Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.169
Peer-review started: September 13, 2023
First decision: November 9, 2023
Revised: November 12, 2023
Accepted: December 18, 2023
Article in press: December 18, 2023
Published online: January 6, 2024
Processing time: 111 Days and 5 Hours
Elizabethkingia miricola is a non-fermenting gram-negative bacterium, which was first isolated from the condensate of the Russian peace space station in 2003. Most studies on this bacterium have been carried out in the laboratory, and clinical case studies are rare. To date, a total of 6 clinical cases have been reported worldwide.
We present the first case of postoperative pulmonary infection in a patient with intracerebral hemorrhage due to Elizabethkingia miricola. The imaging characteristics of pulmonary infection were identified and the formulation and selection of the clinical treatment plan for this patient are discussed.
Elizabethkingia miricola infection is rare. When pulmonary infection occurs, computed tomography imaging may show diffuse distribution of a ground glass density shadow in both lungs, the air containing bronchial sign in local areas, thickening of bronchial vascular bundle, and pleural effusion.
Core Tip:Elizabethkingia miricola infection is rarely reported. We report a 54-year-old male with Elizabethkingia miricola infection in the lungs after surgery for cerebral hemorrhage. The clinical symptoms after infection were nonspecific and could not be timely and accurately diagnosed. Therefore, this report focuses on the imaging characteristics of pulmonary Elizabethkingia miricola infection.
- Citation: Qi PQ, Zeng YJ, Peng W, Kuai J. Lung imaging characteristics in a patient infected with Elizabethkingia miricola following cerebral hemorrhage surgery: A case report. World J Clin Cases 2024; 12(1): 169-175
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/169.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.169
Elizabethkingia miricola is a rare non-fermenting gram-negative bacterium, which was first isolated from the condensate water in the Mir space station[1] in 2003. The original name of the bacterium was Chryseobacterium miricola. The model strain was KCTC 12492 (T) = GTC 862 (T). In 2005, the bacterium was classified into the genus Elizabethkingia together with Elizabethkingia meningoseptica. The bacterium rarely causes clinical infection[2], which was reported in laboratory research. To date, there have only been 6 clinical cases of this bacterial infection worldwide. These clinical reports mainly show that the bacterium can cause bacteremia and sepsis. In addition, infection by this bacterium has also been found in patients with cystic fibrosis and alcoholic pancreatitis. We report the first case of Elizabethkingia miricola infection in a patient who underwent surgery for cerebral hemorrhage. We discuss the imaging characteristics after infection and the disease development and treatment process, in order to provide a reference for the early detection and identification of the bacterium in clinical practice.
A 54-year-old male was admitted to the hospital due to sudden headache and left limb weakness for 3 h.
The patient presented with severe swelling and pain, accompanied by weakness in the left limb, unstable walking, and nausea and vomiting once before admission without any obvious cause.
The patient had a history of hypertension for 1 year, but did not take antihypertensive drugs or monitor his blood pressure regularly.
The patient had a history of occasional smoking and drinking, and his family members had no history of cerebral hemorrhage.
On admission, the patient was lethargic and had difficulty opening his eyes. The Glasgow Coma Scale score was 13 points, the National Institute of Health Stroke Scale score was 17 points, and the left limb muscle strength was grade 0.
Routine blood tests, liver and kidney function, and coagulation tests showed no significant abnormalities.
