Thieblemont C, Baseggio L, Michallet AS, Coiffier B, Salles G. Clinicopathologic characteristics and outcome of diffuse large B-cell lymphomas presenting with an associated low-grade component at diagnosis. J Clin Oncol. 2006;24:5234-5241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Nakamura S, Matsumoto T, Nakamura S, Kusano Y, Esaki M, Kurahara K, Fukuda T. Duodenal mucosa-associated lymphoid tissue lymphoma treated by eradication of Helicobacter pylori: report of 2 cases including EUS findings. Gastrointest Endosc. 2001;54:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Arima N, Tsudo M. Extragastric mucosa-associated lymphoid tissue lymphoma showing the regression by Helicobacter pylori eradication therapy. Br J Haematol. 2003;120:790-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/