Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2803
Revised: March 7, 2024
Accepted: April 11, 2024
Published online: June 6, 2024
Processing time: 99 Days and 20 Hours
The effect of serum iron or ferritin parameters on mortality among critically ill pa
To determine the association between serum iron or ferritin parameters and mor
Web of Science, Embase, PubMed, and Cochrane Library databases were searched for studies on serum iron or ferritin parameters and mortality among critically ill patients. Two reviewers independently assessed, selected, and abstracted data from studies reporting on serum iron or ferritin parameters and mortality among critically ill patients. Data on serum iron or ferritin levels, mortality, and demo
Nineteen studies comprising 125490 patients were eligible for inclusion. We observed a slight negative effect of serum ferritin on mortality in the United States population [relative risk (RR) 1.002; 95%CI: 1.002-1.004). In patients with sepsis, serum iron had a significant negative effect on mortality (RR = 1.567; 95%CI: 1.208-1.925).
This systematic review presents evidence of a negative correlation between serum iron levels and mortality among patients with sepsis. Furthermore, it reveals a minor yet adverse impact of serum ferritin on mortality among the United States population.
Core Tip: This systematic review presents evidence of a negative correlation between serum iron levels and mortality among patients with sepsis. Furthermore, it reveals a minor yet adverse impact of serum ferritin on mortality among the United States population. This guide provides direction for future prognostic assessments in patients with sepsis. Further high-quality cohort studies and experimental studies on molecular mechanisms are needed to confirm our findings.
- Citation: Yang DC, Zheng BJ, Li J, Yu Y. Iron and ferritin effects on intensive care unit mortality: A meta-analysis. World J Clin Cases 2024; 12(16): 2803-2812
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2803.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2803
Iron is an essential nutritional element of the human body with many important biological functions[1]. With the varying cellular environment, the interconversion between the two oxidized states of iron (Fe2+ and Fe3+) keeps iron relevant to a variety of important biochemical reactions, but also with potential hazard risks[2]. Under oxygenic conditions, the Fenton/Haber-Weiss reaction of free iron catalyzes the production of harmful atomic groups, and this mechanism has been hypothesized to underlie iron toxicity in some pathological states[3].
With the progress of clinical and experimental studies, iron metabolism has been shown to play a crucial role not only in metabolic diseases, but also in monitoring disease prognosis using serum iron indicators[4-6]. Changes in iron metabolism indicators occur in critically ill patients, and some of these serum iron metabolism indicators have been reported to be used to predict prognosis in these patients. In a study on multiple serum iron metabolism indicators among 51 critically ill patients after surgery, elevated serum ferritin was associated with poor clinical outcomes[7]. Another study on a prospective cohort of 121 patients under critical care in an integrated intensive care unit (ICU) revealed a significant association between plasma iron levels and increased risk of inpatient 30-d mortality[8]. According to another prospective study, serum iron metabolism indicators are associated with prognosis of ICU patients. In-survival analysis revealed that lower serum iron and iron utilization levels could increase short-term and long-term survival outcomes. Furthermore, after multivariate analysis adjusting for other indicators, iron was found to be an independent predictor of short-term and long-term survival outcomes of mortality among ICU patients[9]. Currently, no conclusions have been established as to whether serum iron metabolism indicators can accurately predict the prognosis of critically ill patients. The relevant underlying mechanisms are still being explored.
Therefore, a better understanding of the importance of iron metabolism among ICU patients is urgently needed. In consequence, we analyzed the effect of iron parameters on mortality among critically ill patients admitted to ICU.
This meta-analysis study was conducted by following the Meta-analysis of Observational Studies in Epidemiology guidelines[10]. A literature search of relevant published studies that analyzed the association between iron parameters and mortality outcomes among critically ill patients was conducted on October 1, 2021. Using literature searches on PubMed, Embase, Web of Science, and Cochrane Library databases, we identified articles using the following terms: Ferritin, iron, anemia, critically ill, sepsis, sepsis shock, and mortality. Only studies published in English were identified for the analysis in this study. By consulting the reference lists in these research articles, we identified additional studies relevant to the subject.
Studies were included, if: (1) They were cohort or case-control studies; (2) the study evaluated the association between iron parameters and mortality among critically ill patients admitted to ICUs; (3) they provided sufficient data on odds ratios (OR); and (4) the Newcastle-Ottawa scale (NOS) score was ≥ 6. All studies containing overlapping data were excluded.
The data extracted included the first author’s name, year of publication, study population and country, period, study type, age, ORs with 95%CI, ICU type, patient types, and NOS scores (Table 1). For publications that did not report the association between iron or ferritin and mortality among critically ill patients, we calculated the ORs, if data on these variables were available.