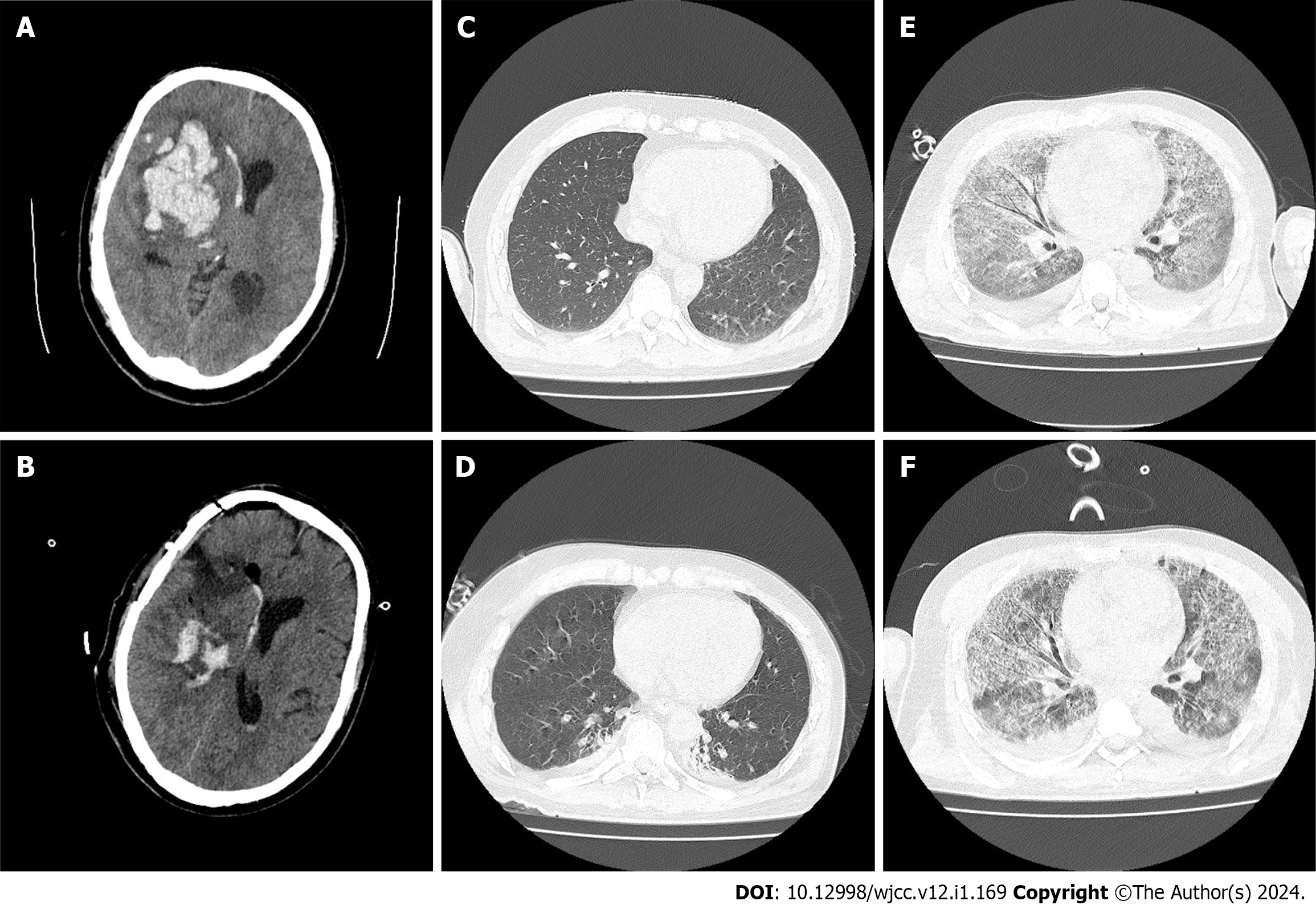
After admission, he underwent head computed tomography (CT) in the emergency department (Figure 1A). Right basal ganglia hemorrhage was observed, and the amount of bleeding was approximately 60 mL.
Right basal ganglia hemorrhage and hypertension.
