Published online Sep 6, 2024. doi: 10.12998/wjcc.v12.i25.5784
Revised: May 22, 2024
Accepted: June 24, 2024
Published online: September 6, 2024
Processing time: 105 Days and 15.5 Hours
Sinonasal teratocarcinosarcoma (SNTCS) is a rare and highly invasive neoplasm originating from the nasal cavity and sinuses. Typically, it exhibits an invasive behavior towards adjacent structures; however, in exceptional instances, it may infiltrate the intracranial compartment. Due to the tumor's rarity and lack of distinctive features on computed tomography (CT) and magnetic resonance imaging (MRI) images, SNTCS is often misdiagnosed.
In this study, we present a case of SNTCS in a 56-year-old patient who exhibited unexplained cognitive impairment before admission. CT and MRI scans revealed the presence of a mass in the right nasal cavity, with lesions extending to the right ethmoid sinus and right frontal region. Subsequently, the patient underwent pathological examination for confirmation and received surgical intervention to excise the tumor. The future advancement in our understanding of this disease will significantly contribute to the precise diagnosis and treatment of SNTCS.
SNTCS is an exceptionally rare malignant tumor that originates from the nasal cavity and paranasal sinuses, presenting a diagnostic challenge due to its non-specific imaging findings. MRI accurately delineates the location, morphological characteristics, size, internal structure, extent of surrounding involvement, and metabolic information of the lesion. These aspects play a pivotal role in the precise localization and qualitative assessment of SNTCS. Nevertheless, a definitive diagnosis still requires a pathological biopsy.
Core Tip: We present a case report of Sinonasal teratocarcinosarcoma involving the nasal cavity, unilateral paranasal sinuses, and extending into the intracranial region in pathology. Our findings underscore the crucial role of integrating radiology results, clinical manifestations, and pathological biopsy outcomes for accurate diagnosis by clinicians.
- Citation: Fu LY, Yang MY, Ye PY, Wang ZC, Chen CJ, Li H, Xu SW. Sinonasal teratocarcinosarcoma involving the nasal cavity, unilateral paranasal sinuses, and intracranial invasion: A case report. World J Clin Cases 2024; 12(25): 5784-5790
- URL: https://www.wjgnet.com/2307-8960/full/v12/i25/5784.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i25.5784
Sinonasal teratocarcinosarcoma (SNTCS) is a rare malignant tumor characterized by the coexistence of both teratoma and carcinosarcoma features within its composition, primarily affecting the nasal cavity and sinuses[1,2]. This disease mainly manifests in the nasal cavity and sinuses, with occasional reports of tumors occurring in other anatomical sites such as the nasopharynx, oral cavity, and even intracranially[3-7]. Clinical manifestations of tumors include: (1) Nasal obstruction and hyposmia resulting from local tumor growth; (2) Epistaxis, fetid odor in the nasal cavity, and increased nasal secretions due to rapid tumor growth leading to necrosis; and (3) Invasion of adjacent tissues by the tumor can result in facial edema, lacrimation, proptosis, visual impairment, headaches, alterations in consciousness and behavior. Physical examination may reveal a nasal mass with an ill-defined morphology that cannot be distinguished from polyps or papillomas. The disease typically follows a relatively short course, lasting 2-10 weeks but occasionally extending up to 2 years[3,8-11]. Shanmugaratnam et al[12] and Heffner et al[13] respectively conducted the description and final nomenclature of SNTCS in 1983 and 1984[12,13]. According to reports, patients with SNTCS have an average survival duration of 1.7 years, accompanied by a three-year mortality rate of 60%[13,14]. Currently, to the best of our knowledge, only approximately 150 cases have been reported, with intracranial involvement observed in a limited subset of these cases. In this study, we present a rare case involving the ethmoidal sinus and intracranial region in SNTCS, elucidating the computed tomography (CT) and magnetic resonance imaging (MRI) findings alongside histopathological analysis. We aim to contribute valuable insights for future clinical diagnosis and treatment strategies.
A 56-year-old male presented with an acute and unexplained impairment of consciousness four hours ago, which necessitated a referral to the emergency department of our hospital.
The primary manifestations included unresponsiveness to stimuli and failure to awaken upon stimulation, accompanied by generalized limb and head tremors, tightly clenched teeth, and frothing at the mouth. These symptoms lasted for approximately 1-2 minutes before spontaneously resolving themselves. After regaining consciousness, the patient reported having a headache and feeling uncomfortable. Subsequently, he was transported by ambulance to the 900th Hospital of the Joint Logistics Support Force for a comprehensive assessment and medical treatment.
