Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1771
Peer-review started: August 10, 2022
First decision: October 21, 2022
Revised: October 26, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: March 16, 2023
Processing time: 208 Days and 15.5 Hours
Endocardial fibroelastosis (EFE) is a diffuse endocardial collagen and elastin hyperplasia disease of unknown etiology, which may be accompanied by myo
A 13-mo-old female child was admitted to hospital with retching. Chest X-ray demonstrated enhanced texture in both lungs and an enlarged heart shadow. Color doppler echocardiography showed an enlarged left heart with ventricular wall hypokinesis and decreased left heart function. Abdominal color ultrasonography revealed a markedly enlarged liver. Pending the result of the endomyo
Our report suggests that EFE-induced pediatric AHF may present in children over 1 year of age without any apparent precipitants, and that the associated clinical presentations are grossly similar to that of pediatric DCM. Nonetheless, it is still possible to be diagnosed effectively on the basis of the comprehensive analysis of auxiliary inspection findings before the result of the end
Core Tip: Prior to the report of endomyocardial biopsy, the diagnosis and treatment of endocardial fibroelastosis (EFE) is highly susceptible to be confounded with other primary cardiomyopathies. Herein, we report a case of pediatric acute heart failure (AHF) caused by EFE mimicking dilated cardiomyopathy, aiming to provide a valuable reference for clinicians to early identify and diagnose EFE-induced AHF.
- Citation: Xie YY, Li QL, Li XL, Yang F. Pediatric acute heart failure caused by endocardial fibroelastosis mimicking dilated cardiomyopathy: A case report. World J Clin Cases 2023; 11(8): 1771-1781
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1771.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1771
Pediatric endocardial fibroelastosis (EFE) is a kind of primary infantile cardiomyopathy, also known as endocardial sclerosis[1]. While various theories have been proposed in recent years in relation to the pathogenesis of EFE, the exact etiology of EFE remains unknown[2]. At present, most scholars believe that it is associated with the immune inflammatory response caused by viral infection[3]. More than 60% of children with onset are younger than 1 year of age[4]. The clinical manifestations of infants under 6 mo of age are principally acute heart failure (AHF), while the clinical manifestations of infants 6 to 12 mo of age are principally chronic heart failure (CHF), and often occurs after respiratory infections[5]. The symptoms and signs of AHF caused by EFE greatly resemble those of the acute exacerbations of dilated cardiomyopathy (DCM) in pediatric patients[5]. However, the treatment options for these two conditions clinically are not exactly identical, and early misdiagnosis may have potentially unintended consequences for the subsequent therapy of the children[6]. More importantly, it is relatively rare for children with EFE over 1 year of age to develop AHF suddenly without any notable triggers or other directly related underlying diseases[7].
Here, we report a case of pediatric AHF caused by EFE mimicking DCM, in which the child was pre
A 13-mo-old female child was admitted to our hospital with retching for 1 wk and worsening condition for 2 d.
The child had experienced retching without apparent triggers for 1 wk prior to presentation at our hospital, but no vomiting. The child’s family denied that the child had a history of fever, rash, cough and expectoration, jaundice, diarrhea, and trauma. Outpatient blood assays revealed hemoglobin (HGB) 84.00 g/L, C-reactive protein (CRP) 0.30 mg/L, and carbon dioxide combining power (CO2CP) 15.70 mmol/L. During the course of the illness, the child had poor appetite, poor sleep, fair mental status, and reduced urine output, but there was no abnormal exhaust and defecation. The child had no other concomitant symptoms, including any signs of upper respiratory tract infection.
The child was hospitalized in our neonatology unit 1 year ago with a diagnosis of neonatal hyperbilirubinemia (NHB), neonatal bilirubin encephalopathy (NBE), congenital hepatic cyst (CHC), and congenital bilateral renal multiple cysts (CRMCs). NHB and NBE recovered favorably after active treatment. CHC and CRMC were treated with conservative observation, and color ultrasonography of the liver and kidneys were conducted every other year. Anemia was detected at 8 mo of age, with a minimum HGB of about 34 g/L. After treatment with oral medication and dietary therapy, the maximum HGB was about 80 g/L. The child had no previous history of surgery or blood transfusion.
The child was born of a gravida 1, parity 1 mother. She was delivered vaginally at 38+2 wk of gestation, with a birth weight of 2750 g. She was breastfed, with normal growth and developmental milestones and no significant history of medication or food allergies. The child’s parents were healthy, and there was no abnormality in the family history.