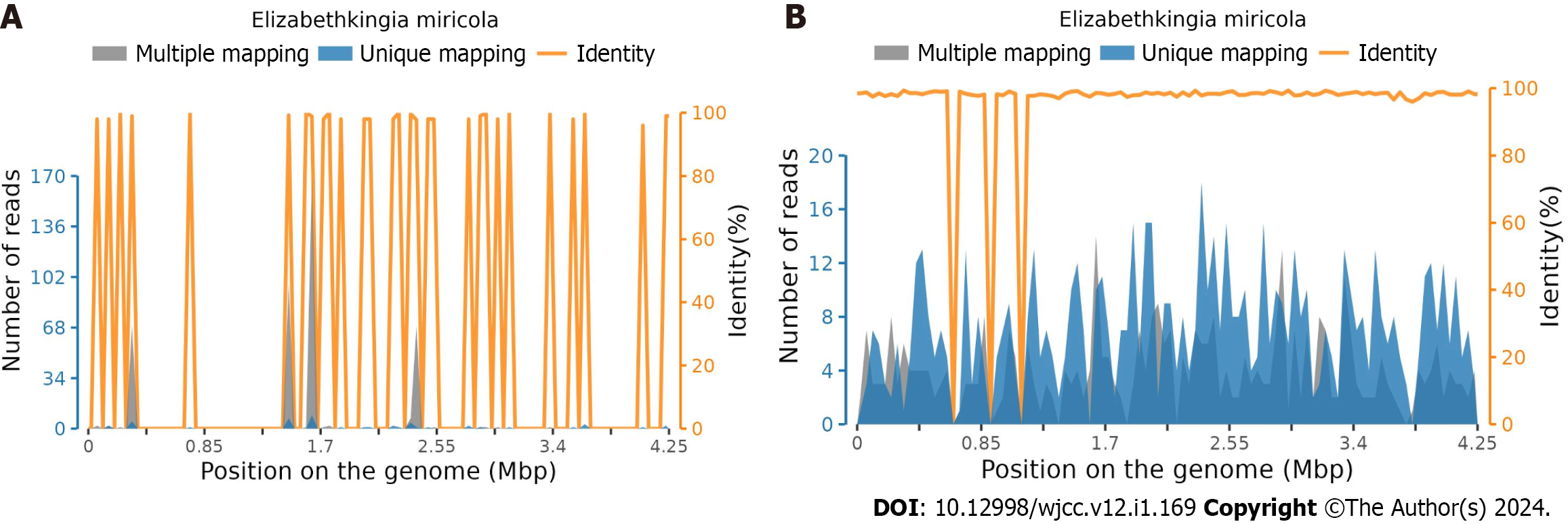
On the day of admission, evacuation of intracerebral hematoma by craniotomy was performed in the emergency department, and the outcome of this procedure was considered satisfactory. The following day, a head CT scan showed that the hematoma was basically cleared (Figure 1B), which resulted in reduced intracranial pressure. The day after surgery, the patient was found to be unconscious, but the sting stimulus induced eye opening. An emergency chest CT (Figure 1C) and a subsequent chest CT re-examination (Figure 1D) showed that the lungs were in good condition. Mannitol and sedation were administered routinely. A small amount of gram-negative bacteria was found in the sputum smear, and ceftizoxime sodium 2 g q8h intravenous drip was given to prevent infection. On the third day after surgery, chest CT re-examination showed a small amount of bilateral pleural effusion with poor air content in adjacent lung tissues. White blood cell count was 16.6 × 109/L and neutrophil percentage was 87.9%, and a small amount of Klebsiella oxytoca were found in the sputum culture. Bacterial infection was considered, the previous antibiotic treatment was continued, and a tracheotomy was performed on the fifth day after surgery. During this period, acid fast staining of the Mycobacterium tuberculosis smear was performed; however, no acid fast bacteria were found. Thus, tuberculous infection was excluded. No fungi were observed in the fungal smear after staining, and fungal infection was excluded. On the ninth day after surgery, procalcitonin and interleukin-6 levels were higher than the normal level due to decreased blood oxygen saturation. Aggravation of pulmonary infection was considered, and the treatment plan was adjusted to piperacillin sulbactam anti-infection treatment, and purulent airway secretions were removed by fiberoptic bronchoscopy on the 10th d after surgery. Two days later, chest CT showed that a ground glass density shadow was diffusely distributed in both lungs, the air containing bronchial sign was seen in local areas, and the trachea and vascular bundle of both pulmonary bronchi were thickened. Considering that the inflammatory changes were obvious, accompanied by pulmonary edema (Figure 1E), and sputum culture on the 13th day after surgery showed infection with Staphylococcus haemolyticus, the treatment scheme was adjusted to oral linezolid 600 mg q12h + cefoperazone sodium sulbactam sodium injection 3 g q8h intravenous drip combined with anti-infection treatment. The results of 19ncov RNA detected by real-time polymerase chain reaction were negative, and coronavirus disease 2019 was eliminated. Chest CT re-examination on the 16th d after surgery showed that the ground glass density shadow was diffusely distributed, the air containing bronchial sign was seen in the local area, and the bronchial vascular bundles of both lungs were thickened. Considering these inflammatory changes, pulmonary edema was not excluded, and there was no significant improvement compared with the previous CT scan findings (Figure 1F). The sputum culture showed that there were more Klebsiella spp., and the blood culture (aerobic + anaerobic) results showed no bacterial growth and elimination of bacteremia. The treatment regimen was adjusted to oral linezolid 600 mg q12h + meropenem 1 g q8h intravenous drip combined with anti-infection treatment. High throughput sequencing technology was used to analyze the nucleic acid sequence of microorganisms in the lung lavage fluid 20 d after surgery. Elizabethkingia miricola was detected. The DNA detection results showed that the total length of the genome was 46062 (BP), the coverage was 1.0839%, the average depth was 1.03 × (Figure 2A), the type was g-, the number of genus sequences was 1183, the relative abundance was 38.28%, and the number of species sequences was 708. The RNA detection results showed that the total length of the genome was 7301 (BP), the coverage was 0.1718%, the average depth was 1.36 ×, the type was g-, the number of genus sequences was 166, the relative abundance was 28.77%, and the sequence number of species was 47 (Figure 2B). Drug sensitivity testing showed that the patient was sensitive to quinolone antibiotics and moxifloxacin was administered. As the patient's condition was severe, he developed respiratory failure 22 d after surgery, and his family members did not permit further treatment.