The patient had no prior medical history. Personal and family histories revealed no relevant information. During the examination of the nervous system, no discernible abnormalities were observed in the patient.
The patient had no relevant personal or family medical history.
The patient's physical examination revealed no evident abnormalities.
Macroexamination revealed a mass of gray-white and gray-brown tissue measuring 7 cm × 5 cm × 1.5 cm, which was soft with some areas of necrosis. Under low magnification of hematoxylin-eosin stain, the examined tissues appeared as solid sheets with an abundance of blood vessels in the stroma, accompanied by large hemorrhages and necrotic areas. The primitive neuroepithelial cells were arranged in nests and surrounded by abundant fibrous septa (Figure 1A). In the tumor, there were glandular epithelial structures present along with focal nests of squamous epithelial components. The cytoplasm appeared transparent without any signs of keratinization (Figure 1B). Localized patchy glandular epithelium structures were also observed (Figure 1C). Under high magnification, tumor cells exhibited significant heterogeneity, with easily visible nuclear division figures (Figure 1D).
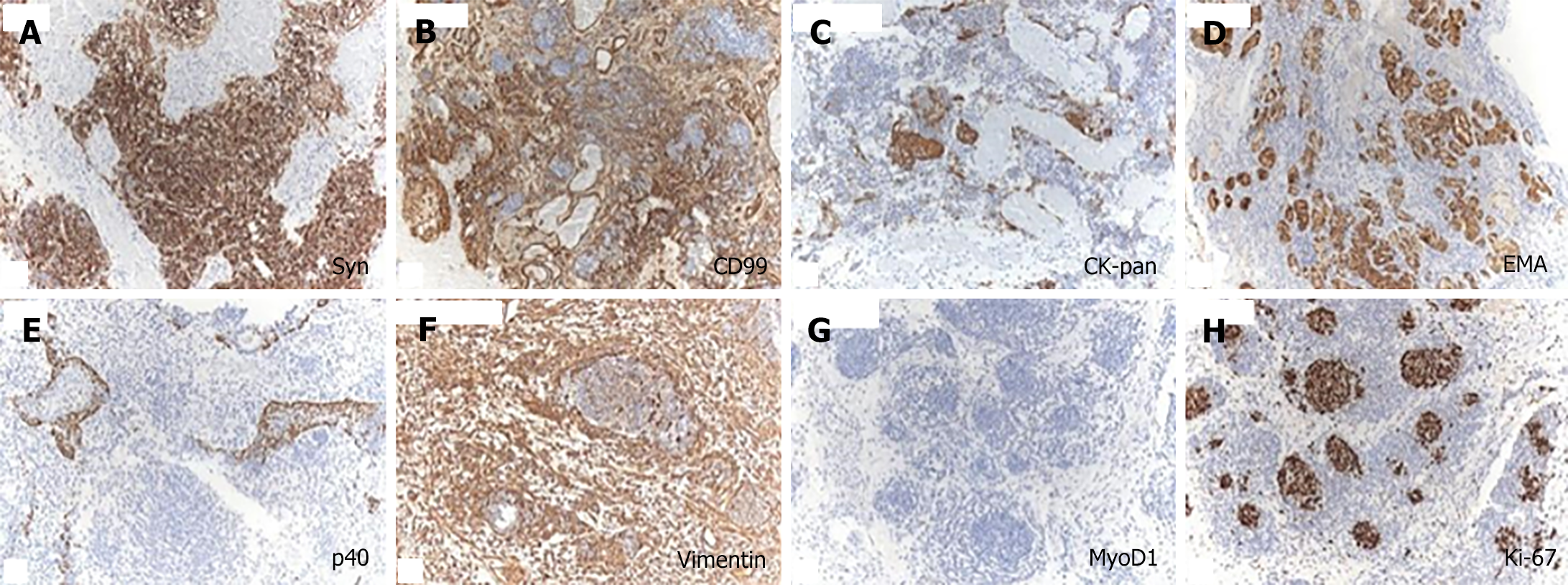
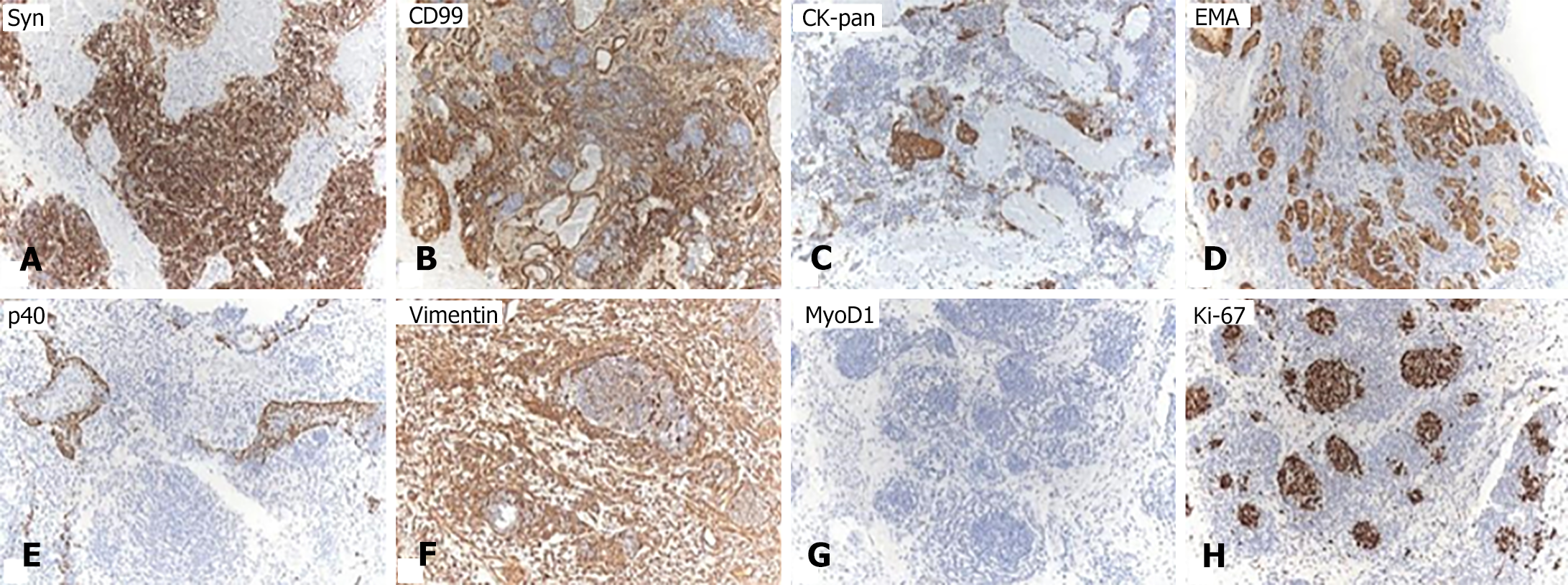
The immunohistochemical staining revealed varying degrees of positivity for neuroendocrine markers (Syn, and CD99 positive) in primitive neuroepithelial cells, while epithelial markers (CK-pan, epithelial membrane antigen, and p40 positive) also showed varying degrees of positivity (Figure 2A-E). Mesenchymal markers (Vimentin positive) were observed in the stromal component (Figure 2F). Ki-67 exhibited 40% positivity, whereas tumor cell MyoD1 was negative (Figure 2G and H). Based on the pathological and immunohistochemical findings, a diagnosis of SNTC was established.
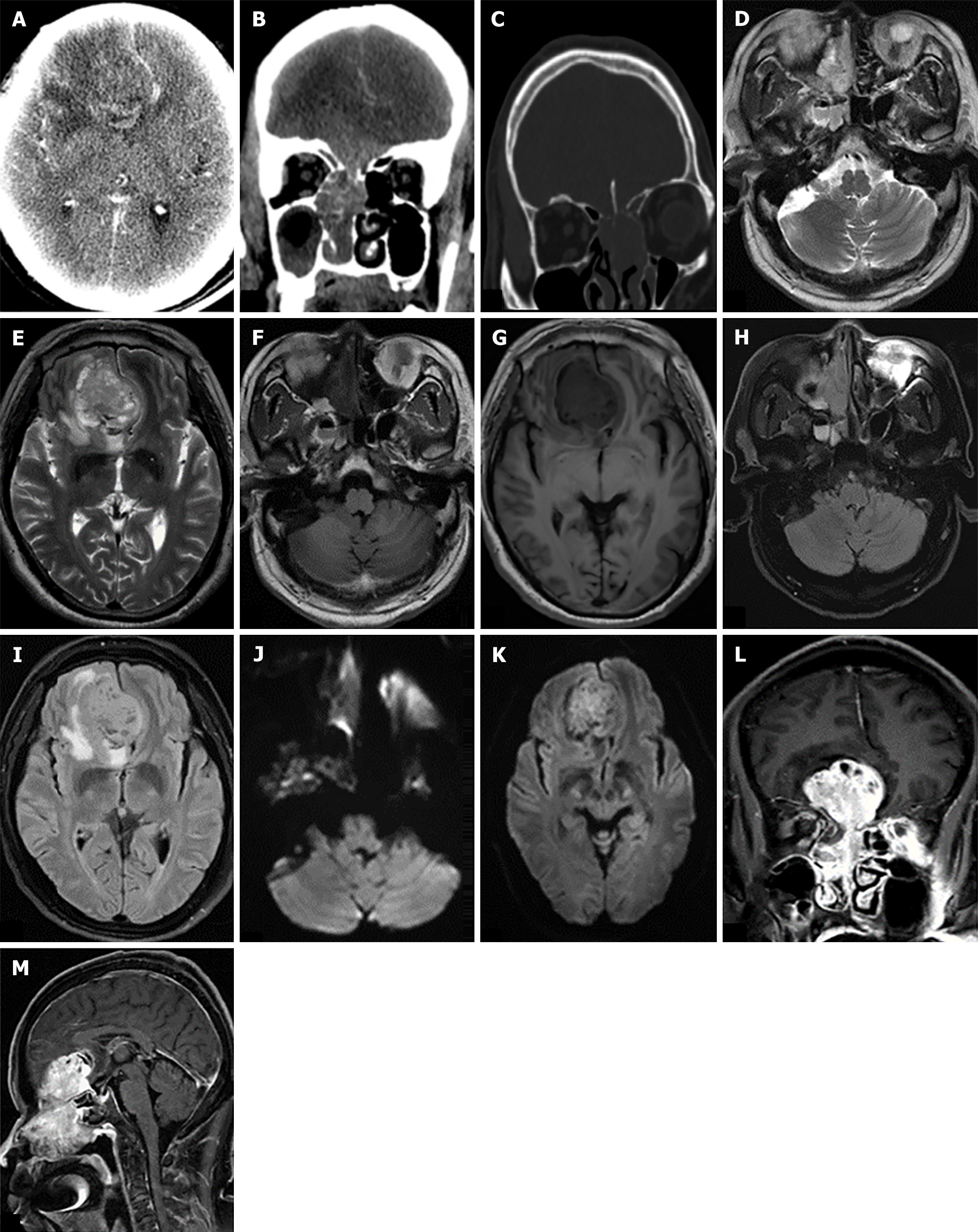
The CT scan reveals a mass-like, slightly hyperdense shadow in the right nasal cavity and frontal region with a measured CT value of approximately 35 HU on the plain scan. During the arterial phase, it measures around 43 HU, and during the venous phase, it increases to about 66 HU indicating significant enhancement. Additionally, surrounding edema formation is also observed. The septum exhibits leftward deviation while the lesion affects the right ethmoid sinus (Figure 3A and B). CT bone window shows multiple bone destruction in the bilateral skull base and ethmoid sinus wall (Figure 3C). MRI reveals a heterogeneous mass with abnormal signals in both the right nasal cavity and forehead region. T2WI demonstrates equal to slightly high signal intensity whereas T1WI shows slightly low signal intensity. FLAIR demonstrates a slightly increased signal while diffusion-weighted imaging also exhibits slight hyperintensity (Figure 3D-K). Following contrast-enhanced imaging, the lesion displays evident heterogeneous enhancement. The adjacent brain gyri demonstrate displacement due to compression from the lesion accompanied by perilesional edema and infiltration. The lesion involves the right ethmoidal sinus (Figure 3L and M).
Combined with the patient's medical history, the final diagnosis was SNTCS involving the nasal cavity, unilateral paranasal sinuses, and an extension into the intracranial region.
Although there is currently no specific therapeutic regimen available for this disease, it has been recommended that the patient undergo intervention resection of malignant tumors in the anterior skull base and reconstruction of the skull base using a combined transnasal and transcranial approach following a comprehensive evaluation. During the nasal endoscopy examination, it was observed that there was tumor infiltration and excessive secretions in the right nasal cavity, with the tumor firmly adhered to the skull base. Under general anesthesia, most of the tumor was removed through nasal endoscopy with a plasma knife. The tumor was completely separated from the top of the ethmoid sinus along its bony surface, and then cleaning of both ethmoid and sphenoid sinuses was performed. Afterward, the opening of the middle turbinate took place followed by coverage of the entire cranial base defect in an upward direction. The nasal cavity was filled with a gelatin sponge; medical gauze was compressed on top while the gelatin sponge was dilated at the bottom.
At 6 months postoperatively, the patient was still alive.
The SNTCS is an extremely rare malignant neoplasm that affects the nasal cavity and sinuses. It is characterized by its highly invasive nature and unfavorable prognosis[15]. This tumor mainly originates in the nasal cavity and often extends into the sinuses, with a preference for involvement of the ethmoid sinus and sphenoid sinus[16,17]. In clinical practice, the most common symptoms include nasal congestion and epistaxis. Additionally, patients may experience headaches, rhinorrhea, lacrimation, maxillary protrusion, changes in visual acuity, and olfactory dysfunction (penetration into the cribriform plate can cause anosmia)[18-20].
The majority of SNTCS primarily manifests in the nasal cavity and sinuses. However, only a limited number of literature reports have documented initial or progressive involvement extending to the orbit/cranium[3,17,21]. In this particular scenario where rapid tumor growth causes damage to surrounding structures, patients frequently experience visual impairment, headaches, cognitive confusion, and aberrant behaviors[22]. The appearance of SNTCS on CT and MRI images often lacks specificity; exhibiting similar characteristics observed in general malignant tumors located in the nasal cavity and sinuses[23], including esthesioneuroblastomas, that should be considered as a diagnostic possibility of a mass in this location demonstrating both expansible and destructive growth properties. Both CT and MRI are useful, each with its characteristics, for accurately staging tumor extent and identifying extranasal tumor invasion, which is fundamental to operative planning. The plain CT scan frequently reveals space-occupying lesions in the nasal cavity and paranasal sinuses, which may exhibit a polypoid or lobulated morphology with homogeneous or heterogeneous lesion density, without evident calcification or cystic degeneration. The enhanced scan demonstrates conspicuous uneven enhancement, while local progressive lesions can infiltrate the surrounding tissues and cause thinning or destruction of adjacent bone structures, potentially extending to invade the skull base. On MRI, T1WI typically exhibits isointense signals, while T2WI demonstrates high signal intensity. The contrast-enhanced scan reveals heterogeneous enhancement. The tumor demonstrates a significant mass effect involving the ethmoid sinus and extending beyond the cranial vault to impact the frontal lobe. Additionally, periosteal reaction and bone destruction are observed in the surrounding region. This case exemplifies the exceptional rarity and distinctiveness of SNTCS, a nasal sinus tumor that is unparalleled in its occurrence. In the field of radiology, MRI accurately depicts the precise location, morphological characteristics, size, internal structure, surrounding involvement, and metabolic activity of lesions. It plays a pivotal role in precisely localizing and qualitatively assessing SNTCS. However, due to histological heterogeneity exhibited by tumors, diagnosing SNTCS remains challenging in clinical practice. Integrating clinical manifestations with radiology imaging information and pathological biopsy remains essential for enhancing the diagnostic accuracy of this disease.
SNTCS is a clinically rare condition, with only a limited number of cases involving the central nervous system. The clinical manifestations are atypical and lack specific imaging findings; therefore, it is crucial to combine pathological biopsy as the gold standard with radiology examinations and clinical presentations for an accurate diagnosis of this disease.
| 1. | Thompson LDR, Bishop JA. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2022;16:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 2. | Misra P, Husain Q, Svider PF, Sanghvi S, Liu JK, Eloy JA. Management of sinonasal teratocarcinosarcoma: a systematic review. Am J Otolaryngol. 2014;35:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Kurmi DJ, Mittal RS, Sharma A, Gandhi A, Singhvi S. Sinonasal teratocarcinosarcoma involving nasal cavity, nasopharynx, and all paranasal sinuses with bilateral orbital and intracranial extension: A rare case report. Asian J Neurosurg. 2017;12:232-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Rotenberg B, El-Hakim H, Lodha A, MacCormick A, Ngan BY, Forte V. Nasopharyngeal teratocarcinosarcoma. Int J Pediatr Otorhinolaryngol. 2002;62:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Crazzolara R, Puelacher W, Ninkovic M, Zelger B, Buchberger W, Meister B, Zimmerhackl LB, Klein-Franke A. Teratocarcinosarcoma of the oral cavity. Pediatr Blood Cancer. 2004;43:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Smith SL, Hessel AC, Luna MA, Malpica A, Rosenthal DI, El-Naggar AK. Sinonasal teratocarcinosarcoma of the head and neck: a report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg. 2008;134:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Nitsche M, Hermann RM, Christiansen H, Berger J, Pradier O. Rationale for individualized therapy in Sinonasal Teratocarcinosarcoma (SNTC): case report. Onkologie. 2005;28:653-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Jin W, Teng Y, Zhao P, Zhang W, Li X, Li Y. Sinonasal teratocarcinosarcoma masquerading as an olfactory neuroblastoma. Int J Clin Exp Pathol. 2018;11:910-915. [PubMed] |
| 9. | Belotti A, Carpenito L, Bulfamante AM, Maccari A, Bulfamante G. Sinonasal teratocarcinosarcoma treated with surgery and proton beam therapy: clinical, histological aspects and differential diagnosis of a new case. Pathologica. 2021;113:469-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Chapurin N, Totten DJ, Morse JC, Khurram MS, Louis PC, Sinard RJ, Chowdhury NI. Treatment of Sinonasal Teratocarcinosarcoma: A Systematic Review and Survival Analysis. Am J Rhinol Allergy. 2021;35:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Sakci Z, Aydin F, Ceylan O, Ogul H. Sinonasal teratocarcinosarcoma mimicking chronic invasive fungal disease of paranasal sinuses. Ann R Coll Surg Engl. 2021;103:e193-e195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 12. | Shanmugaratnam K, Kunaratnam N, Chia KB, Chiang GS, Sinniah R. Teratoid carcinosarcoma of the paranasal sinuses. Pathology. 1983;15:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Heffner DK, Hyams VJ. Teratocarcinosarcoma (malignant teratoma?) of the nasal cavity and paranasal sinuses A clinicopathologic study of 20 cases. Cancer. 1984;53:2140-2154. [PubMed] [DOI] [Full Text] |
| 14. | Budrukkar A, Agarwal JP, Kane S, Siddha M, Laskar SG, Pai P, Murthy V, Sengar M, D'Cruz A. Management and clinical outcome of sinonasal teratocarcinosarcoma: single institution experience. J Laryngol Otol. 2010;124:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Miller M, Newberry CI, Witt B, Oakley GM. Sinonasal Teratocarcinosarcoma-A Rare and Highly Aggressive Neoplasm. JAMA Otolaryngol Head Neck Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Sable M, Kakkar A, Garg K, Suri V. Sinonasal Teratocarcinosarcoma: An Underdiagnosed Entity Posing Diagnostic Challenges. Turk Neurosurg. 2017;27:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 17. | Yoon SY, Park KS, Hwang JH, Park SH, Han MH. Sinonasal Teratocarcinosarcoma, a Rare Tumor Involving Both the Nasal Cavity and the Cranial Cavity. Brain Tumor Res Treat. 2020;8:57-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Sobani ZA, Akhtar S, Junaid M, Salahuddin I. Sinonasal teratocarcinosarcoma. J Pak Med Assoc. 2012;62:633-635. [PubMed] |
| 19. | Foong YC, Murdolo V, Naiman N, Hepner L, Awad R. Sinonasal teratocarcinosarcoma: a case report. J Med Case Rep. 2017;11:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Zhou T, Tian Y, Zhu Z, Yue J, Cheng Q, Niu X, Zhou Y, Fan J, Zhou L, Sun H. Sinonasal Teratocarcinosarcoma Involving Orbital and Intracranial Extension: A Rare Case Report. Ear Nose Throat J. 2022;1455613221135649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Tchoyoson Lim CC, Thiagarajan A, Sim CS, Khoo ML, Shakespeare TP, Ng I. Craniospinal dissemination in teratocarcinosarcoma. J Neurosurg. 2008;109:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Abayie AO, Nyarko KM, Bährle M, Brütting A. The first case report of primary thyroid teratocarcinosarcoma: An analog to sinonasal teratocarcinosarcoma. Rare Tumors. 2021;13:20363613211043662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Nguyen BD. Sinonasal teratocarcinosarcoma: MRI and F18-FDG-PET/CT imaging. Ear Nose Throat J. 2010;89:106-108. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/