| Ref. | Population and country | Period | Study type | Age | Adjustment OR (95%CI) | ICU type | Patient types | NOS |
| Rimmelé et al[13] | 1260, Italy | From February 22 to May 31, 2020 | Cohort | Median age of 63 yr | Ferritin: 1.000 (1.000–1.000) | ICUs | COVID-19 | 7 |
| Deng et al[14] | 100, China | From January 30 to March 30 2020 | Cohort | Median age of 65 yr | Ferritin: 32.63 (8.30–128.32) | ICUs | COVID-19 | 7 |
| Lachmann et al[17] | 116310, Germany | From January 2006 to August 2018 | Cohort | 18 years old or older | Ferritin: 1.518 (1.384–1.665) | Adult ICU | Critically ill patients | 8 |
| Tonial et al[15] | 1407, Brazil | From July 2013 to January 2017 | Cohort | 6 months to 18 yr old | Ferritin: 5.17 (2.619-10.205) | Pediatric ICU | Sepsis | 8 |
| Brandtner et al[18] | 61, Austria | From February 2018 to December 2019 | Cohort | Mean age of 63 yr | Ferritin: 6 (1.1-39) | Adult ICU | Sepsis | 7 |
| Iron: 4.6 (1.5-17) | ||||||||
| Shu et al[16] | 483, United States | From 2001 to 2012 | Cohort | Aged 16 yr or above | Ferritin (90 D): 1.001 (1.000-1.001) | Adult ICU | AKI | 6 |
| Iron (90 D): 1.741 (1.285-2.358) | ||||||||
| Jiang et al[20] | 258, China | From May 1 2016 to November 30 2017 | Cohort | 18 yr old or older | Ferritin: 1.010 (1.000–1.021) | Adult ICU | Sepsis | 6 |
| Xia et al[19] | 250, China | From June 2015 to May 2017 | Cohort | 18 yr old or older | Iron: 0.696 (0.268-0.918) | Adult ICU | Critically ill patients | 6 |
| Lan et al[23] | 1891, United States | From 2001 to 2012 | Cohort | Aged 16 yr or above | Ferritin: 1.000 (0.999–1.000) | Adult ICU | Sepsis | 6 |
| Iron: 1.506 (1.190–1.908) | ||||||||
| Xie et al[21] | 103, China | From August 2016 to January 2017 | Cohort | 18 yr old or older | Ferritin (long-term): 1.002 (1.000–1.003) | NCU | Neuro-critically patients | 6 |
| Lasocki et al[22] | 2087, France | From August 2011 to June 2013 | Cohort | Unlimited | Ferritin: 1.02 (0.51-2.06) | ICUs | Iron deficiency | 7 |
| Ghosh et al[24] | 42, South Asia | From May 2016 to March 2017 | Cohort | 6 months to 12 yr old | Ferritin: 1.94 (0.94–4.02) | Pediatric ICU | Septic shock | 6 |
