Published online Sep 16, 2024. doi: 10.12998/wjcc.v12.i26.5854
Revised: May 12, 2024
Accepted: May 23, 2024
Published online: September 16, 2024
Processing time: 109 Days and 2.7 Hours
In this editorial, we commented on the article by Akers et al published in the recent issue of the World Journal of Clinical Cases. We focused specifically on the role of the transcription factor paired box protein 8 (PAX8) belonging to the family PAX in the carcinogenesis of a gynecologic tumor, endocervical adenocarcinoma, arising from the tissue of mesonephric origin, and the potential diagnostic value for the same type of neoplasms. The global vaccination program of human papillomavirus (HPV) has dramatically reduced the incidence of cervical cancer, including cases of adenocarcinoma. The type of adenoid epithelial origin has a lower frequency of HPV detection but tends to be more aggressive and fatal. Cases of endocervical adenocarcinoma occurring in females of meno
Core Tip: Paired box proteins (PAXs) are a family of transcription factors that play an important role in the embryogenesis of different tissues through the regulation of gene expression. PAX 2, 5, and 8 are expressed in the sites of mesonephric tissues, and their deregulation contributes to the genesis of urogenital tumors such as cervical and ovarian cancers. Immunohistochemical staining and in situ hybridization tests revealed that the specimens of endocervical adenocarcinoma were positive for the transcription factor PAX8 and human papillomavirus. It is proposed that combined with mucin of glandular tumor, PAX8 can be used as a diagnostic marker for cervical adenocarcinoma.
- Citation: Zhou JH, Zhang XN. Paired box proteins as diagnostic biomarkers for endocervical adenocarcinoma. World J Clin Cases 2024; 12(26): 5854-5858
- URL: https://www.wjgnet.com/2307-8960/full/v12/i26/5854.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i26.5854
In 2023, a report by Akers et al[1] published in the World Journal of Clinical Cases described a case of distant metastasis of cervical adenocarcinoma with alterations in several biomarkers including tumor suppressor p16, estrogen receptor, and transcription factor paired box protein 8 (PAX8). The case was also positive for human papillomavirus (HPV)[1]. We recently reported 2 cases of gastric-type endocervical adenocarcinoma; one of the patients was immunohistochemically positive for PAX8[2]. Because PAX protein expression related to the tissue origin of lesions during embryogenesis and published data on the involvement of PAX proteins in the cancers of the ovary and uterine cervix, we aimed to dissect the possible significance of using PAX proteins, especially PAX8, as diagnostic and therapeutic targets of cervical adenocarcinoma.
Gynecologic tumors such as ovarian and cervical cancers arise from the tissues of mesonephric origin during embryogenesis. The transcription factors regulate embryonic development and play a role in organogenesis. The aberrant expression is responsible for the genesis of malignancies. Some members of the PAX family are implicated in these processes.
The PAX family comprises nine members, namely PAX1-9. They are divided into four groups based on their distinct molecular motif composition. The transcription factors are preferentially expressed in different anatomic sites during human embryogenesis to regulate gene expression; in relation to this, the PAX protein family molecules also contribute to carcinogenesis in specific parts of the body.
The members of the PAX family preferentially contribute to the regulation of gene expression of different organs; early expression of PAX2 is essential for the formation of the mesonephric duct[3]. It also compensates for the reduced expression of PAX8 throughout kidney development; the expression of PAX8 is activated in kidney and thyroid tissue during their organogenesis process[4]. In addition, PAX1 and PAX9 are implicated in the development of the skeleton. PAX2, 3, 5, 6, 7, and 8 are implicated in the development of the central nervous system[5], and aberrant expression of these PAX family members contributes to malignancies of the kidneys, thyroid gland, ovary, fallopian tube, and uterine cervix[6-8]. Table 1 summarizes the biological classification and activities of PAX family proteins including their role in tumorigenicity.
| Group in PAX family | Member in PAX family | Expression site during embryogenesis | Involvement in tumorigenicity |
| I | PAX1 | Skeleton, thymus, pharyngeal pouch | |
| PAX9 | Skeleton, teeth, thymus | ||
| PAX2 | Kidney, CNS | Bladder and renal cancers | |
| II | PAX5 | B cells, CNS | Lymphomas |
| PAX8 | Kidneys, thyroid gland, CNS | Thyroid, ovarian, and cervical cancers | |
| III | PAX3 | Neural crest, CNS, muscle | Melanoma, rhabdomyosarcoma |
| PAX7 | Same as above | Rhabdomyosarcoma | |
| IV | PAX4 | Pancreas, gut | |
| PAX5 | Pancreas, gut, and eyes | Gastrointestinal cancers |
Two major types of tumors, squamous carcinoma and adenocarcinoma[9,10], arise in the epithelial lining of the uterine cervix. Cervical cancer predominantly of squamous epithelial origin is associated with infection of high-risk HPV like types 16 and 18. The high incidence of HPV infection in cell cervical carcinoma offers an opportunity for global eradication through HPV vaccination[11]. Cervical adenocarcinomas, however, form a spectrum from well-differentiated adenoma malignum (a mucinous variant of minimal deviation adenocarcinoma) to poorly differentiated, invasive gastric-type adenocarcinoma[12,13]. Endocervical carcinoma, specifically gastric-type cervical adenocarcinoma, has a low incidence of HPV infection.