The patient died on the second day after discharge during telephone follow-up.
Elizabethkingia miricola rarely causes human disease. In previous studies, only 6 cases have been reported. The first clinical case of human disease caused by this bacterium was a mantle cell lymphoma patient who received allogeneic stem cell transplantation and chemotherapy and required ventilator support[3]. In 2008, the bacterium was isolated from the sputum and blood of the patient by the clinical center of the National Institutes of Health. Since then, five clinical cases of infection caused by this bacterium have been reported. In 2015, a young woman hospitalized due to alcoholic pancreatitis was reported to be infected with Elizabethkingia miricola[4] following blood sampling. In 2016, a clinical case of pulmonary abscess caused by this bacterium was reported[5]. In 2017, a patient with urinary tract infection was reported and Elizabethkingia miricola[6] was isolated from the urine sample. In 2018, it was reported that the bacterium was isolated from the blood of one patient with diffuse large B-cell lymphoma and the sputum sample of one patient with cystic fibrosis[7,8]. The clinical characteristics, possible etiology and prognosis of these cases are summarized in Table 1.
| Case number | Clinical features | Etiology | Prognosis | Ref. |
| Previous case 1 | Hemoptysis, dyspnea, persistent fever, pulmonary CT showed diffuse infiltration | Respiratory tract infection and bacteremia caused by severe immune dysfunction after stem cell transplantation and chemotherapy | Death | Green et al[3] |
| Previous case 2 | Abdominal pain, fever, respiratory distress, pulmonary CT showed atelectasis, abdominal CT showed hemorrhage | Chronic liver disease and alcohol abuse, bacteremia | Survived | Rossati et al[4] |
| Previous case 3 | Dry cough, fever, dyspnea, chest CT findings: pulmonary abscess and pleural effusion | Pulmonary infection caused by bacteria | Survived | Gonzalez et al[5] |
| Previous case 4 | Dysuria, oliguria, fever, abdominal pain | Urinary tract infection caused by bacteria | Survived | Gupta et al[6] |
| Previous case 5 | Fever, neutropenia | Decreased immunity and bacteremia after chemotherapy | Survived | Lin et al[7] |
| Previous case 6 | Cough, expectoration, shortness of breath, wheezing, decreased lung function | Long-term oral administration of glucocorticoids reduced immunity | Survived | Frost et al[8] |
| This case | Decreased consciousness, fever, decreased blood oxygen saturation, systemic multiple organ function injury and stressed state. CT showed diffuse distribution of a ground glass density shadow with pulmonary edema in both lungs | Complications after cerebral hemorrhage | Died |
The case in the current report is the first case of postoperative infection with Elizabethkingia miricola in the world. It is also the seventh report of human disease caused by Elizabethkingia miricola to date. This report mainly discusses the imaging characteristics and changes in this patient with pulmonary infection due to Elizabethkingia miricola, and discusses the selection of strategies and schemes during his treatment.