| Tacke et al[9] | 312, Germany | Not provided | Cohort | Median age of 64 yr | Ferritin (long-term): 1.000 (1.000–1.000) | Adult ICU | Critically ill patients | 7 |
| Iron (long-term): 1.040 (1.011–1.071) | ||||||||
| Unal et al[26] | 111, Turkey | Between May 2012 and January 2013 | Cohort | Mean age of 73.79 | Ferritin: 1.002 (1.000-1.004) | Adult ICU | Critically ill patients | 6 |
| Carcillo et al[29] | 100, Pittsburgh | Not provided | Cohort | Age greater than or equal to 44 wk gestation and less than 18 yr | Ferritin: 1.001 (1.000–1.001) | Pediatric ICU | Sepsis | 7 |
| Uscinska et al[25] | 392, Poland | Between 2008 and 2011 | Cohort | Mean age of 70 | Iron: 0.85 (0.78–0.94) | ICUs | Anemia | 7 |
| Leaf et al[8] | 121, United States | Between 2008 and 2012 | Cohort | Age >18 yr | Iron (long-term): 1.38 (0.91-2.10) | ICUs | AKI | 6 |
| Bennett et al[27] | 171, United States | Between September 2, 2003, and August 16, 2007 | Cohort | Age < 18 yr | Ferritin: 4.32 (2.21-8.47) | Pediatric ICU | Critically ill patients | 6 |
| Garcia et al[28] | 31, Brazil | From January 2004 to September 2005 | Cohort | Aged 1 month–16 yr | Ferritin: 3.2 (1.3-7.9) | Pediatric ICU | Sepsis | 6 |
The strength of the association between iron or ferritin and mortality among critically ill patients was reported using ORs and 95%CIs. When adjusted and crude ORs were provided, the most adjusted ORs were extracted. We used the heterogeneity value (I2) test and Q-statistic to detect any possible heterogeneity, as a quantitative measure of inconsistency among studies[11]. Meta-regression analyses were used to investigate the sources of heterogeneity. Pooled ORs and 95%CIs were calculated using a random-effects model[12].
All statistical analyses in the meta-analysis were performed using Statistical software for data science version 13.0. All reported P values were from two-sided statistical tests. Statistical significance was set at P ≤ 0.05. Egger’s and Begg’s regression models were used to evaluate potential publication bias[11].