Generally, adenocarcinoma of the cervix has a poor prognosis. Regarding distant metastasis, cervical cancer typically metastasizes to local structures through direct invasion, hematogenous dissemination, or dissemination through the lymphatic system[14,15]. It was reported that breast metastases of cervical cancer occurred in the case of high-grade adenocarcinoma with mucinous features that we discussed in our case study[2].
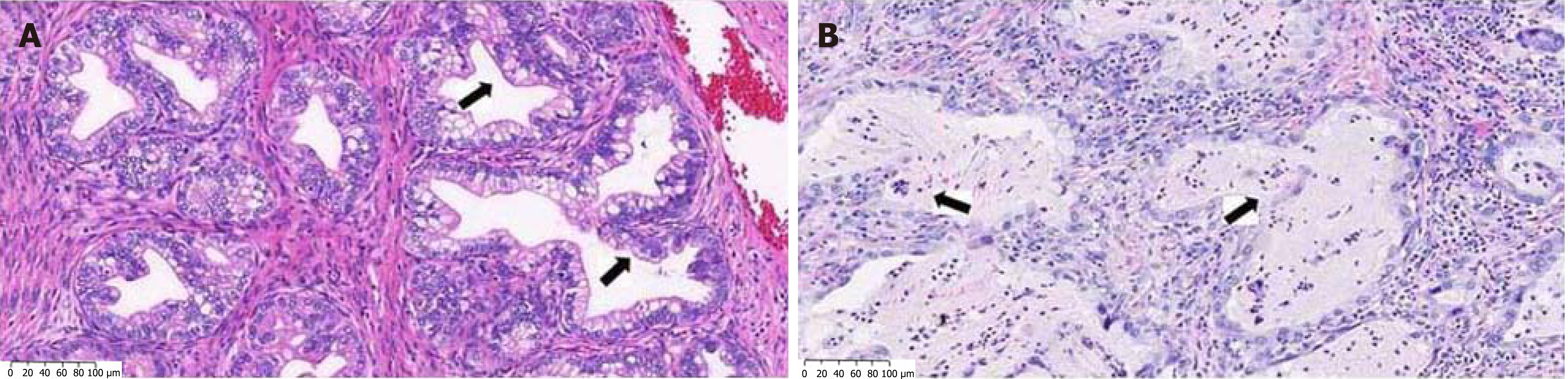
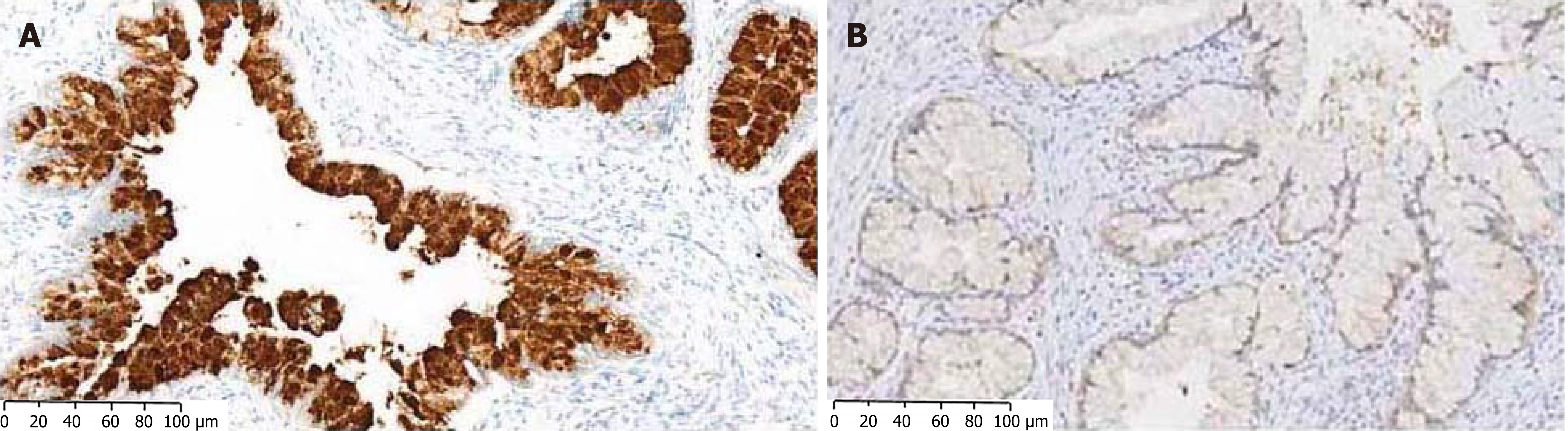
In situ hybridization test revealed that the case of cervical adenocarcinoma was positive for HPV and the transcription factor PAX8. Similarly, we reported 2 cases of gastric-type endocervical adenocarcinoma with one case being positive for PAX8[2]. Our cases presented as lesions of glandular appearance, and microscopically the tumor cells presented as cells of adenoid epithelium (Figure 1). In addition to PAX8 they were positive for a mucin protein, MUC 5AC (Figure 2), and negative for HPV. Furthermore, in contrast to the present report, our cases were negative for both estrogen receptor and the tumor suppressor p16.
Both PAX2 and PAX8 play an important role in the genesis of the anatomic structure of the ovary, uterus cervix, and uterus corpus during embryonic development. It has been shown that PAX8 is expressed in tumors of the thyroid gland, kidneys, and Müllerian tube, such as malignancies of ovary and endometrium, and its incidence of detection is high in ovarian cancer[16-20]. The possible involvement of PAX8 has been revealed in the genesis of cervical cancer[21,22].
PAX 8 has been recognized as a DNA binding transcription factor. A binding site for it has been identified in the promoter region in Wilms’ tumor gene (WT1), coding for a product for the development of kidney and gonadal glands. The data suggested that part of its role in kidney development was as a modulator of WT1 expression in the kidney[23]. It remains to be tested whether tumors of the cervix and ovary have altered expression of WT1. While high PAX8 level is present in thyroid cancers, it has been reported that the coding region of PAX8 is mutated in patients of thyroid dysgenesis, and it is responsible for elevated thyroid-stimulating hormone levels in congenital hypothyroidism[24]. Given this, the molecules of particular members of the PAX family are proposed to be indicators or biomarkers for the diagnosis of cervical adenocarcinoma or cancer of the cervix in general.
The expression of PAX8 has been noted in tumors of thyroid, renal, and Müllerian tube origins, like neoplasms originating from ovary and endometrium. Meanwhile, however, PAX8 was reported to be expressed in all 20 cases (100%) of benign endocervical epithelium specimens. Additionally, it was expressed in 97%-100% of adenocarcinoma in situ and in 0%-87% of endocervical adenocarcinoma specimens[25,26]. These findings suggest that the incidence of PAX proteins is low with the progression of the tumor and that the aberrant expression profile of tissue antigen due to the differentiation status in malignancy may account for negative PAX8 expression in 1 of our reported cases. The study of numerous clinical samples is needed to clarify this issue.
Multiple drivers of cancer involve the genesis of cervical cancer. We immunohistochemically detected the presence of mutant p53 in our reports. However, the oncogenicity-related changes could be highly heterogeneous; our study revealed the absence of mutant k-ras as assayed using a PCR-based amplification refractory mutant system[2]. Our early work showed that in HPV-positive cervical cancer cases, oncogene c-myc was overexpressed due to exon alteration[27] and the change in c-myc, which cooperates with mutant k-ras, results in malignancy, as reported in another study[28]. The gain-of-function mutations of oncogenes together with the mutation of tumor suppressor genes are common changes in malignancies but less specific to particular neoplasms of certain histologic origins. It is therefore proposed that molecules implicated in organogenesis related to tumors can be used as therapeutic targets for adenocarcinomas. The PAX family proteins may fulfill such demands[29,30].
| 1. | Akers A, Read S, Feldman J, Gooden C, English DP. Diagnostic challenges and individualized treatment of cervical adenocarcinoma metastases to the breast: A case report. World J Clin Cases. 2024;12:412-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 2. | Zhou J, Zhang X, Mao W, Zhu Y, Yan L, Jiang J, Zhang M. Pathological features of gastric-type endocervical adenocarcinoma: A report of two cases. Oncol Lett. 2024;27:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 3. | Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature. 1993;362:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 198] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 420] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 221] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Daniel L, Lechevallier E, Giorgi R, Sichez H, Zattara-Cannoni H, Figarella-Branger D, Coulange C. Pax-2 expression in adult renal tumors. Hum Pathol. 2001;32:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Heidarpour M, Tavanafar Z. Diagnostic utility of PAX8 in differentiation of mullerian from non-mullerian tumors. Adv Biomed Res. 2014;3:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Laury AR, Hornick JL, Perets R, Krane JF, Corson J, Drapkin R, Hirsch MS. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol. 2010;34:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Adegoke O, Kulasingam S, Virnig B. Cervical cancer trends in the United States: a 35-year population-based analysis. J Womens Health (Larchmt). 2012;21:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Guo F, Cofie LE, Berenson AB. Cervical Cancer Incidence in Young U.S. Females After Human Papillomavirus Vaccine Introduction. Am J Prev Med. 2018;55:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Bewley S. HPV vaccination and cervical cancer screening. Lancet. 2022;399:1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Cree IA, White VA, Indave BI, Lokuhetty D. Revising the WHO classification: female genital tract tumours. Histopathology. 2020;76:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 13. | Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, Nishimura R. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 14. | Kumar L, Pokharel YH, Dawar R, Thulkar S. Cervical cancer metastatic to the breast: a case report and review of the literature. Clin Oncol (R Coll Radiol). 1999;11:414-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Cholmondeley K, Callan L, Sangle N, D'Souza D. Metastatic cervical adenocarcinoma to the breast: A case report and literature review. Gynecol Oncol Rep. 2019;28:33-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Maulbecker CC, Gruss P. The oncogenic potential of Pax genes. EMBO J. 1993;12:2361-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Corona RI, Seo JH, Lin X, Hazelett DJ, Reddy J, Fonseca MAS, Abassi F, Lin YG, Mhawech-Fauceglia PY, Shah SP, Huntsman DG, Gusev A, Karlan BY, Berman BP, Freedman ML, Gayther SA, Lawrenson K. Non-coding somatic mutations converge on the PAX8 pathway in ovarian cancer. Nat Commun. 2020;11:2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Fu DJ, De Micheli AJ, Bidarimath M, Ellenson LH, Cosgrove BD, Flesken-Nikitin A, Nikitin AY. Cells expressing PAX8 are the main source of homeostatic regeneration of adult mouse endometrial epithelium and give rise to serous endometrial carcinoma. Dis Model Mech. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Di Palma T, Lucci V, de Cristofaro T, Filippone MG, Zannini M. A role for PAX8 in the tumorigenic phenotype of ovarian cancer cells. BMC Cancer. 2014;14:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 20. | Ramachandran D, Wang Y, Schürmann P, Hülse F, Mao Q, Jentschke M, Böhmer G, Strauß HG, Hirchenhain C, Schmidmayr M, Müller F, Runnebaum I, Hein A, Koch M, Ruebner M, Beckmann MW, Fasching PA, Luyten A, Dürst M, Hillemanns P, Dörk T. Association of genomic variants at PAX8 and PBX2 with cervical cancer risk. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Wu Y, Li H, Wang H, Zhang F, Cao H, Xu S. MSK2 promotes proliferation and tumor formation in squamous cervical cancer via PAX8/RB-E2F1/cyclin A2 axis. J Cell Biochem. 2019;120:11432-11440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Li CG, Nyman JE, Braithwaite AW, Eccles MR. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene. 2011;30:4824-4834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 23. | Fraizer GC, Shimamura R, Zhang X, Saunders GF. PAX 8 regulates human WT1 transcription through a novel DNA binding site. J Biol Chem. 1997;272:30678-30687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, Souabni A, Baserga M, Tassi V, Pinchera A, Fenzi G, Grüters A, Busslinger M, Di Lauro R. PAX8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 304] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Shukla A, Thomas D, Roh MH. PAX8 and PAX2 expression in endocervical adenocarcinoma in situ and high-grade squamous dysplasia. Int J Gynecol Pathol. 2013;32:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Goyal A, Yang B. Differential patterns of PAX8, p16, and ER immunostains in mesonephric lesions and adenocarcinomas of the cervix. Int J Gynecol Pathol. 2014;33:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Huang SL, Liu ZQ, Zhang XN, Li MJ. Human papillomavirus, human cytomegalovirus and oncogene C-myc in cervical carcinoma and cervicitis. Chin Med J (Engl). 1993;106:208-210. [PubMed] |
| 28. | Stenzel A, Semczuk A, Rózyńskal K, Jakowicki J, Wojcierowski J. "Low-risk" and "high-risk" HPV-infection and K-ras gene point mutations in human cervical cancer: a study of 31 cases. Pathol Res Pract. 2001;197:597-603. [PubMed] |
| 29. | Yemelyanova A, Gown AM, Wu LS, Holmes BJ, Ronnett BM, Vang R. PAX8 expression in uterine adenocarcinomas and mesonephric proliferations. Int J Gynecol Pathol. 2014;33:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Danialan R, Assaad M, Burghardt J, Newcomb P, Cartun RW, Mandavilli S. The utility of PAX8 and IMP3 immunohistochemical stains in the differential diagnosis of benign, premalignant, and malignant endocervical glandular lesions. Gynecol Oncol. 2013;130:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/