The child was 74 cm tall and weighed 9.5 kg, with body temperature 36.4 °C, pulse rate 150/min, res
The relevant blood tests of the child after admission are illustrated in Table 1. A number of laboratory test indicators, including HGB, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration and ferritin concentration, were decreased. Biomarkers of heart failure such as N-Terminal pro-brain natriuretic peptide were remarkably elevated. No abnormal results were found in urine and stool analyses.
| Test item | Day 1 | Day 3 | Day 7 |
| WBC | 10.11 × 109/L (4.4-11.0) | 9.32 × 109/L (4.4-11.0) | 8.68 × 109/L (4.4-11.0) |
| RBC | 4.84 × 1012/L (4.0-5.5) | 4.77 × 1012/L (4.0-5.5) | 4.82 × 1012/L (4.0-5.5) |
| HGB | 84 g/L (112-149) | 82 g/L (112-149) | 83 g/L (112-149) |
| HCT | 30.7% (34-43) | 33.5% (34-43) | 38.1% (34-43) |
| MCV | 63.4 fL (76-88) | 62.8 fL (76-88) | 63.0 fL (76-88) |
| MCH | 17.4 pg (24-30) | 16.9 pg (24-30) | 17.2 pg (24-30) |
| MCHC | 274 g/L (310-355) | 271 g/L (310-355) | 273 g/L (310-355) |
| PLT | 522 × 109/L (188-472) | 487 × 109/L (188-472) | 445 × 109/L (188-472) |
| LYMPH% | 56.3% (23-69) | 44.1% (23-69) | 40.7% (23-69) |
| MONO% | 7.2% (2-11) | 8.1% (2-11) | 8.6% (2-11) |
| NEUT% | 34.0% (22-65) | 36.7% (22-65) | 33.15% (22-65) |
| LYMPH# | 5.69 × 109/L (1.8-6.3) | 4.42 × 109/L (1.8-6.3) | 4.05 × 109/L (1.8-6.3) |
| MONO# | 0.73 × 109/L (0.12-0.80) | 0.80 × 109/L (0.12-0.80) | 0.87 × 109/L (0.12-0.80) |
| NEUT# | 3.44 × 109/L (1.2-7.0) | 3.65 × 109/L (1.2-7.0) | 3.34 × 109/L (1.2-7.0) |
| RDW-CV | 20.4% (10.9-15.0) | 19.4% (10.9-15.0) | 17.6% (10.9-15.0) |
| FER | 8.11 ng/mL (15.0-150.0) | 8.10 ng/mL (15.0-150.0) | 8.10 ng/mL (15.0-150.0) |
| VB12 | 362.40 pg/mL (197.00-771.00) | 348.67 pg/mL (197.00-771.00) | 384.22 pg/mL (197.00-771.00) |
| Folate | 18.77 ng/mL (3.89-26.80) | 18.03 ng/mL (3.89-26.80) | 19.55 ng/mL (3.89-26.80) |
| CRP | 0.3 mg/L (0.0-1.0) | 0.2 mg/L (0.0-1.0) | 0.05 mg/L (0.0-1.0) |
| IgG | 10.43 g/L (7.00-16.00) | 8.27 g/L (7.00-16.00) | 9.01 g/L (7.00-16.00) |
| IgA | 1.30 g/L (0.70-4.00) | 1.55 g/L (0.70-4.00) | 1.41 g/L (0.70-4.00) |
| IgM | 1.73 g/L (0.40-2.30) | 1.66 g/L (0.40-2.30) | 1.80 g/L (0.40-2.30) |
| C3 | 1.56 g/L (0.90-1.80) | 1.31 g/L (0.90-1.80) | 1.45 g/L (0.90-1.80) |
| C4 | 0.32 g/L (0.10-0.40) | 0.30 g/L (0.10-0.40) | 0.29 g/L (0.10-0.40) |
| ASO | 96 IU/mL (0-170) | 83 IU/mL (0-170) | 79 IU/mL (0-170) |
| ESR | 16 mm/h (0-20) | 14 mm/h (0-20) | 12 mm/h (0-20) |
| PCT | 0.12 ng/mL (< 0.05) | 0.07 ng/mL (< 0.05) | 0.03 ng/mL (< 0.05) |
| NT-proBNP | 893.15 ng/L | 517.40 ng/L | 190.64 ng/L |
| Ca | 2.65 mmol/L (2.10-2.80) | 2.50 mmol/L (2.10-2.80) | 2.69 mmol/L (2.10-2.80) |
| K | 4.36 mmol/L (3.70-5.20) | 4.62 mmol/L (3.70-5.20) | 4.57 mmol/L (3.70-5.20) |
| Na | 139.70 mmol/L (135.00-145.00) | 142.74 mmol/L (135.00-145.00) | 140.32 mmol/L (135.00-145.00) |
| Cl | 102.80 mmol/L (96.00-108.00) | 105.71 mmol/L (96.00-108.00) | 103.40 mmol/L (96.00-108.00) |
| AMY | 50.0 U/L (35.0-135.0) | 62.0 U/L (35.0-135.0) | 57.0 U/L (35.0-135.0) |
| CO2CP | 15.7 mmol/L (22.0-29.0) | 21.1 mmol/L (22.0-29.0) | 25.8 mmol/L (22.0-29.0) |
| ALT | 11.0 U/L (7.0-30.0) | 10.3 U/L (7.0-30.0) | 9.0 U/L (7.0-30.0) |
| AST | 39.0 U/L (15.0-40.0) | 36.7 U/L (15.0-40.0) | 29.4 U/L (15.0-40.0) |
| AST/ALT | 3.55 | 3.56 | 3.27 |
| LDH | 394.0 U/L (120.0-250.0) | 376.0 U/L (120.0-250.0) | 285.0 U/L (120.0-250.0) |
| CK | 100.0 U/L (50.0-310.0) | 84.0 U/L (50.0-310.0) | 77.0 U/L (50.0-310.0) |
| CK-MB | 23.5 U/L (0.0-25.0) | 20.9 U/L (0.0-25.0) | 18.4 U/L (0.0-25.0) |
| α-HBDH | 331.0 U/L (72.0-182.0) | 255.4 U/L (72.0-182.0) | 191.1 U/L (72.0-182.0) |
| GLU | 5.35 mmol/L (3.90-6.10) | 5.62 mmol/L (3.90-6.10) | 5.05 mmol/L (3.90-6.10) |
| BUN | 3.2 mmol/L (2.7-7.0) | 2.9 mmol/L (2.7-7.0) | 2.7 mmol/L (2.7-7.0) |
| CREA | 22.2 μmol/L (19.0-44.0) | 21.4 μmol/L (19.0-44.0) | 20.1 μmol/L (19.0-44.0) |
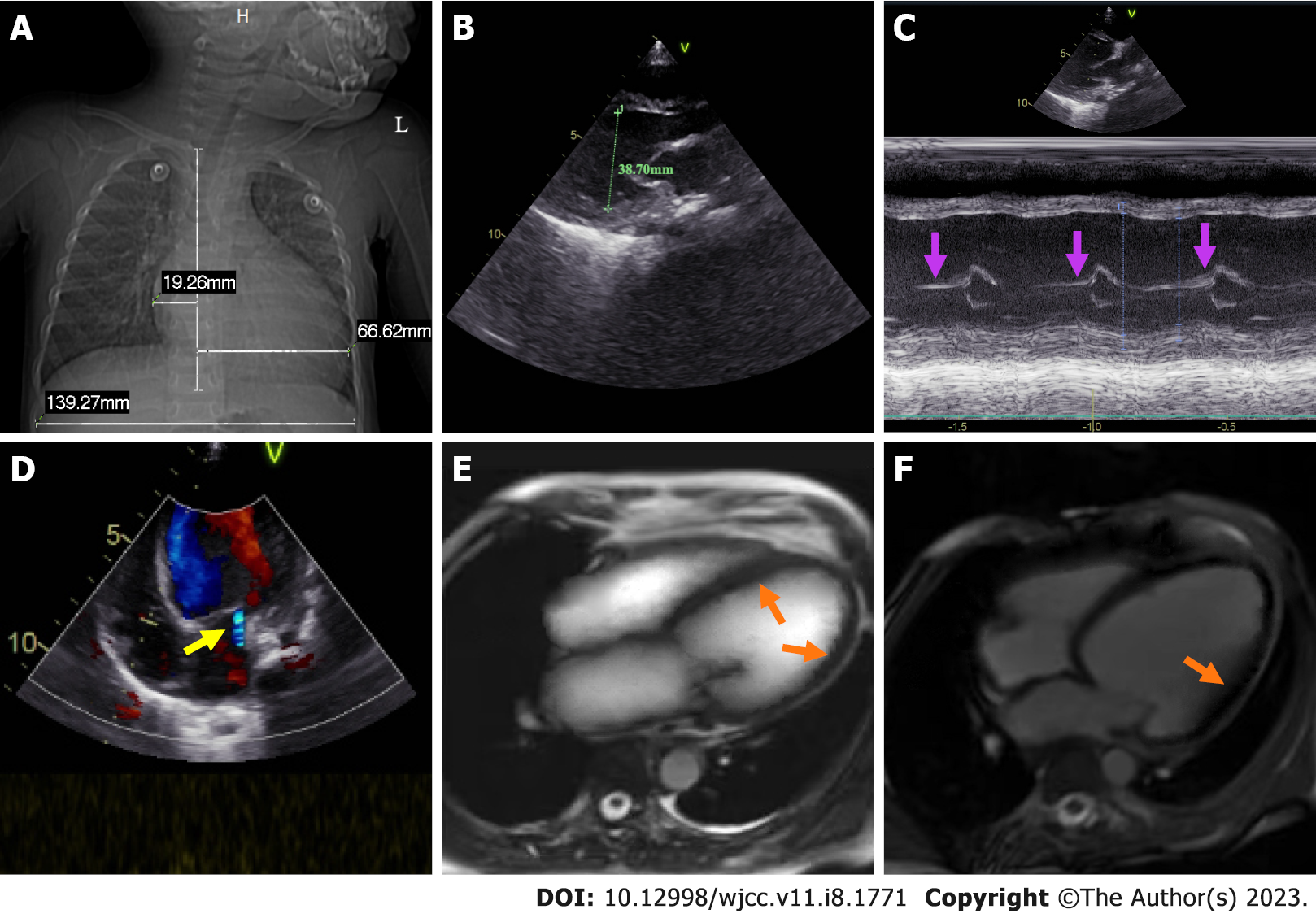
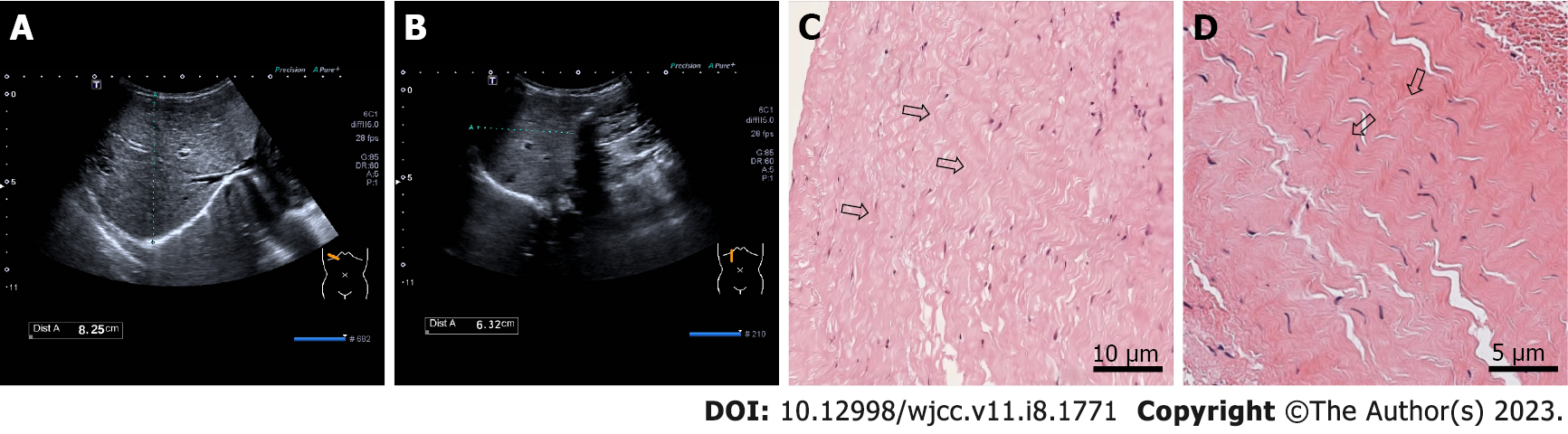
Chest X-ray demonstrated enhanced texture in both lungs and enlarged heart shadow, with a cardiothoracic ratio of about 0.62 (Figure 1A). Electrocardiogram (ECG) suggested left axis deviation, paroxysmal supraventricular tachycardia with a heart rate of 150/min and ST-T segment changes. Color doppler echocardiography (CDE) displayed enlarged left heart, thickening of the posterior wall of the left ventricle, minor amounts of mitral regurgitation, ventricular wall hypokinesis and decreased left heart function (Figure 1B-D). Cardiac magnetic resonance (CMR) revealed slight endocardial thickening of the left ventricle, minor thickening of the myocardium, and diffuse motion reduction of the ventricular wall with no apparent delayed enhancement abnormalities (Figure 1E and F). Color ultrasonography of the abdomen showed hepatic cyst (size: 0.93 cm × 0.75 cm), bilateral renal multiple cysts (the largest one size: 1.1 cm × 1.7 cm) and a markedly enlarged liver with a right oblique diameter of 8.25 cm and an inferior hepatic border 6.32 cm from the right inferior costal arch border of the midclavicular line (Figure 2A and B).
We arranged emergency consultations with multiple disciplines encompassing respiratory medicine, cardiology, hematology, and nursing. According to the clinical symptoms, signs, laboratory examinations, imaging examinations, relevant medical history, age of onset, and rapid development of the condition, the child was preliminarily diagnosed with pediatric AHF caused by EFE. And the result of the final endomyocardial biopsy report also confirmed our diagnosis (Figure 2C and D).
As per the standard pediatric advanced life support guidelines, the child was immediately resuscitated and monitored for vital signs, and administered oxygen by nasal cannula (oxygen flow rate: 6 L/min), sedated with chlorpromazine 1 mg/kg and promethazine 1 mg/kg by intramuscular (i.m.) injection, enhanced cardiac contractility with 10 μg/kg cedilanid by intravenous (i.v.) push (half of the total amount given for the first time and the remaining amount given in two divided doses, every 6 h), prompted diuresis with 0.1 mg/kg furosemide by i.v. push (given every 6 h), and relieved bron
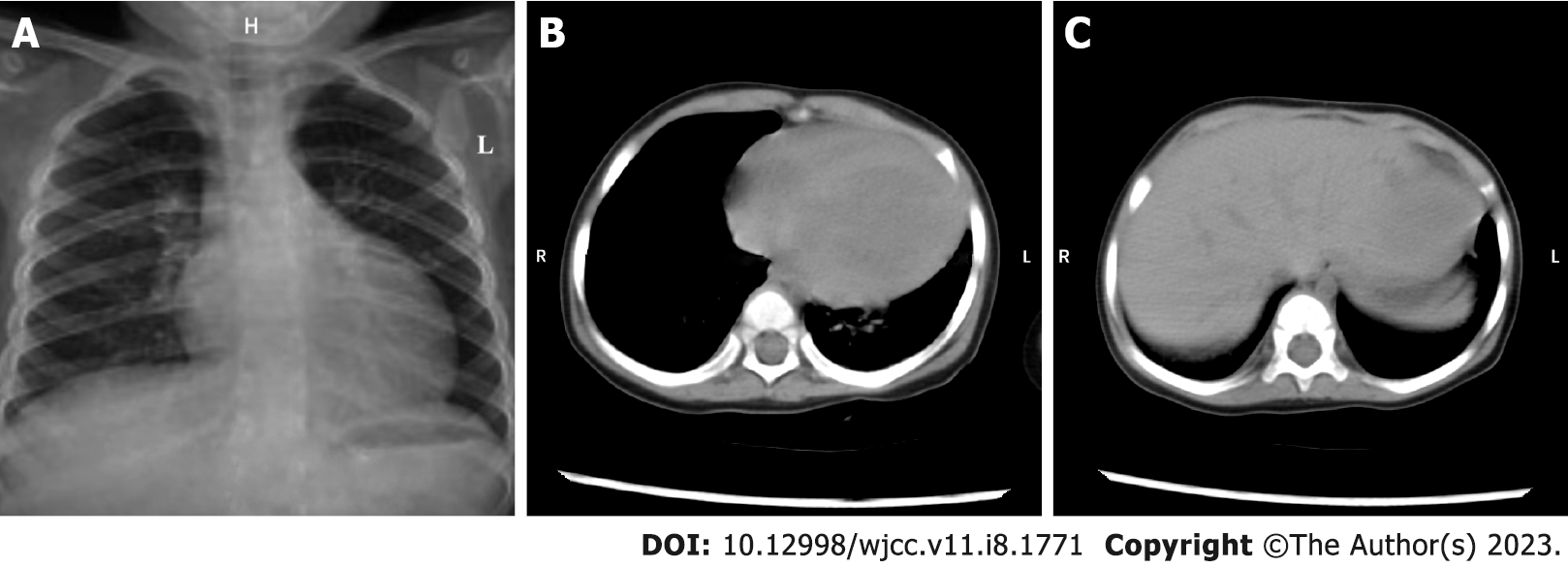
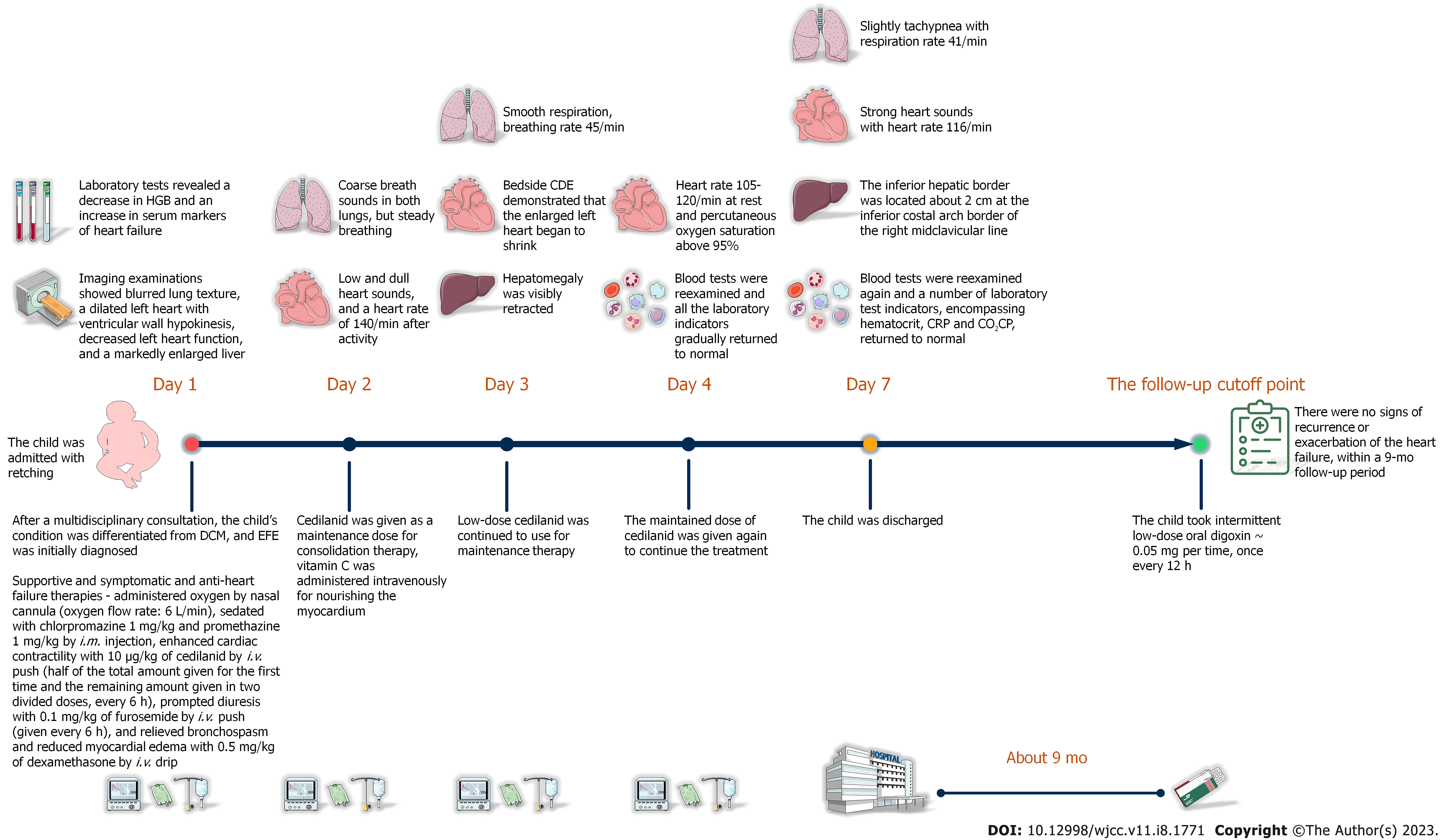
After correct, timely, and effective treatment, the child was discharged on day 7 after admission without any complications. The reexamination result of chest X-ray showed that the enlarged heart shadow was slightly retracted (Figure 3A). Similarly, the computed tomography (CT) reexamination results of thorax and abdomen revealed that the size of enlarged heart was marginally retracted, and the hepatomegaly was visibly retracted (Figure 3B and C). After the child was discharged from our hospital, her anemia improved dramatically with a combination of oral drug (ferrous gluconate: 3 mg per time, three times 1 d) and dietary iron supplementation manners. Except for this, the child took intermittent low-dose oral digoxin (0.05 mg per time, once every 12 h) with no signs of recurrence or exacerbation of the heart failure, within a 9-mo follow-up period. The timeline of the child’s visit is illustrated in Figure 4.
EFE is one of the important causes of heart failure in infants and toddlers. It occurs in 1/5000 live births and accounts for 1% to 2% of congenital heart disease, which has an unknown etiology and genetic characteristics, can occur in the absence of cardiac malformations[6,9]. The younger the age of onset of the children with EFE, the worse the prognosis and the higher the mortality[10]. Due to the lack of specificity in the presentation of this disease, it is prone to be confounded with pneumonia and myoca
Evidence-based medicine has indicated that AHF caused by EFE has a devastating course and severe prognosis[11]. The gold standard for the diagnosis and differential diagnosis of EFE is the endomy
Children younger than 1 year of age or even younger with primary EFE are more likely to be exactly diagnosed according to the age distribution of the onset of pediatric heart diseases[14-16]. Yet, in rare-onset children older than 1 year of age, the manifestation of EFE’s cardiac imaging examinations are almost identical to those of early onset DCM in infants and toddlers[17]. Both show a sudden dilatation of a single heart cavity (e.g., the left ventricle) on chest X-ray or chest CT. On a more accurate CDE inspection, both also exhibit the same ventricular wall hypokinesis, varying degrees of ventricular wall thickening, cardiac valve regurgitation, and left heart dysfunction. And more important, the blood-related tests of both present with assay indicators of AHF without any specificity. These things together make it easy for pediatric AHF caused by EFE to be masqueraded as DCM[10].
In our case, this 13-mo-old female child suddenly developed AHR without any triggers and without any history of infection, cardiac malformation, and other underlying cardiovascular disease. This is relatively rare in clinical practice, as many studies suggest that respiratory infections and congenital cardiovascular malformations are important triggers and potential initiators in the development of AHF caused by EFE[10]. We had considered pediatric DCM before the available endomyocardial biopsy result was returned, yet the echocardiographic findings just showed a diffuse and uniform slight thickening of the left ventricular endocardium with echo dense and enhanced, and a clear demarcation between ventricular endocardium and myocardium, a slight thickening of the myocardium, only a small amount of mitral regurgitation, a left ventricular end-diastolic dimension of 42 mm (< 50 mm). In parallel, both bright-blood T1-weighted image sequences and black-blood T2-weighted image sequences of the CMR examination of the child demonstrated a general thickening of the endocardium. In fact, for DCM, the possible thickening of the ventricular wall will not appear on the endocardium, but rather more on the thickening of the myocardium, and there are no non-functional fibrotic changes similar to EFE with this thickening. In such cases, when DCM presents with AHF, it is possible that the indicators of left ventricular systolic function on the CDE may not be much decreased. Furthermore, though the ECG manifestations of both DCM and EFE in infants and toddlers are left ventricular high voltage, poor R-wave progression, ST-T segment changes, and various different arrhythmias, the primary characteristics of the former are typically atrial tachycardia and atrial fibrillation. These are all distinctly different from EFE. Of note, the bedside CDE on the 3rd day of admission suggested that the dilated left heart had begun to show retraction, but this is almost impossible in DCM. Because despite the fact that DCM can be relieved, it is difficult to reduce the size of the heart to normal. Combined with the child’s age of onset, family history, and the findings of consultations with physicians from different departments, we unanimously agreed that EFE should be given a higher diagnostic priority. With more evidence pointing to EFE, we made a preliminary diagnosis of pediatric AHF caused by EFE prior to the result of the tissue biopsy was reported, which bought valuable time for early timely and correct intervention in the progression of the child’s disease. More importantly, both the rapid improvement of the child’s condition after treatment and the result of the endomyocardial biopsy report ultimately confirmed that our judgment was correct.
Unfortunately, no directed therapeutic approach for EFE is known since the rarity of the condition[18]. Yet, recovery of the enlarged heart and lost cardiac function in children with EFE is possible, and current clinically recommended treatment typically follows a standardized and vigorous decongestive therapy based on cardiac glycosides[19]. The child followed such a treatment principle from admission to the 9-mo follow-up cut-off point after discharge, and this hospital and domiciliary treatment has helped her well to maintain the sustainability of the decongestive therapy. In recent years, long-term follow-up studies have shown that bioimmunotherapy increases the clinical benefit of children with EFE and helps to improve endocardial hyperplasia and fibrosis[20]. Given the risk of future infection of the child’s hepatic and renal cysts and the underlying physical conditions, there was no opportunity to apply steroid hormones or other biological immunomodulators to her. However, we still recommend the use of biological immunomodulators for those children with EFE who have indications, because they may improve the prognosis of heart failure. Compared with EFE, while DCM is also treated in the acute exacerbation phase using a heart failure management approach, it is generally managed with long-term diuretics, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and beta-blockers to control its progression of CHF and ventricular remodeling due to the irreversibility of cardiac enlargement and decline in cardiac function[21]. Additionally, though bioimmunotherapy for adult DCM seems to benefit a proportion of patients, to date such treatments have not raised sufficient promise for pediatric DCM, and there is no evidence that steroid hormones use reduce mortality and morbidity in children with DCM[22]. As a result, the treatment regimens for EFE and DCM in infants and toddlers are not always identical, and early misdiagnosis improper treatment may have potentially unintended consequences for the children’s prognosis.
In infant and young childhood, EFE frequently presents with DCM and unfavorable progression, which makes it difficult for clinicians to distinguish these two diseases[6,23]. While the case we report is successful in differentiating EFE from DCM prior to the pathology report result, this is based more on extensive clinical work experience[24,25]. Limited by the objective one-sidedness of individual information, the early identification of such pediatric AHF caused by EFE masquerading as DCM still requires more clinical medical record information and data support.
Here, we report a case of pediatric AHF caused by EFE masquerading as DCM, which was accurately identified early in the course of the disease, for which we demonstrate that a primary diagnosis for EFE before the result of the endomyocardial biopsy is entirely possible. We hope that our report will give clinicians more decision support and attract sufficient attention when diagnosing similar diseases.
The information in this article does not necessarily reflect the government’s position or government policy, and no official endorsement should be inferred. We thank Hulunbuir City People’s Hospital for data and technical support. Also, a huge thanks to Miss. Li Qiu-Li for providing the audio, and I wish her all the best in her future wishes and happiness.
| 1. | Ren MY, Ren LH. Research progress of endocardial fibroelastosis[J]. Xinxueguan Kangfu Yixue Zazhi. 2020;29:723-726. [DOI] [Full Text] |
| 2. | Xu X, Friehs I, Zhong Hu T, Melnychenko I, Tampe B, Alnour F, Iascone M, Kalluri R, Zeisberg M, Del Nido PJ, Zeisberg EM. Endocardial fibroelastosis is caused by aberrant endothelial to mesenchymal transition. Circ Res. 2015;116:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Seki A, Patel S, Ashraf S, Perens G, Fishbein MC. Primary endocardial fibroelastosis: an underappreciated cause of cardiomyopathy in children. Cardiovasc Pathol. 2013;22:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Zhang Yong, Sun Lingli and Li Tao. Clinical features and outcomes of endocardial fibroelastosis in 60 children[J]. Zhongguo Fuyou Jiankang Yanjiu. 2014;5:790-792. [DOI] [Full Text] |
| 5. | Pan Y, Liu XY. Prevalence and predictors of left ventricular reverse remodeling after drug therapy in children with new-onset dilated cardiomyopathy[J]. Journal of Clinical Pediatrics. 2020;38:851-856. [DOI] [Full Text] |
| 6. | Luca AC, Lozneanu L, Miron IC, Trandafir LM, Cojocaru E, Pădureţ IA, Mihăilă D, Leon-Constantin MM, Chiriac Ş, Iordache AC, Ţarcă E. Endocardial fibroelastosis and dilated cardiomyopathy - the past and future of the interface between histology and genetics. Rom J Morphol Embryol. 2020;61:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Zhao Y, Wang LK, Eskin A, Kang X, Fajardo VM, Mehta Z, Pineles S, Schmidt RJ, Nagiel A, Satou G, Garg M, Federman M, Reardon LC, Lee SL, Biniwale R, Grody WW, Halnon N, Khanlou N, Quintero-Rivera F, Alejos JC, Nakano A, Fishbein GA, Van Arsdell GS, Nelson SF, Touma M. Recessive ciliopathy mutations in primary endocardial fibroelastosis: a rare neonatal cardiomyopathy in a case of Alstrom syndrome. J Mol Med (Berl). 2021;99:1623-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 8. | Jiao M, Han L, Wang HL, Jin M, Wang XF, Zheng K, Liang YM, Xiao YY. A long-term follow-up study on the clinical treatment of 75 cases with primary endocardial fibroelastosis [J]. Zhonghua Erke Zazhi. 2010;48:603-609. [DOI] [Full Text] |
| 9. | Ren SH, Qu R, Xiao YJ. Progress of endocardial fibroelastosis[J]. Zhongguo Xiaoer Jijiu Yixue. 2012;19:437-439. [DOI] [Full Text] |
| 10. | Arya SO, Karpawich PP, Gupta P, Buddhe S, Singh HR, Hussein Y, Gowda ST. Primary endocardial fibroelastosis presenting in a young child as incessant ventricular tachycardia and dilated cardiomyopathy. Tex Heart Inst J. 2012;39:714-718. [PubMed] |
| 11. | Jarrar M, Vaksmann G, Godart F, Rey C, Dupuis C. [Natural history and prognostic factors in primary endocardial fibroelastosis in infants]. Arch Mal Coeur Vaiss. 1994;87:653-656. [PubMed] |
| 12. | Narayan R, Menahem S, Chow CW, Dennett X. Endomyocardial biopsy in infants and children with cardiomyopathy. Clin Cardiol. 1991;14:903-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Ponce CC, Dinamarco PV. Primary endocardial fibroelastosis and nonimmune hydrops fetalis: case report with autopsy. Fetal Pediatr Pathol. 2015;34:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Moons P, Bratt EL, De Backer J, Goossens E, Hornung T, Tutarel O, Zühlke L, Araujo JJ, Callus E, Gabriel H, Shahid N, Sliwa K, Verstappen A, Yang HL, Thomet C. Transition to adulthood and transfer to adult care of adolescents with congenital heart disease: a global consensus statement of the ESC Association of Cardiovascular Nursing and Allied Professions (ACNAP), the ESC Working Group on Adult Congenital Heart Disease (WG ACHD), the Association for European Paediatric and Congenital Cardiology (AEPC), the Pan-African Society of Cardiology (PASCAR), the Asia-Pacific Pediatric Cardiac Society (APPCS), the Inter-American Society of Cardiology (IASC), the Cardiac Society of Australia and New Zealand (CSANZ), the International Society for Adult Congenital Heart Disease (ISACHD), the World Heart Federation (WHF), the European Congenital Heart Disease Organisation (ECHDO), and the Global Alliance for Rheumatic and Congenital Hearts (Global ARCH). Eur Heart J. 2021;42:4213-4223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48:455-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 852] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 16. | Liu Y, Chen S, Zühlke L, Babu-Narayan SV, Black GC, Choy MK, Li N, Keavney BD. Global prevalence of congenital heart disease in school-age children: a meta-analysis and systematic review. BMC Cardiovasc Disord. 2020;20:488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Writing Group; Sachdeva R, Valente AM, Armstrong AK, Cook SC, Han BK, Lopez L, Lui GK, Pickard SS, Powell AJ; Rating Panel, Bhave NM, Sachdeva R, Valente AM, Pickard SS, Baffa JM, Banka P, Cohen SB, Glickstein JS, Kanter JP, Kanter RJ, Kim YY, Kipps AK, Latson LA, Lin JP, Parra DA, Rodriguez FH 3rd, Saarel EV, Srivastava S, Stephenson EA, Stout KK, Zaidi AN; Solution Set Oversight Committee, Gluckman TJ, Aggarwal NR, Bhave NM, Dehmer GJ, Gilbert ON, Kumbhani DJ, Price AL, Winchester DE, Gulati M; Appropriate Use Criteria Task Force, Dehmer GJ, Doherty JU, Bhave NM, Daugherty SL, Dean LS, Desai MY, Gillam LD, Mehrotra P, Sachdeva R, Winchester DE. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 Appropriate Use Criteria for Multimodality Imaging During the Follow-Up Care of Patients With Congenital Heart Disease: A Report of the American College of Cardiology Solution Set Oversight Committee and Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. J Am Soc Echocardiogr. 2020;33:e1-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Ozdemir D, Cortopassi IO, McNamara RL. An illustrative case of endocardial fibroelastosis and recalcitrant intracardiac thrombosis: A case report. Thromb J. 2019;17:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Manning JA, Sellers FJ, Bynum RS, Keith JD. The Medical Management of Clinical Endocardial Fibroelastosis. Circulation. 1964;29:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Jiao M, Han L, Wang HL, Jin M, Wang XF, Zheng K, Liang YM, Xiao YY. [A long-term follow-up study on the clinical treatment of 75 cases with primary endocardial fibroelastosis]. Zhonghua Er Ke Za Zhi. 2010;48:603-609. [PubMed] |
| 21. | Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet. 2017;390:400-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 466] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 22. | Soares P, Rocha G, Pissarra S, Soares H, Flôr-de-Lima F, Costa S, Moura C, Dória S, Guimarães H. Neonatal dilated cardiomyopathy. Rev Port Cardiol. 2017;36:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Shimada S, Robles C, Illigens BM, Casar Berazaluce AM, del Nido PJ, Friehs I. Distention of the Immature Left Ventricle Triggers Development of Endocardial Fibroelastosis: An Animal Model of Endocardial Fibroelastosis Introducing Morphopathological Features of Evolving Fetal Hypoplastic Left Heart Syndrome. Biomed Res Int. 2015;2015:462469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Raboisson MJ, Fouron JC, Sonesson SE, Nyman M, Proulx F, Gamache S. Fetal Doppler echocardiographic diagnosis and successful steroid therapy of Luciani-Wenckebach phenomenon and endocardial fibroelastosis related to maternal anti-Ro and anti-La antibodies. J Am Soc Echocardiogr. 2005;18:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Weiner Z, Shalev E. Doppler fetal echocardiography in endocardial fibroelastosis. Obstet Gynecol. 2001;98:933-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Batta A, India; Gupta P, United States; Ong H, Malaysia S-Editor: Liu XF L-Editor: Filipodia P-Editor: Liu XF