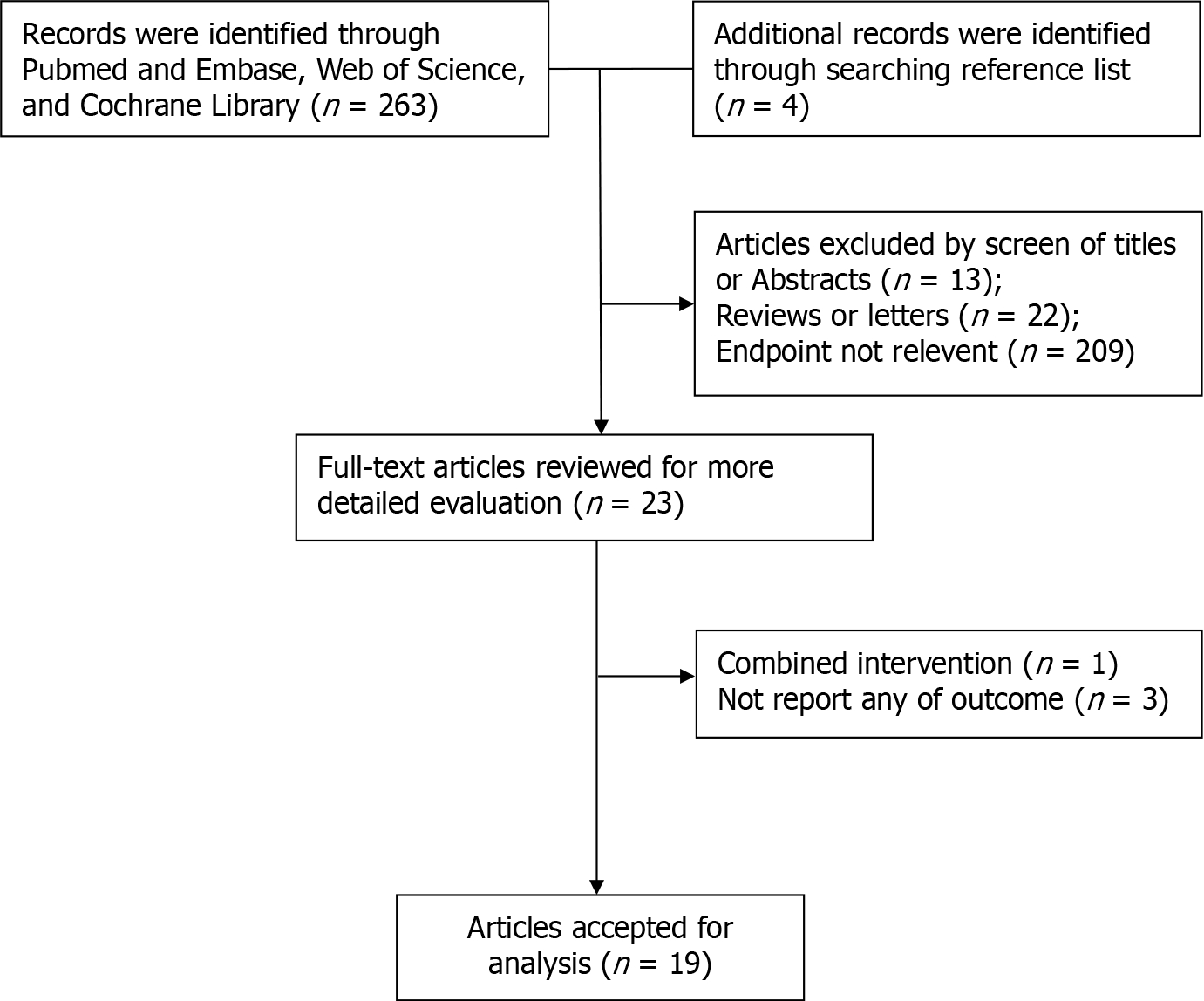
The process for selecting the studies is outlined in Figure 1. After the literature search, 267 potentially relevant records were reviewed. Out of this number, 19 studies, including 125490 patients, were included in the meta-analysis (Table 1). Subsequently, 247 studies were excluded, because they were either duplicated reports or were of relatively low quality. All 19 selected studies were cohort studies[8,9,13-29].
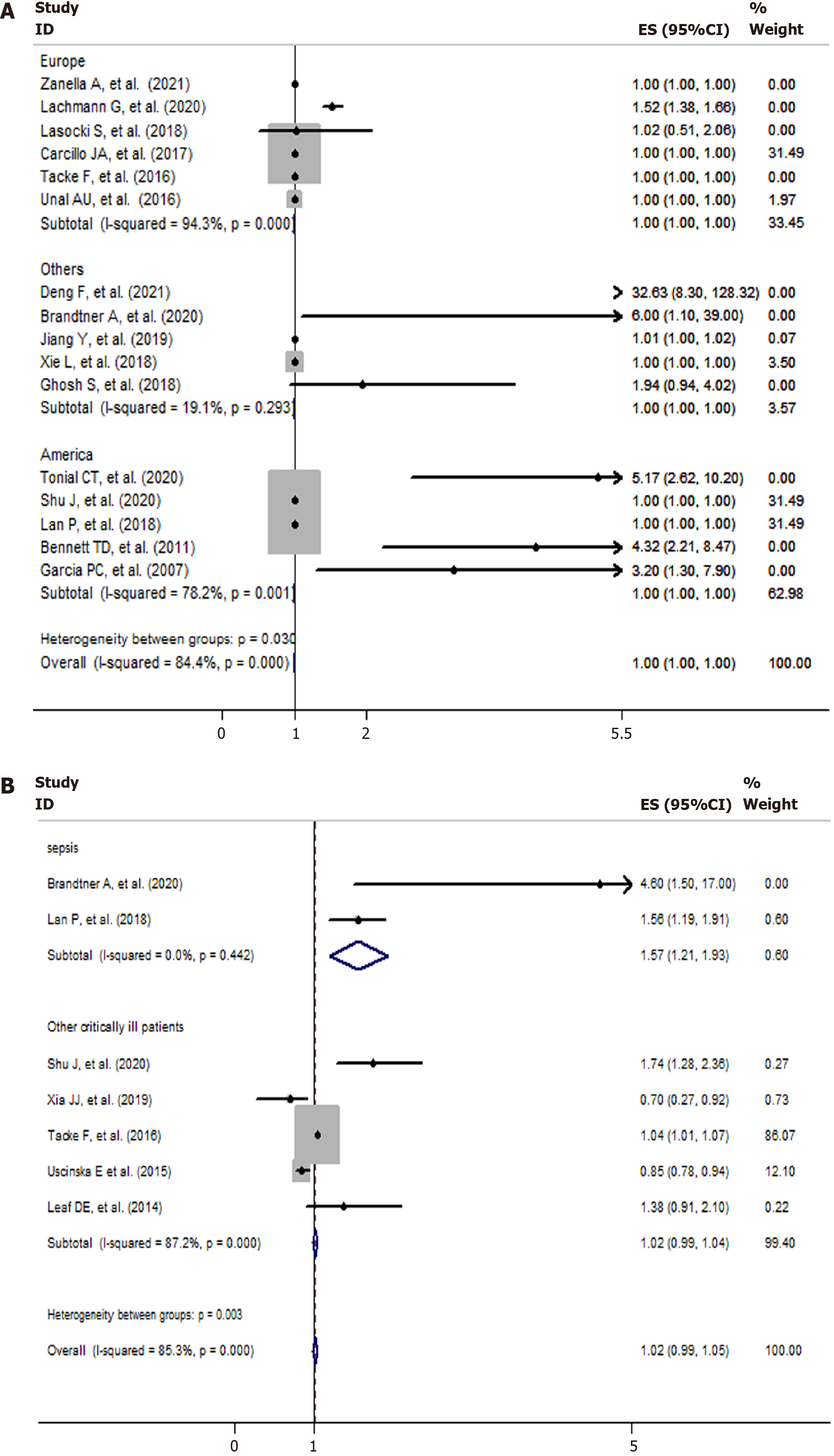
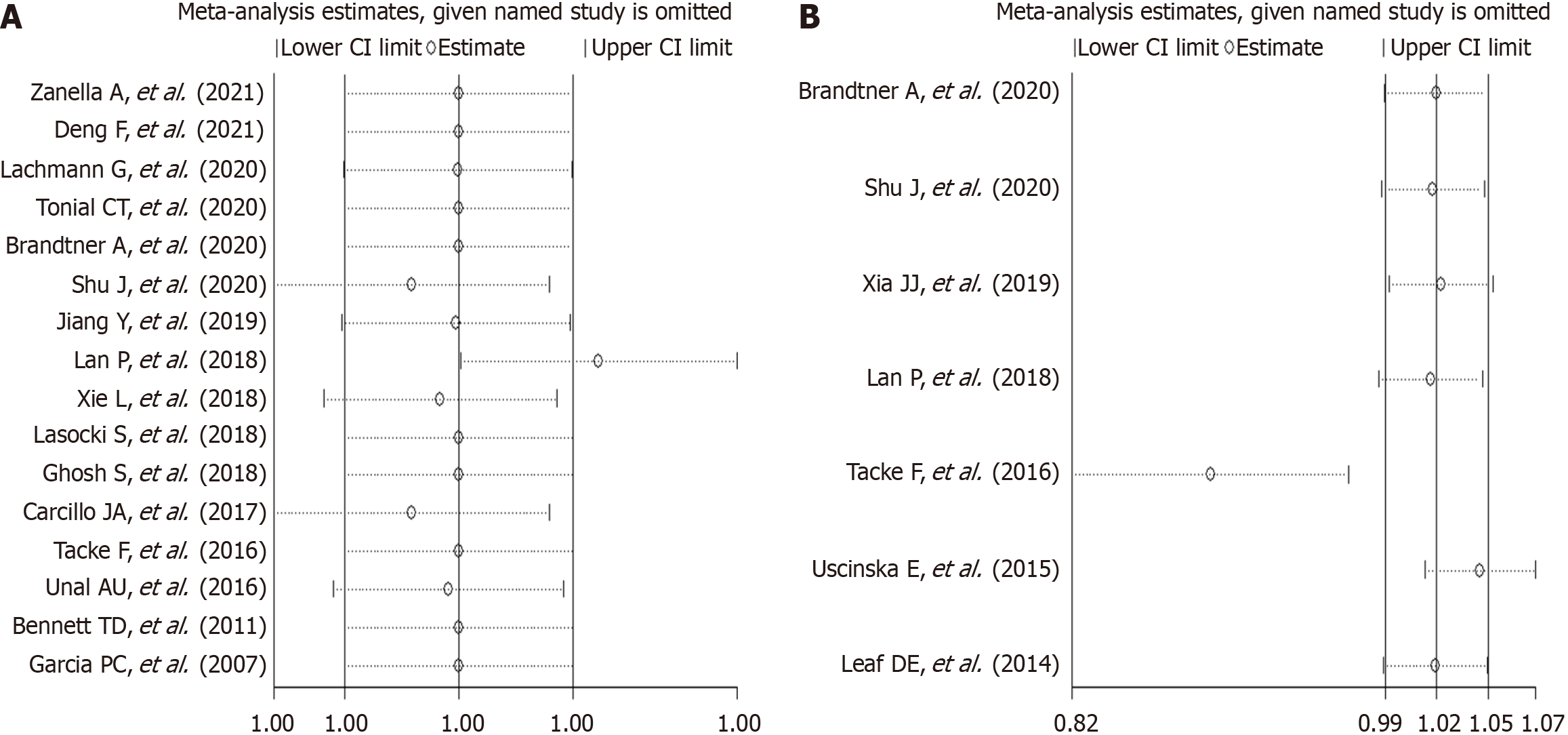
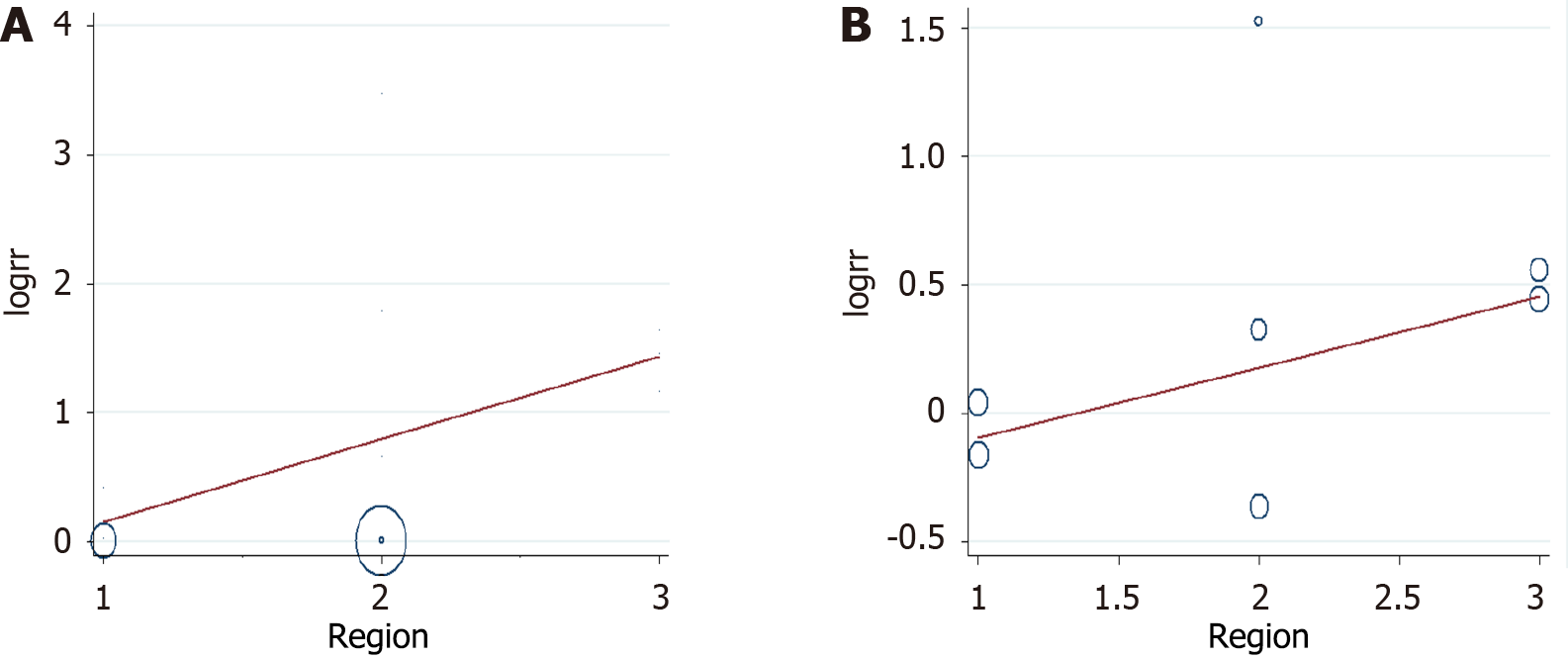
Six studies were conducted in Europe; five studies were conducted in America; and five studies were conducted in other regions (Asia, Australia). Four studies presented data on mortality and iron and ferritin separately. The NOS scores of eight, six, and two studies were 6, 7, and 8, respectively (Table 2). The data on mortality and serum iron and ferritin were extracted separately. Most of the included studies had contrasting findings. Six studies reported that high ferritin levels were associated with high mortality among critically ill patients, whereas the other 10 studies reported no association. Similarly, the studies on serum iron had contrasting findings. Four studies reported that high serum iron levels were associated with high mortality among critically ill patients, whereas the other three studies reported no association or corresponding decrease in mortality among critically ill patients. The analysis of the studies yielded a combined risk estimate, relative risk (RR) of 1.00 (95%CI: 1.00–1.00; P = 0.001) with I2 of 84.4% for ferritin (Figure 2A), and a combined risk estimate, RR of 1.02 (95%CI: 0.99–1.05; P < 0.001) with I2 of 85.3% for iron (Figure 2B). We also assessed the stability and explored the sources of heterogeneity of the results using sensitivity analysis for serum ferritin and iron (Figure 3). After a meta-regulation test, geographical area was associated with 50.51% heterogeneity reduction across the studies for serum ferritin and 41.53% heterogeneity reduction across the studies for serum iron (Figure 4).