The CT scans of this patient showed that during pulmonary infection, the imaging features included diffuse distribution of a ground glass density shadow in both lungs, the air containing bronchial sign in local areas, thickening of bronchial vascular bundle in both lungs, and pleural effusion. Here we needed to distinguish Elizabethkingia miricola infection from the following diseases: (1) New type coronavirus infectious pneumonia: The CT imaging features of new coronavirus infectious pneumonia are in the early stage, multiple small patchy shadows or ground glass shadows, and infiltrating shadows in both lungs can be seen in the peripheral distribution of the lung. For severe and critical patients, lung consolidation shadows can be seen, which are generally not accompanied by pleural effusion. The differential diagnosis is based on the results of nucleic acid detection; (2) mycoplasma pneumonia: This disease is characterized by ground glass, lobular core nodules, and airway wall thickening is often seen. Generally, it can be identified in combination with a positive immunoglobulin M laboratory examination; and (3) Pneumocystis pneumoniae pneumonia: The CT imaging manifestations in these patients are ground glass with interlobular septal thickening, and most of them are "empty" under the pleura. A detailed history should be obtained for these patients, and timely use of high-throughput sequencing technology to analyze the nucleic acid sequence of microorganisms in the alveolar lavage fluid should be performed to help identification and treatment.
There were some problems during the treatment of this case which are worth noting: (1) Early identification: In the early stage of pulmonary infection, the CT imaging features of this patient were not fully displayed, and the sputum smear and sputum culture failed to detect Elizabethkingia miricola, which led to the failure of early diagnosis; thus, the administration of ceftizoxime sodium anti-infection treatment was ineffective. At present, there are few reported clinical cases of Elizabethkingia miricola infection, and there is still no unified reference standard for its imaging characteristics and clinical manifestations. Therefore, our findings may provide a new reference for the early identification of possible infection by this bacterium, combined with the imaging characteristics of the patient, and establish an early under
Elizabethkingia miricola infection is relatively rare. When it leads to pulmonary infection, it has the CT imaging characteristics of diffuse distribution of a ground glass density shadow in both lungs, the air containing bronchial sign in local areas, thickening of bronchial vascular bundle in both lungs, pleural effusion and so on, but needs to be differentiated from new coronavirus pneumonia. When possible Elizabethkingia miricola infection is indicated, early empirical use of quinolones may be effective in patients. In addition, it is suggested that microbial nucleic acid sequence analysis and other techniques should be used for early diagnosis and identification.
In the future, with continuous research on infection by Elizabethkingia spp., early detection and drug treatment of this new pathogen will be improved. Further research should include a comparison of the therapeutic effect of combined antibiotic therapy and single antibiotic therapy, early detection and identification of the pathogen using high-throughput sequencing technology, and various new technologies that are currently being developed. For example, the use of gene sequence targeted therapy for the bacterium, artificial intelligence detection methods and other directions may become the research focus and direction in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Arboix A, Spain; Ciarambino T, Italy S-Editor: Qu XL L-Editor: Webster JR P-Editor: Zhang XD
| 1. | Fuerst TR, de la Cruz VF, Bansal GP, Stover CK. Development and analysis of recombinant BCG vector systems. AIDS Res Hum Retroviruses. 1992;8:1451-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005;55:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Green O, Murray P, Gea-Banacloche JC. Sepsis caused by Elizabethkingia miricola successfully treated with tigecycline and levofloxacin. Diagn Microbiol Infect Dis. 2008;62:430-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Rossati A, Kroumova V, Bargiacchi O, Brustia D, Luigi Garavelli P. Elizabethkingia miricola bacteriemia in a young woman with acute alcoholic pancreatitis. Presse Med. 2015;44:1071-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Gonzalez C, Coolen-Allou N, Allyn J, Estève JB, Belmonte O, Allou N. [Severe sepsis and pulmonary abscess with bacteremia due to Elizabethkingia miricola]. Med Mal Infect. 2016;46:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Gupta P, Zaman K, Mohan B, Taneja N. Elizabethkingia miricola: A rare non-fermenter causing urinary tract infection. World J Clin Cases. 2017;5:187-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Lin JN, Lai CH, Yang CH, Huang YH, Lin HH. Complete Genome Sequence of Elizabethkingia miricola Strain EM798-26 Isolated from the Blood of a Cancer Patient. Genome Announc. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Frost F, Nazareth D. Case Report: First report of Elizabethkingia miricola infection in a patient with cystic fibrosis. F1000Res. 2018;7:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Arboix A, Jiménez C, Massons J, Parra O, Besses C. Hematological disorders: a commonly unrecognized cause of acute stroke. Expert Rev Hematol. 2016;9:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |