Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1761
Peer-review started: October 9, 2022
First decision: October 24, 2022
Revised: November 19, 2022
Accepted: January 28, 2023
Article in press: January 28, 2023
Published online: March 16, 2023
Processing time: 148 Days and 9.6 Hours
The coronavirus disease 2019 (COVID-19) pandemic has become a major health concern worldwide. In that context, the understanding of epidemiological and clinical features associated with the disease and its severity is crucial for the establishment of strategies aimed at disease control and remedy.
To describe epidemiological features, signs, symptoms, and laboratory findings among severely ill COVID-19 patients from an inten
This is a prospective single-center study that evaluated 115 patients admitted to the intensive care unit in a northeastern Brazilian hospital.
The patients had a median age of 65.60 ± 15.78 years. Dyspnea was the most frequent symptom, affecting 73.9% of the patients, followed by cough (54.7%). Fever was reported in approximately one-third of patients and myalgia in 20.8% of the patients. At least two comorbidities were found in 41.7% of the patients, and hypertension was the most prevalent (57.3%). In addition, having two or more comorbidities was a predictor of mortality, and lower platelet count was positively associated with death. Nausea and vomiting were two symptoms that were predictors of death, and the presence of a cough was a protective factor.
This is the first report of a negative correlation between cough and death in severely ill severe acute respiratory syndrome coronavirus 2-infected individuals. The associations between comorbidities, advanced age, and low platelet count and the outcomes of the infection were similar to the results of previous studies, highlighting the relevance of these features.
Core Tip: This is a prospective study carried out in a hospital in Brazil with 115 patients admitted to the intensive care unit with a positive diagnosis for severe acute respiratory syndrome coronavirus 2. The epidemiological features, signs, symptoms, and laboratory findings among severely ill coronavirus disease 2019 patients and the predictive factors for disease outcomes were evaluated. This is the first report of a negative correlation between cough and death in severely ill severe acute respiratory syndrome coronavirus 2-infected individuals.
- Citation: Pinheiro FD, Lopes LW, Dórea RSDM, Araújo GRL, Silva FAFD, de Brito BB, Cordeiro Santos ML, Júnior GMS, de Lorenzo Barcia MTA, Marques RA, Botelho AB, Dantas ACS, Costa DT, Teixeira AF, Souza CL, Marques LM, Campos GB, Oliveira MV, de Magalhães Queiroz DM, Freire de Melo F. Epidemiological and clinical characteristics of COVID-19 in a Brazilian public hospital. World J Clin Cases 2023; 11(8): 1761-1770
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1761.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1761
Since its first records at the end of 2019 in Wuhan, capital city of Hubei province, China, the coronavirus disease 2019 (COVID-19) has caused numerous challenges with regard to a better understanding of its immunology, pathophysiology, clinical manifestations, diagnosis, and treatment[1]. The pathogen was identified as a novel enveloped RNA betacoronavirus that has a phylogenetic similarity to the severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1). In that context, the so-called SARS-CoV-2 has become a global health concern, and on March 11, 2020 the World Health Organization declared the COVID-19 outbreak a pandemic[2].
The incubation period for COVID-19 is generally within 14 d following exposure, and the onset of symptoms occurs 4-5 d after inoculation[3]. In a study including 1084 COVID-19 patients from China, the median period of incubation was 7.8 d, with 5% to 10% of patients experiencing the first symptoms after 14 d of exposure[3]. Recent meta-analyses about the topic corroborate this finding, highlighting averages of 5.1 and 5.6 d for disease incubation[4,5]. Among those affected by the disease, about 17.9% to 33.3% remain asymptomatic, highlighting the difficult control of the transmission of the disease[6].
Pneumonia is the most frequent serious finding, and it usually occurs with fever, cough, expectoration, and dyspnea. Other common symptoms are myalgia, diarrhea, anosmia and dysgeusia, and upper respiratory tract symptoms[7-9]. Of note, dysgeusia and anosmia disorders are more common in COVID-19 than in other viral infections[10]. The prevalence of SARS-CoV-2 infection-related manifestations can vary depending on the level of severity of the illness. In this sense, the prevalence of fever was higher among hospitalized patients in a trial with 1099 patients when compared to non-hospitalized COVID-19 patients. Only 44% of the patients had fever at admission, whereas 89% were febrile during hospitalization[11]. When compared to severe and mild disease groups, patients with moderate involvement had higher rates of dysgeusia and anosmia (88.70% among patients with moderate disease and 45.83% in severely ill individuals)[12].
As prognostic markers of the disease, clinical and epidemiological features have been reported as predictors of severity among patients in hospital care. While dyspnea is also an important finding for a poor prognosis, studies suggest that peripheral oxygen saturation is the respiratory factor to be assessed, as it excludes the subjective factor from the assessment. These studies suggest that an oxygen saturation below 92% was associated with a poor prognosis. Furthermore, it takes about 5 d for the patient to develop dyspnea after the first symptoms, and it can be quickly followed by acute respiratory distress syndrome. In this sense, adequate monitoring of saturation levels in COVID-19 patients is indicated[13].
Efforts have been directed towards the understanding of the relationship between comorbidities and the severity and mortality in SARS-CoV-2 infection. In this sense, studies have shown that diabetes, hypertension, obesity, and cardiovascular disease are important risk factors for severity and mortality[14-16]. In addition, various abnormalities in serum laboratory parameters have been associated with COVID-19, and some of them have been related to an increased risk of mortality[17,18].
To date, SARS-CoV-2 has infected almost 200 million people worldwide, from which 4 million died[2]. In Brazil, 30639130 cases of the disease have been registered so far, with 664641 deaths associated with the disease. The northeast region of Brazil, in which the current study was carried out, has reported 6256874 cases, with the occurrence of 128829 deaths among them[19]. The aim of this study was to describe epidemiological features, signs, symptoms, and laboratory findings at the moment of intensive care unit (ICU) admission among severely ill COVID-19 patients in a city from northeastern Brazil. We also evaluated predictive factors for disease outcomes in the study sample. This study showed some differences in the clinical and epidemiological profiles of COVID-19 patients when compared with previous studies, mainly regarding the frequency of symptoms. We report, for the first time, a negative association between cough and death among severely ill SARS-CoV-2-infected patients.
This single-center prospective study included 225 consecutive COVID-19 patients admitted to the ICU, General Hospital of Vitória da Conquista city, Bahia State, Brazil, from July 30, 2020 to August 28, 2021. The Vitória da Conquista General Hospital is a regional hospital in the third largest city in the Bahia State, providing an extensive multidisciplinary teaching service and attends to a large number of highly complex cases from more than 70 surrounding cities. All included patients had SARS-CoV-2 infection confirmed by real-time (RT-) PCR of nasopharyngeal swab specimens. Subjects were selected according to the criteria for ICU admission recommended by the Bahia State Department of Health. This study was approved by the Ethics Committee of the Brazilian National Research Ethics Commission (No. 4.155.234), with an informed consent form obtained from the relatives responsible for the enrolled patients.
Epidemiological, clinical, and laboratory data of the included patients were obtained both at admission and during hospitalization. Clinical outcomes were monitored in the inpatient system, which indicated discharge, transfer, or death. Data collection comprised clinical, epidemiological, and demographic data as well as exposure history to infected individuals, date of symptom onset, RT-PCR result, and presence of comorbidities. Comorbidities included: (1) Hypertension; (2) Diabetes; (3) Cardiovascular disease; (4) Chronic kidney disease; (5) Obesity; (6) Chronic obstructive pulmonary disease; and (7) Autoimmune diseases.
Blood samples were obtained from the patients for assessment of white blood cell [reference values: (4-10) × 10³ mm³] and platelet [reference values: (150-450) × 10³ g/L] count, hemoglobin levels [reference values 11.5-18.0 mg/dL (female) or 13.0-18.0 mg/dL (male)], C-reactive protein (reference value: ≤ 6 g/L), serum sodium (reference values: 130-150 mEq/L), aspartate aminotransferase (reference values: 10-37 U/I), alanine aminotransferase (reference values: 10-45 U/I), and lactic dehydrogenase (reference values: 120-246 U/I). All laboratory evaluations were completed by conventional methods.
Statistical analysis data were analyzed by the public domain statistical software Epi Info 7 and the SPSS statistical software package version 26.0 (SPSS Inc., Chicago, IL, United States). For the comparisons, Kolgomorov-Smirnov or Shapiro-Wilk test were used to assess the normality of the data as indicated. Two-tailed student’s t test or Mann-Whitney U test as well as χ2 test with Yates’ correction or Fisher’s exact test were employed as indicated. The level of significance was set at P ≤ 0.05. Forward binary logistic regression was performed during analysis.
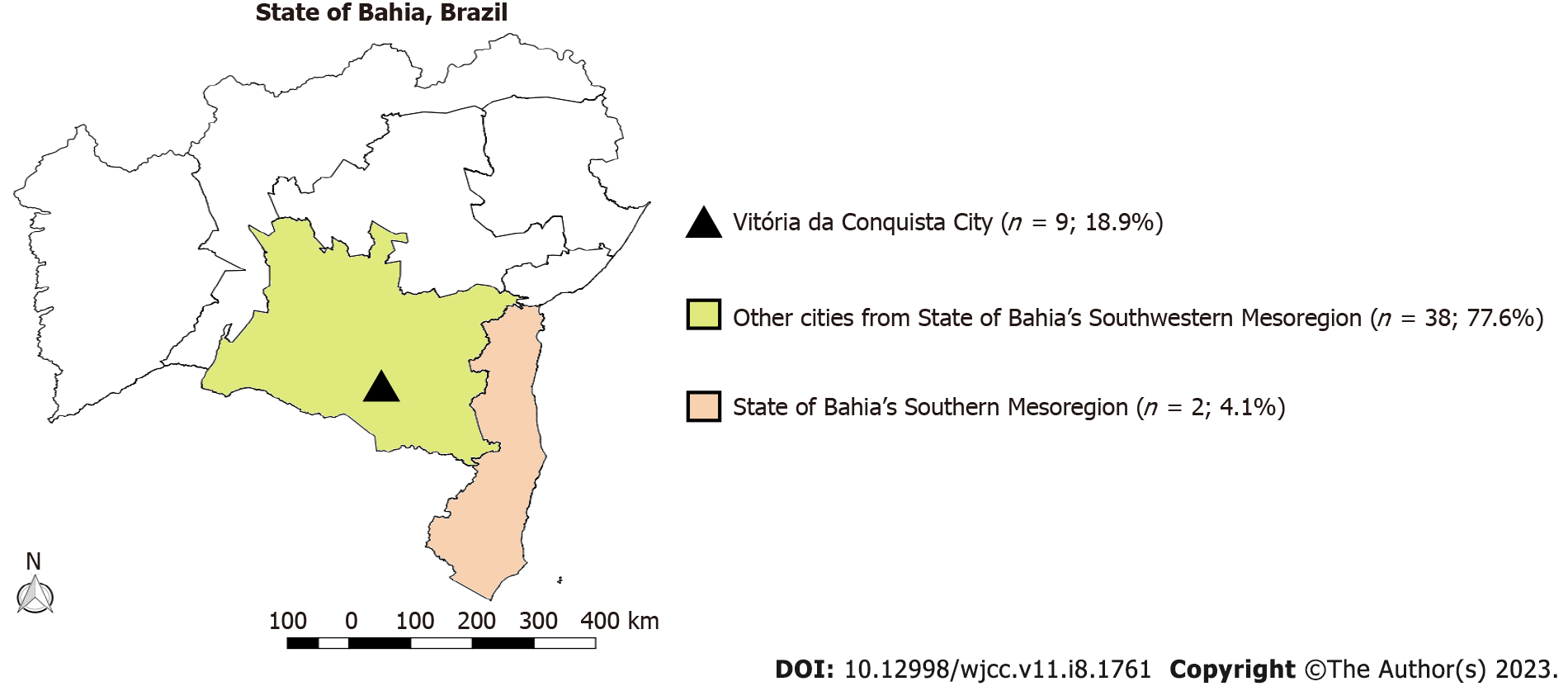
All subjects were positive for SARS-CoV-2 RNA detected by RT-PCR. Their mean age [standard deviation (SD)] was 65.60 ± 15.78 years. Patients included 69 (60%) males (mean age = 67.09, SD = 14.09) and 46 (40%) females (mean age = 63.37, SD = 17.96). A total of 38 patients (33.1%) were from Vitória da Conquista and 36 from adjacent cities (Figure 1).
The most frequent clinical symptom was dyspnea, present in more than two-thirds of the included patients (85 patients, 73.9%). Cough was the second most frequent symptom (63 patients, 54.7%) followed by fever (38 patients, 33.0%), myalgia (24 patients, 20.8%), diarrhea (9 patients, 7.8%), nausea (8 patients, 6.9%), vomiting (7 patients, 6.0%), headache, dysgeusia, and anosmia (5 patients, 4.3% for each symptom). Regarding comorbidities, 66 patients (57.3%) had hypertension, 32 patients (27.8%) had diabetes, 23 patients (20.0%) had cardiovascular disease, 15 patients (13.0%) had obesity, 12 patients (10.4%) had chronic kidney disease, 4 patients (3.4%) had chronic obstructive pulmonary disease, and 1 patient (0.8%) had an autoimmune disease. In the group of patients from whom the information was obtained, no comorbidities were present in 15 patients, whereas 36 and 29 patients had one and two comorbidities, respectively. The others had either three (n = 13) or four (n = 5) comorbidities.
Respiratory rate was increased in the majority of patients (n = 39; 52.0%), whereas 2 patients (2.7%) presented with bradypnea and 34 patients (45.3%) were eupneic. The white blood cell count was elevated in 61.9% of the patients (n = 60), and leukopenia was observed in 5 patients (5.2%) (reference value = 5000-11000 leukocytes/mm³). The inflammatory marker C-reactive protein was found to be altered in 74 patients (96.10%; reference value ≤ 3 mg/L). Regarding hepatic lesion markers, aspartate aminotransferase and alanine aminotransferase levels were elevated in 13 patients (54.2%) and 15 patients (68.2%), respectively.
When the group of patients who died were compared with those who had a favorable disease outcome, increasing age was significantly associated with death (69.28 ± 15.16 vs 61.54 ± 17.08, P = 0.02). The presence of two or more comorbidities was a positive predictor of mortality (P = 0.01). The presence of at least two concomitant illnesses was observed in 60.9% of the patients who died compared with the other group (39.1%). No association was observed for gender (female, male, P = 0.16) and a plethora of patient factors including demographic, clinical, immunologic, hematological, biochemical, and radiographic findings, may be of utility to clinicians to predict COVID-19 severity and mortality. In addition, the mean platelet count among patients who died was 141.49 × 10³ g/L. A statistically significant relationship was observed between the lower platelet count and death (P ≤ 0.001). No statistically significant results were obtained when other comorbidities and laboratory results were assessed.
When evaluating the main outcomes and the symptoms of the patients in the ICU, we found that nausea (P = 0.02) and vomiting (P = 0.05) were predictors of death, and the presence of a cough was a protective factor. There was no statistically significant association between other symptoms and the evaluated outcomes. Tables 1-4 summarize the findings of this study.
| Prevalence of outcomes/destinations | n (%) |
| Discharge or transfer from the ICU | 48 (47.5) |
| Death | 47 (46.5) |
| Transfer | 6 (5.9) |
| Hematologic values | With death, mean ± SD | Without death, mean ± SD | P value |
| Hemoglobin, mg/dL | 11.31 ± 2.96 | 11.70 ± 2.79 | 0.55 |
| Leukocytes, mm³ | 14.45 ± 6.88 | 14.29 ± 8.71 | 0.92 |
| CRP, g/L | 143.46 ± 150.76 | 147.53 ± 201.60 | 0.77 |
| Platelets, g/L | 141.49 ± 9.55 | 154.88 ± 72.91 | < 0.001 |
| Comorbidities | Death, n | Discharged, n | P value | Death, mean ± SD | Discharged median ± SD |
| Hypertension | 33 (53.2) | 29 (46.8) | 0.15 | ||
| Diabetes | 18 (58.1) | 13 (41.9) | 0.14 | ||
| Cardiovascular disease | 11 (50.0) | 11 (50.0) | 0.54 | ||
| Obesity | 6 (50.0) | 6 (50.0) | 0.58 | ||
| Chronic kidney disease | 8 (66.7) | 4 (33.3) | 0.15 | ||
| Chronic obstructive pulmonary disease | 2 (50.0) | 2 (50.0) | 0.67 | ||
| Autoimmune diseases | 0 (0) | 1 (100) | 0.51 | ||
| At least 2 comorbidities | 28 (60.9) | 18 (39.1) | 0.01 | ||
| Laboratory tests | |||||
| Hemoglobin, mg/dL | 0.55 | 11.31 ± 2.96 | 11.70 ± 2.79 | ||
| Leukogram, mm³ | 0.93 | 14.448 ± 6.878 | 14.292 ± 8.705 | ||
| CRP, g/L | 0.18 | 143.46 ± 150.76 | 147.53 ± 201.60 | ||
| Platelets, g/L | < 0.001 | 141.49 ± 9.55 | 154.88 ± 72.91 | ||
| Serum sodium, mEq/L | 0.35 | 143.00 ± 9.79 | 140.62 ± 10.70 | ||
| AST, U/L | 0.22 | 54.20 ± 32.41 | 92.83 ± 24.10 | ||
| ALT, U/L | 0.7 | 26.00 ± 13.78 | 81.90 ± 90.50 | ||
| Lactic dehydrogenase, U/L | 486.00 ± 48.08 | 274 | 0.17 |
| Symptom | Death | Discharged | P value |
| Dyspnea | 36 (46.8) | 41 (53.2) | 0.09 |
| Cough | 23 (40.4) | 34 (59.6) | 0.01 |
| Fever | 14 (41.2) | 20 (58.8) | 0.15 |
| Myalgia | 9 (42.9) | 12 (57.1) | 0.30 |
| Diarrhea | 6 (66.6) | 3 (33.3) | 0.24 |
| Headache | 4 (44.0) | 5 (55.0) | 0.02 |
| Nausea | 7 (87.5) | 1 (12.5) | 0.02 |
| Vomiting | 6 (85.7) | 1 (14.3) | 0.05 |
| Chills | 1 (20.0) | 4 (80.0) | 0.18 |
| Taste changes | 1 (50.0) | 1 (50.0) | 0.75 |
| Anosmia | 1 (50.0) | 1 (50.0) | 0.75 |
This study evaluated the clinical and epidemiological profiles of hospitalized individuals from an ICU in a Brazilian public hospital. Some symptom data found in this investigation diverge from the pattern observed in previous studies. In an investigation in China, fever was the most prevalent symptom among the 1099 laboratory-confirmed COVID-19 patients at admission (43.8%) or during hospitalization (88.7%)[20], in contrast to the approximately one-third of patients observed in this study. Furthermore, a high prevalence of fever (88.7% of the 656 patients) was also identified in a meta-analysis study[21].
Notably, cough was not associated with poor outcomes among the severely ill COVID-19 patients we evaluated. To the best of our knowledge, this is the first study to demonstrate a negative association between cough and death in patients infected with SARS-CoV-2 in the ICU, in contrast a meta-analysis study evaluating 10014 patients with COVID-19[22]. In addition, a high prevalence of this symptom was observed in the present study, corroborating an investigation from the Center for Disease Control and Prevention, which evaluated 373883 individuals and found that the aforementioned symptom was the most common manifestation in COVID-19 (50%)[23].
Seeking explanations for this finding, the hypothesis of coughing as an alarm signal was raised. This sign would make patients seek emergency care earlier when compared to critically ill patients without cough. It is plausible that the patients without cough but desaturation and severe condition did not understand that they should be evaluated by a doctor. During the peak of the epidemic, health systems were overcrowded, and government entities were oriented on a national network to seek assistance only in a serious condition. Faced with these situations, we may be dealing with a needy population with a low availability of health services and with government guidelines to avoid emergency services. This culminates in critically ill patients staying at home for too long and arriving at hospitals in very serious conditions. This could also explain the high mortality rates in the study.
Nausea and vomiting were positively associated with death in the present study in contrast to previous investigations that have associated gastrointestinal symptoms with milder SARS-CoV-2 infections. The frequency of gastrointestinal symptoms found here was lower than in other surveys. In a systematic review performed by our group that included 43 studies and 18246 COVID-19 patients, the prevalence of diarrhea was higher than in this investigation[8]. The most common symptom in our investigation was dyspnea, a pivotal finding among severely ill COVID-19 patients. In a meta-analysis study evaluating 1813 patients, only dyspnea was able to predict severe disease and ICU admission[24]. Headache was more infrequent in our patients than in the aforementioned investigation by Center for Disease Control and Prevention[23], likely due to the administration of analgesics in the emergency room or in other hospitals before admission to the ICU. Similarly, there was a low prevalence of anosmia and taste change, which was expected because these symptoms have been observed in better prognoses[21].
Leukocytosis was found in most individuals included in this investigation, which was in agreement with the higher frequencies of this manifestation reported among critically ill individuals[25,26]. Red blood cell count abnormality was another relevant laboratory finding in this study, with the detection of hemoglobin levels below 11 g/dL in 38.7% of the patients, also in accordance with previous studies showing reduced levels of serum hemoglobin in SARS-CoV-2-infected individuals with severe disease[21]. The levels of serum lactate dehydrogenase were found to be increased in our samples, a common finding among COVID-19 patients[27]. Moreover, serum sodium levels were increased in the patients we evaluated. This was similar to the increased odds of in-hospital death among hypernatremic individuals compared to normonatremic persons[28].
Furthermore, a decreased platelet count was positively associated with mortality in our investigation. Previous studies suggested that the occurrence of thrombocytopenia in SARS-CoV-2 infection can be associated with serious conditions such as intravascular coagulation and sepsis[29]. Another cause of low platelet count in patients with COVID-19 may be drug-induced[3].
This study demonstrated a higher mortality rate among critically ill SARS-CoV-2-infected individuals than most studies have. Of note, ICU mortality due to COVID-19 around the world has ranged from 20% to 62%[30-32]. The difficulties faced by the Brazilian public health system to make enough ICU beds available for COVID-19 patients during the pandemics should be emphasized. In the period from 2020 to 2021, there were approximately 70 ICU beds in the study city, which is the main healthcare center in a region that embraces 2 million inhabitants[33]. This scenario contributed to the occurrence of delays in providing adequate life support for critically ill individuals, which potentially contributed to the high mortality rate observed in this study.
A high prevalence of comorbidities was observed in our patients. Hypertension was the most prevalent comorbidity, which was in agreement with a meta-analysis study. Moreover, the presence of two or more comorbidities as well as older age were associated with higher mortality in our study, similarly to a previous study[34]. The circulating variants of SARS-CoV-2 is an important issue to be accounted for when considering the clinical manifestations of COVID-19 among individuals from a given geographical area[35]. Although the differentiation of the SARS-CoV-2 strains that infected the patients could not be performed in this study, the co-circulation of 13 different strains has been reported to date in the Brazilian state where the study was carried out. The first subline identified was B.1.1.162[36]. Of note, the variants P.1 and P.2, which have been identified in the Brazilian cities Manaus and Rio de Janeiro, respectively, have been detected in the aforementioned state as well[37,38]. Lastly, the Peruvian lineage C.14 was also detected in the study region after its introduction through a ship traveler. Until February 2021, the variants circulating in Bahia state were limited to the A, B, C and P types[39,40].
There are a few limitations to this study. This was an epidemiological investigation conducted at a single health care system with a limited number of participants and in a confined geographic area, thus limiting the generalizability of the results. Future research should be made to identify and predict further factors associated with mortality in COVID-19 populations admitted to the ICU.
This study revealed differences in the clinical profile of COVID-19 patients when compared with previous studies, such as the observation of a negative association between cough and death in severely ill individuals. On the other hand, the associations between comorbidities, advanced age, and low platelet count and the outcomes of the infection were similar to the results of previous studies, highlighting the relevance of these features. Further investigations are needed in order to better characterize risks of poor outcomes among severely ill COVID-19 patients.
Coronavirus disease 2019 (COVID-19) has been a health concern around the world since it was first identified in 2019. An understanding of the epidemiological and clinical features related to the infection is very important for the development of prevention and treatment of the disease.
There is a lack of studies evaluating the clinical and epidemiological characteristics of patients with severe COVID-19 in the study region. Moreover, the data regarding the infection features in that population can contribute to the understanding of the disease.
The objectives were to describe epidemiological characteristics, signs, symptoms, and laboratory findings in individuals with severe COVID-19 from an intensive care unit in the state of Bahia in northeastern Brazil and to analyze predictive features for the disease outcomes.
In this prospective, single-center study, 115 patients with severe COVID-19 admitted to an intensive care unit in northeastern Brazil were evaluated. Epidemiological, clinical, and laboratory data of the included patients were obtained. Clinical outcomes were monitored in the inpatient system.
The patients had a median age of 65.60 ± 15.78 years. Dyspnea was the most frequent symptom, affecting 73.9% of the patients, followed by cough (54.7%). Fever was reported in approximately one-third of patients and myalgia in 20.8% of patients. At least two comorbidities were found in 41.7% of the patients, and hypertension was the most prevalent one (57.3%). In addition, having two or more comorbidities was a predictor of mortality, and lower platelet count was positively associated with death as well. Nausea and vomiting were predictors of death, and the presence of a cough was a protective factor.
This is the first report of a negative correlation between cough and death in severely ill severe acute respiratory syndrome coronavirus 2-infected individuals. The associations between comorbidities, advanced age, low platelet count, and the outcomes of the infection were similar to the results of previous studies, highlighting the relevance of these features.
In future analyses, we will evaluate the role of various cytokine profiles in the inflammatory response in the population of this study. Moreover, the relationship between comorbidities and infection outcomes might be further explored in the next steps of the research.
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30475] [Article Influence: 5079.2] [Reference Citation Analysis (13)] |
| 2. | World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19. [cited 16 July 2022]. Available from: https://vietnam.un.org/en/38806-who-director-generals-opening-remarks-media-briefing-covid-19. |
| 3. | Qin J, You C, Lin Q, Hu T, Yu S, Zhou XH. Estimation of incubation period distribution of COVID-19 using disease onset forward time: a novel cross-sectional and forward follow-up study. medRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Alene M, Yismaw L, Assemie MA, Ketema DB, Gietaneh W, Birhan TY. Serial interval and incubation period of COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 5. | McAloon C, Collins Á, Hunt K, Barber A, Byrne AW, Butler F, Casey M, Griffin J, Lane E, McEvoy D, Wall P, Green M, O'Grady L, More SJ. Incubation period of COVID-19: a rapid systematic review and meta-analysis of observational research. BMJ Open. 2020;10:e039652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 383] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 6. | Quesada JA, López-Pineda A, Gil-Guillén VF, Arriero-Marín JM, Gutiérrez F, Carratala-Munuera C. [Incubation period of COVID-19: A systematic review and meta-analysis]. Rev Clin Esp (Barc). 2021;221:109-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). 2022 Oct 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 8. | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 886] [Cited by in RCA: 854] [Article Influence: 142.3] [Reference Citation Analysis (0)] |
| 9. | Silva FAFD, Brito BB, Santos MLC, Marques HS, Silva Júnior RTD, Carvalho LS, Vieira ES, Oliveira MV, Melo FF. COVID-19 gastrointestinal manifestations: a systematic review. Rev Soc Bras Med Trop. 2020;53:e20200714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 10. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4761] [Cited by in RCA: 4765] [Article Influence: 794.2] [Reference Citation Analysis (1)] |
| 11. | Mullol J, Alobid I, Mariño-Sánchez F, Izquierdo-Domínguez A, Marin C, Klimek L, Wang DY, Liu Z. The Loss of Smell and Taste in the COVID-19 Outbreak: a Tale of Many Countries. Curr Allergy Asthma Rep. 2020;20:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Eastin C, Eastin T. Clinical Characteristics of Coronavirus Disease 2019 in China. J Emerg Med. 2020;58:711-712. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Mendonça CV, Mendes Neto JA, Suzuki FA, Orth MS, Machado Neto H, Nacif SR. Olfactory dysfunction in COVID-19: a marker of good prognosis? Braz J Otorhinolaryngol. 2022;88:439-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Jang JG, Hur J, Choi EY, Hong KS, Lee W, Ahn JH. Prognostic Factors for Severe Coronavirus Disease 2019 in Daegu, Korea. J Korean Med Sci. 2020;35:e209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Cai Z, Yang Y, Zhang J. Obesity is associated with severe disease and mortality in patients with coronavirus disease 2019 (COVID-19): a meta-analysis. BMC Public Health. 2021;21:1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 16. | Mahamat-Saleh Y, Fiolet T, Rebeaud ME, Mulot M, Guihur A, El Fatouhi D, Laouali N, Peiffer-Smadja N, Aune D, Severi G. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open. 2021;11:e052777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 17. | de Almeida-Pititto B, Dualib PM, Zajdenverg L, Dantas JR, de Souza FD, Rodacki M, Bertoluci MC; Brazilian Diabetes Society Study Group (SBD). Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 18. | Velavan TP, Meyer CG. Mild vs severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 342] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 19. | Tjendra Y, Al Mana AF, Espejo AP, Akgun Y, Millan NC, Gomez-Fernandez C, Cray C. Predicting Disease Severity and Outcome in COVID-19 Patients: A Review of Multiple Biomarkers. Arch Pathol Lab Med. 2020;144:1465-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 20. | Coronavírus Brasil. Painel Coronavirus - Brasil. [cited 20 July 2022]. Available from: https://covid.saude.gov.br. |
| 21. | Guan W, Ni Z, Yu Hu, Liang W, Ou C, He J, Liu L, Shan H, Lei C, Hui DSC, Du B, Li L, Zeng G, Yuen KY, Chen R, Tang C, Wang T, Chen P, Xiang J, Li S, Wang J, Liang Z, Peng Y, Wei L, Liu Y, Hu Y, Peng P, Liu J, Chen Z, Li G, Zheng Z, Qiu S, Luo J, Ye C, Zhu S, Zhong N. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 19019] [Article Influence: 3169.8] [Reference Citation Analysis (9)] |
| 22. | Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK, Franco-Paredes C, Henao-Martinez AF, Paniz-Mondolfi A, Lagos-Grisales GJ, Ramírez-Vallejo E, Suárez JA, Zambrano LI, Villamil-Gómez WE, Balbin-Ramon GJ, Rabaan AA, Harapan H, Dhama K, Nishiura H, Kataoka H, Ahmad T, Sah R; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19). Electronic address: https://www.lancovid.org. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1647] [Cited by in RCA: 1440] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 23. | Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: A meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6:e05684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | Czeisler MÉ, Marynak K, Clarke KEN, Salah Z, Shakya I, Thierry JM, Ali N, McMillan H, Wiley JF, Weaver MD, Czeisler CA, Rajaratnam SMW, Howard ME. Delay or Avoidance of Medical Care Because of COVID-19-Related Concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1250-1257. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 533] [Cited by in RCA: 1102] [Article Influence: 183.7] [Reference Citation Analysis (0)] |
| 25. | Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65:533-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 26. | Sayad B, Afshar ZM, Mansouri F, Rahimi Z. Leukocytosis and alteration of hemoglobin level in patients with severe COVID-19: Association of leukocytosis with mortality. Health Sci Rep. 2020;3:e194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Yamada T, Wakabayashi M, Yamaji T, Chopra N, Mikami T, Miyashita H, Miyashita S. Value of leukocytosis and elevated C-reactive protein in predicting severe coronavirus 2019 (COVID-19): A systematic review and meta-analysis. Clin Chim Acta. 2020;509:235-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 28. | Hirsch JS, Uppal NN, Sharma P, Khanin Y, Shah HH, Malieckal DA, Bellucci A, Sachdeva M, Rondon-Berrios H, Jhaveri KD, Fishbane S, Ng JH; Northwell Nephrology COVID-19 Research Consortium. Prevalence and outcomes of hyponatremia and hypernatremia in patients hospitalized with COVID-19. Nephrol Dial Transplant. 2021;36:1135-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 29. | Asakura H, Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int J Hematol. 2021;113:45-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (6)] |
| 30. | Bomhof G, Mutsaers PGNJ, Leebeek FWG, Te Boekhorst PAW, Hofland J, Croles FN, Jansen AJG. COVID-19-associated immune thrombocytopenia. Br J Haematol. 2020;190:e61-e64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 31. | Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, Tie Y, Fullerton KE. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 987] [Article Influence: 164.5] [Reference Citation Analysis (0)] |
| 32. | Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1636] [Article Influence: 272.7] [Reference Citation Analysis (0)] |
| 33. | Ganesan R, Mahajan V, Singla K, Konar S, Samra T, Sundaram SK, Suri V, Garg M, Kalra N, Puri GD. Mortality Prediction of COVID-19 Patients at Intensive Care Unit Admission. Cureus. 2021;13:e19690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Thakur B, Dubey P, Benitez J, Torres JP, Reddy S, Shokar N, Aung K, Mukherjee D, Dwivedi AK. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 2021;11:8562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 35. | Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, Pillay S, San EJ, Msomi N, Mlisana K, von Gottberg A, Walaza S, Allam M, Ismail A, Mohale T, Glass AJ, Engelbrecht S, Van Zyl G, Preiser W, Petruccione F, Sigal A, Hardie D, Marais G, Hsiao NY, Korsman S, Davies MA, Tyers L, Mudau I, York D, Maslo C, Goedhals D, Abrahams S, Laguda-Akingba O, Alisoltani-Dehkordi A, Godzik A, Wibmer CK, Sewell BT, Lourenço J, Alcantara LCJ, Kosakovsky Pond SL, Weaver S, Martin D, Lessells RJ, Bhiman JN, Williamson C, de Oliveira T. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature. 2021;592:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 1211] [Article Influence: 242.2] [Reference Citation Analysis (0)] |
| 36. | Fundação Ezequiel Dias. Boletim Informativo - Vigilância Laboratorial dos Vírus Respiratórios. Fundação Ezequiel Dias, 2021 [cited 30 July 2022]. Available from: http://www.funed.mg.gov.br/wp-content/uploads/2021/12/Informe-Virus-Respiratorios.pdf/. |
| 37. | Hitchings MDT, Ranzani OT, Torres MSS, de Oliveira SB, Almiron M, Said R, Borg R, Schulz WL, de Oliveira RD, da Silva PV, de Castro DB, Sampaio VS, de Albuquerque BC, Ramos TCA, Fraxe SHH, da Costa CF, Naveca FG, Siqueira AM, de Araújo WN, Andrews JR, Cummings DAT, Ko AI, Croda J. Effectiveness of CoronaVac among healthcare workers in the setting of high SARS-CoV-2 Gamma variant transmission in Manaus, Brazil: A test-negative case-control study. Lancet Reg Health Am. 2021;1:100025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 38. | Moreira FRR, D'arc M, Mariani D, Herlinger AL, Schiffler FB, Rossi ÁD, Leitão IC, Miranda TDS, Cosentino MAC, Tôrres MCP, da Costa RMDSC, Gonçalves CCA, Faffe DS, Galliez RM, Junior ODCF, Aguiar RS, Dos Santos AFA, Voloch CM, Castiñeiras TMPP, Tanuri A. Epidemiological dynamics of SARS-CoV-2 VOC Gamma in Rio de Janeiro, Brazil. Virus Evol. 2021;7:veab087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Laboratório Central de Saúde Pública - Bahia. Relatório dos resultados parciais referente ao sequenciamento de nova geração das amostras de SARS-CoV-2 positivas realizado no Laboratório Central de Saúde Pública Profº Gonçalo Moniz. Secretaria Estadual de Saúde. [cited 1 August 2022]. Available from: http://www.saude.ba.gov.br/wp-content/uploads/2021/05/11.05-BOLETIM-INFORMATIVO-SEQUENCIAMENTO-LACEN-EDICAO-05.pdf/. |
| 40. | Padilla-Rojas C, Barcena-Flores L, Vega-Chozo K, Galarza-Perez M, Bailon-Calderon H, Lope-Pari P, Balbuena-Torres J, Huaringa-Nuñez M, Caceres-Rey O, Rojas-Serrano N. Near-Complete Genome Sequence of a SARS-CoV-2 VOC 202012/01 Strain in Peru. Microbiol Resour Announc. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ganguli S, Bangladesh; Su C, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