| Group | Number of the study | RR (95%CI) | P value | I2 (%) |
| Ferritin | ||||
| Geographic region | ||||
| Europe | 6 | 1.001 (1.001-1.002) | < 0.01 | 94.3 |
| America | 5 | 1.002 (1.002-1.004) | 0.293 | 19.1 |
| Others | 5 | 1.001 (1-1.001) | 0.001 | 78.2 |
| NOS | ||||
| 6 | 8 | 1.001 (1.001-1.001) | 0.001 | 70.5 |
| 7 | 6 | 1.001 (1.001-1.001) | 0.72 | 0 |
| 8 | 2 | 1.523 (1.383-1.663) | 0.059 | 71.9 |
| Patient types | ||||
| Sepsis | 7 | 1.001 (1.001-1.001) | 0.004 | 68.2 |
| Other critically ill patients | 9 | 1.001 (1.001-1.002) | < 0.01 | 89.9 |
| ICU types | ||||
| Adult ICU | 8 | 1.001 (1-1.001) | 0 | 91.3 |
| Pediatric ICU | 5 | 1.001 (1.001-1.002) | 0.017 | 66.9 |
| General ICU | 3 | 1.025 (0.25-1.8) | 0.302 | 6.2 |
| Iron | ||||
| Geographic region | ||||
| Europe | 2 | 1.017 (0.988-1.045) | < 0.01 | 94.7 |
| America | 3 | 0.858 (0.573-1.143) | 0.09 | 58.4 |
| Others | 2 | 1.616 (1.318-1.914) | 0.583 | 0 |
| NOS | ||||
| 6 | 4 | 1.217 (1.011-1.424) | 0.001 | 82.7 |
| 7 | 3 | 1.017 (0.989-1.045) | < 0.01 | 89.9 |
| Patient types | ||||
| Sepsis | 2 | 1.567 (1.208-1.925) | 0.442 | 0 |
| Other critically ill patients | 5 | 1.017 (0.989-1.045) | < 0.01 | 87.2 |
| ICU types | ||||
| Adult ICU | 5 | 1.043 (1.013-1.073) | 0.001 | 79.7 |
| General ICU | 2 | 0.859 (0.780-0.939) | 0.084 | 66.6 |
Due to the differences in geographic area (Europe, America, and others), NOS (6, 7, or 8), patient category (sepsis, other critically ill patients), and ICU type [adult ICU, pediatric ICU (PICU), or general ICU] among the studies, we further conducted subgroup analyses to determine the effect of these factors in our analyses (Table 2). We found a significant negative effect of serum ferritin on mortality in the United States population (RR = 1.002; 95%CI: 1.002-1.004) and in the general ICU (RR = 1.025; 95%CI: 0.25-1.8). We obtained a statistically negative effect on patients with sepsis (RR = 1.567; 95%CI: 1.208-1.925) for serum iron.
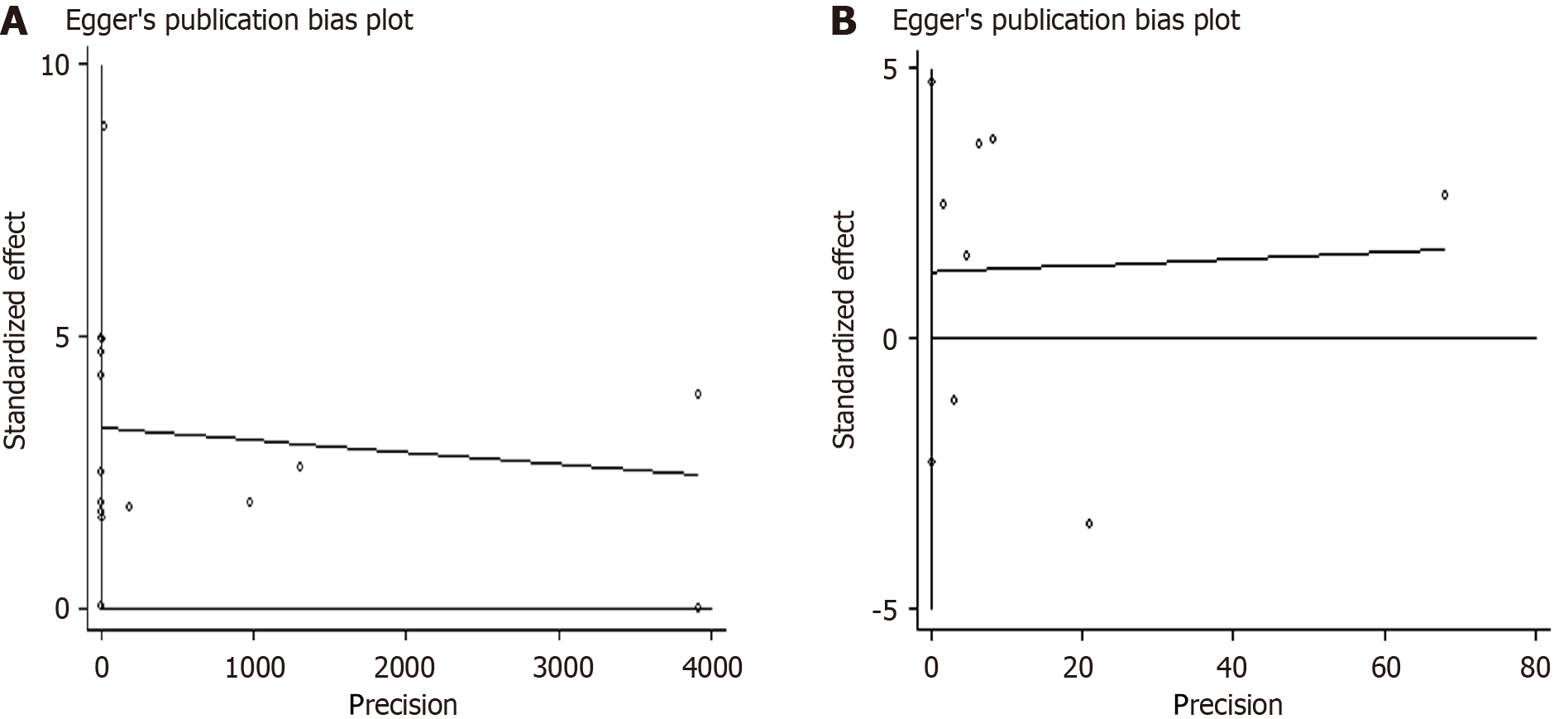
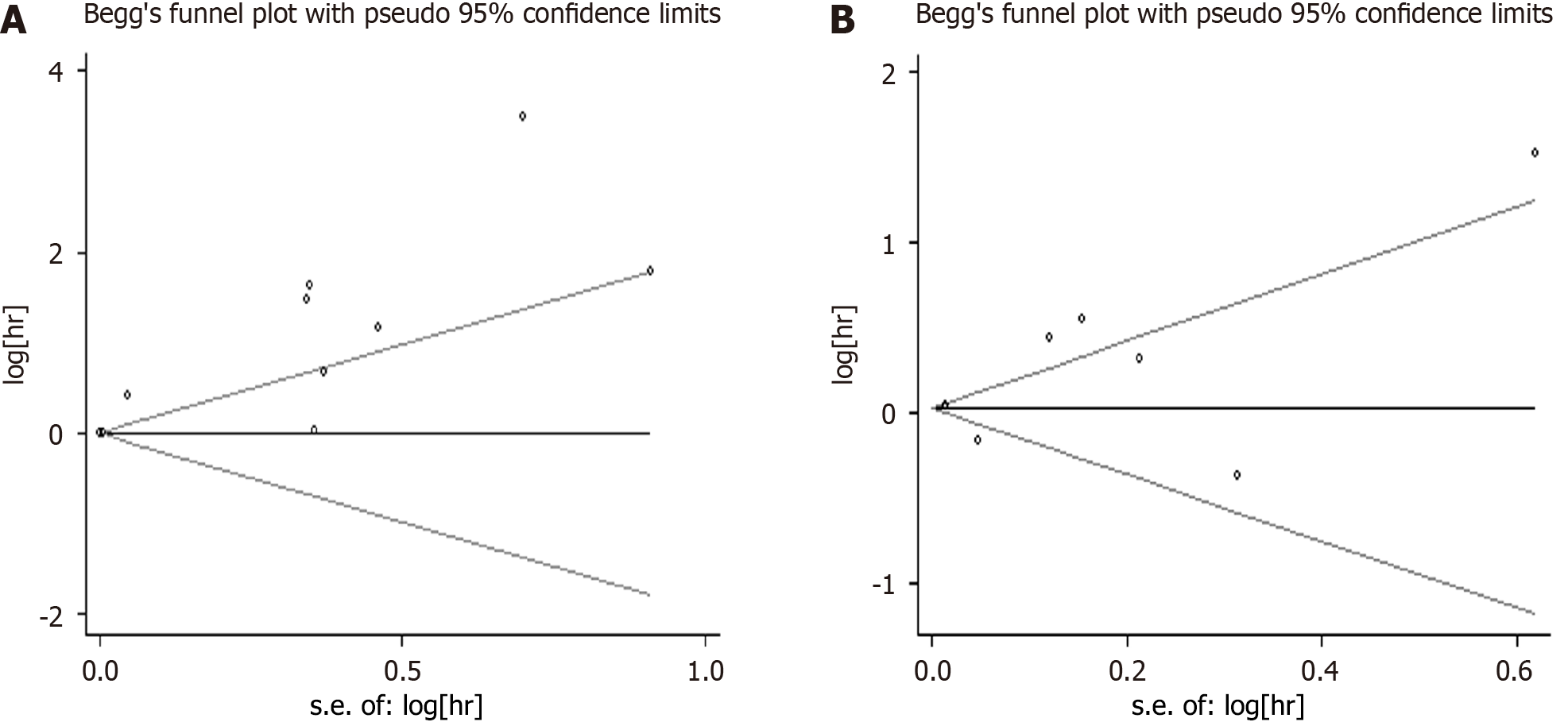
Based on Egger’s and Begg’s regression models, evidence of publication bias (Figures 5 and 6) were noted for iron or ferritin, in relation to mortality. Egger’s funnel plot and Begg’s linear regression test revealed P values < 0.05.
To the best of our knowledge, this study is the first systematic review examining the role of iron parameters on mortality in critically ill patients. High iron parameters had no association with mortality among critically ill patients. Due to the heterogeneity of the meta-analysis, we performed subgroup analysis, sensitivity analysis, and regression analysis to explore the source of heterogeneity. Subgroup analyses were used to determine the effect of geographic area (Europe, America, or others), NOS score (6, 7, or 8), patient types (sepsis, other critically ill patients), and ICU types (Adult ICU, PICU, or general ICU) in our analyses (Table 2). For iron, sepsis had a statistically negative effect (RR = 1.567; 95%CI: 1.208-1.925). Sensitivity analysis revealed stable conclusions and no apparent source of heterogeneity for serum ferritin and iron (Figure 3). From the meta-regulation test, geographical area was associated with 50.51% heterogeneity reduction across studies on serum ferritin and 41.53% heterogeneity reduction across studies on serum iron (Figure 4). Due to publication bias, further studies of higher quality are needed to confirm our findings.
The meta-analysis concluded that serum ferritin had no association with mortality among critically ill patients. How
The meta-analysis concluded that no association existed between iron level and mortality in critically ill patients, al
This study had few limitations. First, only studies published in English journals were included. However, a significant portion of the literature were studies conducted in Asia, where the official language is not English. Second, predicting the effect of misclassification of cohort studies on the results was challenging. Third, systematic confounding or the risk of bias in observation studies cannot be ruled out easily. Fourth, due to heterogeneity across the studies, regression analysis was used to explain the source of heterogeneity, which may be due to differences in study geographical areas. In this study, analyses of the association between concentration of ferritin and duration of ferritin abnormality and mortality among critically ill patients were not included. The original studies lacked data on these parameters for this analysis.
In summary, this systematic review presents evidence of a negative correlation between serum iron levels and mortality among patients with sepsis. Additionally, it identifies a minor but adverse effect of serum ferritin on mortality within the United States population. Further high-quality cohort studies and experimental studies on molecular mechanisms are needed to confirm our findings.
| 1. | Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61:933-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 2. | Venkataramani V. Iron Homeostasis and Metabolism: Two Sides of a Coin. Adv Exp Med Biol. 2021;1301:25-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 3. | Kohli SK, Handa N, Bali S, Khanna K, Arora S, Sharma A, Bhardwaj R. Current Scenario of Pb Toxicity in Plants: Unraveling Plethora of Physiological Responses. Rev Environ Contam Toxicol. 2020;249:153-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Kumar A, Brookes MJ. Iron Therapy in Inflammatory Bowel Disease. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 5. | Ueda N, Takasawa K. Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 6. | Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD; CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. 2015;36:657-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 916] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 7. | Bobbio-Pallavicini F, Verde G, Spriano P, Losi R, Bosatra MG, Braschi A, Iotti G, Chiaranda M, Villa S. Body iron status in critically ill patients: significance of serum ferritin. Intensive Care Med. 1989;15:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Leaf DE, Rajapurkar M, Lele SS, Mukhopadhyay B, Waikar SS. Plasma catalytic iron, AKI, and death among critically ill patients. Clin J Am Soc Nephrol. 2014;9:1849-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Tacke F, Nuraldeen R, Koch A, Strathmann K, Hutschenreuter G, Trautwein C, Strnad P. Iron Parameters Determine the Prognosis of Critically Ill Patients. Crit Care Med. 2016;44:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17221] [Article Influence: 662.3] [Reference Citation Analysis (0)] |
| 11. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 12. | DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 2073] [Article Influence: 188.5] [Reference Citation Analysis (0)] |
| 13. | Rimmelé T, Pascal L, Polazzi S, Duclos A. Organizational aspects of care associated with mortality in critically ill COVID-19 patients. Intensive Care Med. 2021;47:119-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Deng F, Zhang L, Lyu L, Lu Z, Gao D, Ma X, Guo Y, Wang R, Gong S, Jiang W. Increased levels of ferritin on admission predicts intensive care unit mortality in patients with COVID-19. Med Clin (Barc). 2021;156:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Tonial CT, Costa CAD, Andrades GRH, Crestani F, Einloft PR, Bruno F, Miranda AP, Fiori HH, Garcia PCR. Prediction of Poor Outcomes for Septic Children According to Ferritin Levels in a Middle-Income Setting. Pediatr Crit Care Med. 2020;21:e259-e266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Shu J, Hu Y, Yu X, Chen J, Xu W, Pan J. Elevated serum iron level is a predictor of prognosis in ICU patients with acute kidney injury. BMC Nephrol. 2020;21:303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Lachmann G, Knaak C, Vorderwülbecke G, La Rosée P, Balzer F, Schenk T, Schuster FS, Nyvlt P, Janka G, Brunkhorst FM, Keh D, Spies C. Hyperferritinemia in Critically Ill Patients. Crit Care Med. 2020;48:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Brandtner A, Tymoszuk P, Nairz M, Lehner GF, Fritsche G, Vales A, Falkner A, Schennach H, Theurl I, Joannidis M, Weiss G, Pfeifhofer-Obermair C. Linkage of alterations in systemic iron homeostasis to patients' outcome in sepsis: a prospective study. J Intensive Care. 2020;8:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Xia JJ, Wang F, Jiang XN, Jiang TT, Shen LJ, Liu Y, You DL, Ding Y, Ju XF, Wang L, Wu X, Hu SY. Serum iron levels are an independent predictor of in-hospital mortality of critically ill patients: a retrospective, single-institution study. J Int Med Res. 2019;47:66-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Jiang Y, Jiang FQ, Kong F, An MM, Jin BB, Cao D, Gong P. Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: a preliminary observational study. Ann Intensive Care. 2019;9:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Xie L, Peng Y, Huang K, Wu Y, Wang S. Predictive value of iron parameters in neurocritically ill patients. Brain Behav. 2018;8:e01163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Lasocki S, Lefebvre T, Mayeur C, Puy H, Mebazaa A, Gayat E; FROG-ICU study group. Iron deficiency diagnosed using hepcidin on critical care discharge is an independent risk factor for death and poor quality of life at one year: an observational prospective study on 1161 patients. Crit Care. 2018;22:314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Lan P, Pan KH, Wang SJ, Shi QC, Yu YX, Fu Y, Chen Y, Jiang Y, Hua XT, Zhou JC, Yu YS. High Serum Iron level is Associated with Increased Mortality in Patients with Sepsis. Sci Rep. 2018;8:11072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Ghosh S, Baranwal AK, Bhatia P, Nallasamy K. Suspecting Hyperferritinemic Sepsis in Iron-Deficient Population: Do We Need a Lower Plasma Ferritin Threshold? Pediatr Crit Care Med. 2018;19:e367-e373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Uscinska E, Sobkowicz B, Sawicki R, Kiluk I, Baranicz M, Stepek T, Dabrowska M, Szmitkowski M, Musial WJ, Tycinska AM. Parameters influencing in-hospital mortality in patients hospitalized in intensive cardiac care unit: is there an influence of anemia and iron deficiency? Intern Emerg Med. 2015;10:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Unal AU, Kostek O, Takir M, Caklili O, Uzunlulu M, Oguz A. Prognosis of patients in a medical intensive care unit. North Clin Istanb. 2015;2:189-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Bennett TD, Hayward KN, Farris RW, Ringold S, Wallace CA, Brogan TV. Very high serum ferritin levels are associated with increased mortality and critical care in pediatric patients. Pediatr Crit Care Med. 2011;12:e233-e236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Garcia PC, Longhi F, Branco RG, Piva JP, Lacks D, Tasker RC. Ferritin levels in children with severe sepsis and septic shock. Acta Paediatr. 2007;96:1829-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Carcillo JA, Sward K, Halstead ES, Telford R, Jimenez-Bacardi A, Shakoory B, Simon D, Hall M; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network Investigators. A Systemic Inflammation Mortality Risk Assessment Contingency Table for Severe Sepsis. Pediatr Crit Care Med. 2017;18:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Haschka D, Hoffmann A, Weiss G. Iron in immune cell function and host defense. Semin Cell Dev Biol. 2021;115:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 31. | Marshall JC. Inflammation, coagulopathy, and the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med. 2001;29:S99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 288] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 704] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 33. | Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 2012;185:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 34. | Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2133] [Cited by in RCA: 2213] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 35. | Liu Q, Wu J, Zhang X, Wu X, Zhao Y, Ren J. Iron homeostasis and disorders revisited in the sepsis. Free Radic Biol Med. 2021;165:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 36. | Boshuizen M, Binnekade JM, Nota B, van de Groep K, Cremer OL, Tuinman PR, Horn J, Schultz MJ, van Bruggen R, Juffermans NP; Molecular Diagnosis and Risk Stratification of Sepsis (MARS) Consortium. Iron metabolism in critically ill patients developing anemia of inflammation: a case control study. Ann Intensive Care. 2018;8:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Retter A, Wyncoll D, Pearse R, Carson D, McKechnie S, Stanworth S, Allard S, Thomas D, Walsh T; British Committee for Standards in Haematology. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160:445-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 38. | Heming N, Montravers P, Lasocki S. Iron deficiency in critically ill patients: highlighting the role of hepcidin. Crit Care. 2011;15:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Darveau M, Denault AY, Blais N, Notebaert E. Bench-to-bedside review: iron metabolism in critically ill patients. Crit Care. 2004;8:356-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Wizorek JJ, Turnbull IR, Buchman TG. Iron overload before cecal ligation and puncture increases mortality. Shock. 2003;20:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade D
Novelty: Grade B
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Wu H, China S-Editor: Liu H L-Editor: A P-Editor: Zhao S