Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1684
Peer-review started: October 22, 2022
First decision: December 26, 2022
Revised: January 8, 2023
Accepted: February 17, 2023
Article in press: February 17, 2023
Published online: March 16, 2023
Processing time: 135 Days and 9 Hours
Diabetic foot ulcer (DFU) is a debilitating and severe manifestation of uncontrolled and prolonged diabetes that presents as ulceration, usually located on the plantar aspect of the foot. Approximately 15% of individuals with diabetes will eventually develop DFU, and 14%-24% of them will require amputation of the ulcerated foot due to bone infection or other ulcer-related complications. The pathologic mechanisms underlying DFU are comprise a triad: Neuropathy, vascular insufficiency, and secondary infection due to trauma of the foot. Standard local and invasive care along with novel approaches like stem cell therapy pave the way to reduce morbidity, decrease amputations, and prevent mortality from DFU. In this manuscript, we review the current literature with focus on the pathophysiology, preventive options, and definitive management of DFU.
Core Tip: Diabetic foot ulcer: Pathophysiology: - Neuropathy including sensory and motor - Vascular insufficiency leading to ischemia - Secondary infection with inflammation. Overview of management: (1) Preventive care including self-screening, health care screening, insoles, podiatric care; (2) Noninvasive modalities including wound dressing, human skin equivalent, topical growth factors, shock wave therapy, stem cell therapy, hyperbaric oxygen, negative pressure, shock wave therapy, maggot therapy, antibiotics; and (3) Invasive modalities including debridement, revascularization, skin grafting, amputation.
- Citation: Raja JM, Maturana MA, Kayali S, Khouzam A, Efeovbokhan N. Diabetic foot ulcer: A comprehensive review of pathophysiology and management modalities. World J Clin Cases 2023; 11(8): 1684-1693
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1684.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1684
Diabetes mellitus affects approximately 422 million people worldwide and is responsible for an estimated 2 million deaths per year[1]. It affects 11.3% of the United States population[2]. Diabetic foot ulcer (DFU) is a debilitating and severe manifestation of uncontrolled and prolonged diabetes that presents as an ulceration, usually located at the plantar aspect of the foot. Approximately 15% of individuals with diabetes will eventually develop one of these ulcers, and out of these individuals, 14%-24% of them will require amputation of the ulcerated foot due to bone infection or other ulcer-related complications[3]. With such a high level of morbidity stemming from debilitating osteomyelitis and amputation in patients with DFU, it is of the utmost importance to properly address and treat the underlying causes of DFU. In this paper, we review the current literature with focus on the pathophysiology, preventive options, and definitive management of DFU.
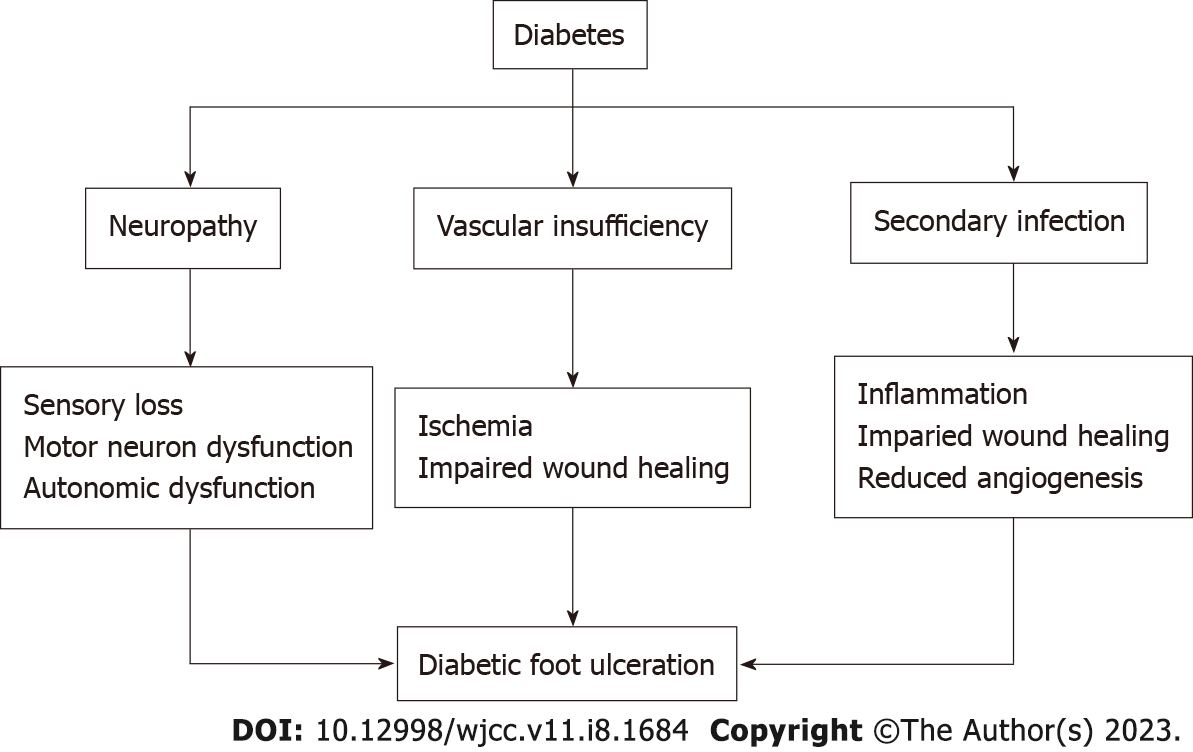
DFU comprises a full-thickness wound involving the dermis, located in the weight-bearing or exposed area below the ankle. The Wagner system aids in categorizing the severity of the ulcer, ranking it on a scale of 1 to 5 (Table 1). The pathologic mechanisms of DFU are described in terms of a triad. This triad includes neuropathy, vascular insufficiency, and secondary infection due to trauma of the foot[4] (Figure 1).
| Grade | Characteristic |
| Wagner grade 1 | Partial- or full-thickness ulcer (superficial) |
| Wagner grade 2 | Deep ulcer extending to ligament, tendon, joint capsule, bone, or deep fascia without abscess or OM |
| Wagner grade 3 | Deep abscess, OM, or joint sepsis |
| Wagner grade 4 | Partial-foot gangrene |
First, the lack of protective sensation in the feet predisposes patients with diabetes to developing trauma and ulcers. This sensory impairment occurs due to hyperglycemia-induced upregulation of aldose reductase and sorbitol dehydrogenase, which in turn increase the production of fructose and sorbitol. These glucose products accumulate and induce osmotic stress, thereby reducing nerve cell myoinositol synthesis and nerve conduction[5]. Also, from a pathological stance, advanced glycation end-products (AGEs) must be considered. AGEs are non-enzymatic protein, amino acid, and DNA adducts which form from dicarbonyls and glucose. AGE formation is enhanced in diabetes and is associated with the development of diabetic complications[6]. In addition to sensory neuropathy, diabetes can induce neuronal autonomic dysfunction that results in impaired sweat production, leaving the foot susceptible to dryness, skin cracking, and fissuring[7]. Furthermore, motor neuron dysfunction can give rise to muscle wasting and structural abnormalities of the foot[8]. This causes focally elevated pressures at various zones of the plantar foot and increases the risk of ulceration[9].
In addition to the triad, impaired wound healing has been established as a key means of DFU progression[10]. Importantly, molecular changes at the site of DFU precede the grossly visualized tissue abnormalities[11]. In fact, the route from hyperglycemia to DFU involves complex molecular dysfunctions in wound healing. Ordinarily, wounds undergo several healing stages involving hemostasis, inflammation, proliferation, and remodeling. Acute wounds advance linearly through these stages; however, chronic nonhealing DFUs stall in 1 or more phases. In the early phases of wound healing, neutrophils normally release granular molecules to kill foreign pathogens in a process known as neutrophil extracellular traps (NETosis)[12]. However, in a diabetic microenvironment, NETosis becomes dysregulated, causing a proinflammatory cascade and overproduction of cytokines and superoxide, which delay wound healing[13,14]. Moreover, hyperglycemia induces formation of AGEs that cause structural and functional changes in key proteins[15]. Specifically, AGEs can bind to the receptor of advanced glycation end-products (RAGE), which is normally minimally expressed in normoglycemic conditions[16]. This in turn activates nuclear factor kappa-B (NF-κB). Ultimately, cytokine release is enhanced with a self-sustaining cascade that prolongs inflammation and favors apoptosis[17]. Overall, hyperglycemia induces a proinflammatory environment largely due to the dysregulation of cytokine release, NETosis, and AGE production.
Along with inflammation, substantial alterations of the extracellular matrix (ECM) also play a significant role in perpetuating the non-healing DFU. In cases of normal wound healing, the production and degradation of ECM proteins such as collagen and fibrin are tightly regulated[18]. Collagen comprises most of the soft tissue ECM, and thus, abnormalities of collagen metabolism have significant consequences on wound healing. Specifically, collagen-degrading enzymes known as matrix metalloproteinases (MMPs) become hyperactive, resulting in a highly-proteolytic environment with reduced collagen content[19,20]. Overall, the ECM becomes disorganized and insufficient to support wound healing. Alongside elevated MMP activity, the accumulation of AGEs results in a reduction of fibroblast growth factor (FGF) and transforming growth factor-beta[21,22]. This has a similar effect of reducing the collagen content via the induction of fibroblast apoptosis[23].
Lastly, impaired angiogenesis plays a key role in the disruption of diabetic wound healing. Angiogenesis ordinarily occurs during the proliferative phase of wound healing, and is responsible for both the formation of granulation tissue and delivery of nutrition and oxygen to the wound[24]. In the case of DFU, there is a reduction of angiogenic growth factors such as vascular endothelial growth factor (VEGF) 20 and FGF-2[25]. Essentially, VEGF initiates angiogenesis and mediates endothelial cell proliferation while FGF-2 facilitates migration of new blood vessels through the ECM[26,27]. When VEGF and FGF-2 expression is compromised, wound healing declines. Furthermore, endothelial progenitor cells (EPCs) have been implicated as expressors of proangiogenic factors and receptors including VEGF and FGF[28]. A deficiency of function and number of EPCs has been demonstrated in patients with type 2 diabetes mellitus, which is attributed to AGE accumulation[29-31]. Overall, the dysfunction of EPCs and circulating growth factors contributes significantly to the development and progression of DFU by way of disrupting angiogenesis.
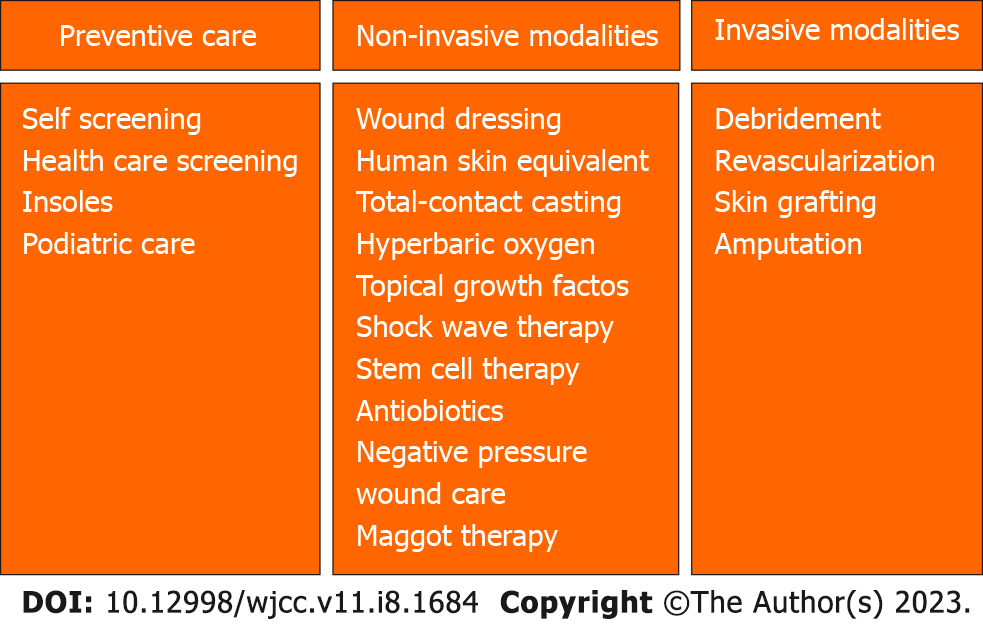
The management of DFU involves preventative care as well as various treatment modalities, including both noninvasive and invasive measures (Figure 2).
Due to diabetes being a risk factor for the development of underlying peripheral vascular disease, the majority of DFUs are asymptomatic until advanced enough to recognize more severe signs and symptoms. During the diagnosis of DFU, neuropathy may mask ischemia and vice versa. Therefore, the primary preventative strategy is regular diabetic foot screening to allow early identification of DFU, followed by initiation of treatment if appropriate. Ultimately, early detection and management work to avoid further complications such as gangrene and amputation[32]. Screening comprises the patient checking his or her own feet for trauma or ulceration every day and routine screening during health care visits.
The most prevalent management modality for DFU is local care, in which many potential avenues of treatment can be utilized. These include wound dressings, human skin equivalents (HSEs), pressure offloading, total-contact casting (TCC), systemic hyperbaric oxygen, larvae therapy (maggot therapy), and topical growth factors.
Wound dressings are the most basic and common treatment measure, and although they serve a vital purpose in the management of DFU, other methods have proven vastly more effective in comparison to or in adjunct with wound dressings.
HSE is more effective compared to the standard treatment of saline-moistened gauze in reducing the rates of amputation and infection and in improving the rate of ulcer healing. One randomized controlled trial (RCT) assessed the effectiveness of Graftskin, a living skin equivalent indicated for use in noninfected, nonischemic DFU. In this study, Graftskin was applied weekly for a maximum of 4 wk or until complete healing occurred. The results of the trial highlighted the increased effectiveness of HSE in comparison to the control group, in which ulcers were treated only with saline-moistened gauze. The use of HSE resulted in an 18% increase in complete wound healing when compared to the control group[33]. Despite these impressive results, one limitation to this treatment is that HSE may have limited availability or accessibility.
Pressure offloading serves as one of the primary treatments of DFU, primarily in ulcers accompanied by neuropathy, with many variants being utilized. For ischemic DFUs, however, revascularization is more commonly used. Common methods of offloading include bed rest, wheelchair use, implementation of a crutch-assisted gait, total contact casting, use of felted foam, use of therapeutic shoes, and use of removable cast walkers[34]. The most effective offloading treatment is TCC, in which full casts are applied by an experienced physiotherapist and are changed weekly for 2-3 wk or until healing has occurred. One RCT found that TCC was extremely effective in increasing ulcer healing and reducing infection when compared to traditional dressing changes and other offloading methods. The study reported a 91% rate of healing within the TCC population, compared to a 32% rate of healing in the control group. This rate was reported following a 65 d period. Furthermore, the TCC group reported a 0% incidence of infection, with the same in the control group reported as 26%[35]. Multiple other studies have reported similar results, with TCC being an extremely effective treatment to DFU, particularly when compared to traditional dressing changes. One adverse effect of this treatment, however, is fungal infection, but this was addressed with topical treatment and did not prevent continued casting. Despite the evident success of TCC, one national survey in the United States evaluating 901 foot clinics in 48 states and the District of Columbia indicated that TCCs were used only by 1.7% of centers; this is potentially due to the tedious nature of this treatment option. TCC requires an experienced physiotherapist, and constant replacement and care. The application of the cast is a timely and intricate endeavor, and tends to cause patients discomfort according to the survey. The survey also indicated that the primary treatment across the foot clinics was shoe modifications.
Another treatment for DFU is systemic hyperbaric oxygen therapy (HBOT), which is reserved for advanced cases and aimed at reducing the risk of amputation. This treatment is prevalent particularly in the treatment of infected DFU, where 1 systematic review identified 6 RCTs that evaluated chronic DFU. Systemic HBOT sessions are usually conducted in 45 to 120 min sessions once or twice daily at pressures between 1.5-3.0 ATA. This method resulted in significantly reduced rates of major amputation compared with usual care of DFU. HBOT is typically used as an adjunctive therapy to normal wound care measures[36]. However, this treatment modality is quite expensive, is still not fully researched, and may warrant further trials.
Maggot therapy is another well-researched technique with respect to the treatment of chronic wounds in which maggots are placed on the wound area. This treatment method has been shown to significantly facilitate debridement. In one study, maggot therapy also enabled faster development of granulation tissue and more significantly decreased wound surface area compared to other topical treatments such as hydrogel dressings. Maggot therapy also had no effect on disinfection or complete healing rate for the wound[37].
Topical growth factors, particularly platelet-derived growth factors, have also proven effective in increasing ulcer healing rates when compared with placebo. Growth factors serve as principal immediate mediators of wound healing, and when applied in the setting of DFU, accelerate ulcer healing. One meta-analysis evaluated 26 RCTs with 2088 participants, and focused on recombinant epidermal growth factor, autologous platelet rich plasma, and recombinant human platelet-derived growth factor. Overall, each of the 3 treatments significantly improved rate of healing when used alongside standard treatment, with recombinant human epidermal growth factor slightly favored when compared to other growth factors[38].
Extracorporeal shockwave therapy (ESWT) has been reported to accelerate the healing of soft tissue wounds when treating DFU. ESWT is utilized to stimulate osteoblasts and in turn facilitate soft tissue healing. There have been promising clinical trial results, indicating that ESWT is more effective in the treatment of DFU when compared to traditional methods. Two multi-national RCTs were conducted to compare the efficacy of ESWT when used adjunctively with standard care and other DFU treatments. The trials both lasted 12 wk and showed reduction of wound volume by more than 50% with the use of ESWT when compared to standard treatment alone[39].
The cornerstone of available treatment options currently includes treatment of infection, surgical debridement, and revascularization[40]. Better understanding of the tissue remodeling process, which comprises inflammation, cell migration, neovascularization, and tissue proliferation, has paved the way for stem cell-based therapy to become viable for the treatment of DFU[41]. Stem cells aid wound healing by secretion of cytokines that play an important role in cell migration, angiogenesis, remodeling of extracellular matrix, and regeneration of nerves[42]. Also, stem cell capacity for differentiation into various cell types, including myofibroblasts and endothelial cells, optimizes wound healing[43].
The stem cell types that have been studied to aid in diabetic foot treatment are mainly adult stem cells (ASCs). Bone marrow-derived mesenchymal stem cells (BM-MSC) are the most extensively studied among the different ASCs; other types include adipose-derived stem cells, umbilical cord-derived mesenchymal stem cells (UC-MSC), and peripheral blood-derived mesenchymal stem cells[44] (Table 2). The use of BM-MSC in the treatment of DFU demonstrated more effective ulcer healing, with improvements in Ankle-Brachial Index (ABI), angiogenesis, and blood flow when compared to local treatment[45-47]. Even functional improvement with a decrease in rest pain and an increase in claudication distance was demonstrated. Decreased amputation when compared to conventional treatment was also seen. Furthermore, combining UC-MSC stem cell therapy with traditional angioplasty resulted in improvements in ABI, claudication distance, and skin temperature[48].
| Stem cell type | Stem cell sub-types | Administration route |
| Adult stem cell | (1) Bone marrow-derived mesenchymal stem cells; (2) Adipose-derived stem cells; Human umbilical cord-derived; (3) Mesenchymal stem cells; and (4) Peripheral blood-derived mesenchymal stem cells | Local: Intramuscular and subcutaneous; Systemic: Intravenous and intraarterial |
| Embryonic stem cell | Cell mass of blastocyst by in vitro fertilization | Proposed local or systemic administration |
Embryonic stem cells (ESCs) are usually derived from blastocysts from the inner cell mass grown via in vitro fertilization[49]. The controversial ethics behind obtaining ESC and their inherent high rate of proliferation and the risks of tumor formation or immunological rejection has limited them from widespread research[50]. However, in one study using an animal model, the use of ESCs did not increase chances of tumor formation in rats[51]; however, further clinical studies are required to test the efficacy of ESC treatment in diabetic feet. Stem cell therapy shows promise as a viable therapeutic option in the treatment of DFU. Stem cells can be used alongside conventional therapies like angioplasty to achieve more desirable outcomes.
The use of systemic and local antibiotics serves as a noninvasive treatment in the management of DFU. Antibiotics can be administered topically through sponge applications or through gauze wrapping, as well as with use of a circulator boot. The presence of infection is defined by the presence of more than 2 classic findings of inflammation or purulence. There are 3 classifications of infection severity: (1) Mild (superficial and limited in size and depth); (2) moderate (deeper and larger in area); and (3) severe (overexpressed and beginning to affect metabolic perturbations). Most DFUs have a microbial cause, with aerobic Gram-positive cocci and staphylococci being the most common implicated microbes. Wounds that lack signs of infection typically do not require antibiotic therapy. If the wound is infected, a post-debridement specimen must be collected for both aerobic and anaerobic cultures. Following testing and potential imaging (including radiographs and MRI, if necessary), antibiotics may be prescribed[52]. If the infection is mild or moderate, narrow-spectrum oral antibiotics may be administered. If infection severity is moderate, high, or severe, broad-spectrum parenteral antibiotics should be utilized[53].
One of the most recent developments in DFU treatment is the utilization of negative pressure wound therapy (NPWT). NPWT utilizes vacuum pressure to draw fluid from the wound and increase blood flow to the affected area, thus stimulating the healing process. While primarily used in burn patients, NPWT has also recently been used in DFU patients with promising results. NPWT results in 2 primary types of tissue deformation: Macro deformation, which is exemplified by wound contraction; and micro deformation, which occurs on the microscopic level. Both deformations stimulate blood flow and promote a wound healing cascade that includes tissue granulation, vessel proliferation, neoangio
Debridement is a major component in the treatment of DFU, particularly due to its ability to alter the environment of the chronic wound through the removal of necrotic and nonviable tissue and foreign debris, which impede the healing process. Debridement may not always lead to complete healing of the DFU, but it serves as an important preliminary step in the treatment. Following debridement, the wound is further analyzed and if necessary, other treatment paths are pursued[55]. Debridement is commonly used in conjunction with other treatment modalities.
When patients with DFU also have a history of peripheral arterial disease (PAD), delayed healing, higher complication rates, and an increased chance of potential amputation may be observed. Thus, when patients have both DFU and chronic limb ischemia, revascularization can serve as a promising treatment option. According to various studies, the ulcer healing rate following revascularization ranges from 46% to 91%, representing a higher rate of healing compared to PAD patients that do not undergo revascularization[56]. Revascularization options include stenting and surgical bypass if other intervention is not possible. Atherectomy, shockwave treatment for calcified lesions, and balloon revascularization (cutting, drug coated, cryoplasty) can also be used alone or with stenting[57]. In a clinical trial in which 80 patients who underwent foot revascularization procedures, promising results were also shown. All patients in this study underwent an endovascular procedure (balloon angioplasty). The patients were followed for 12 mo after the procedure, and results showed that 56.2% of the patients fully recovered, 58.7% had minor amputations, and only 16.2% required major amputations. Overall, revascularization is an effective treatment for DFU, especially when the patient is at risk of amputation[58]. However, the effectiveness of the vascular procedure differs among patients, and it also does not reduce the risk of death associated with PAD. It is important to consider the role of complex therapy (including medical management) in conjunction with revascularization in the treatment of DFU. This includes close monitoring of glucose, lipids, and blood pressure, and the use of antiplatelet therapy following the surgical procedure. Compared with initial supervised exercise training (SET) alone, endovascular therapy in combination with SET is associated with significant improvements in total walking distance, ABI, and risk of future revascularization or amputation. On the other hand, endovascular therapy alone was not associated with an improvement in functional capacity[59]. It is also important to note that post-endovascular procedure patients must be started on dual antiplatelet therapy, including aspirin plus clopidogrel or ticagrelor for several months. Statin therapy has also been proven to stabilize any plaques present before and after revascularization.
Skin grafting may serve as a solution when DFUs become more severe, offering a chance to replace the infected skin and promote the healing process. There are a variety of skin grafting techniques that may be used, including bioengineered or artificial skin, autografts (taken from the patient), allografts (taken from another person), or xenografts (taken from animals). A review article that analyzed 17 RCTs concluded that skin grafting and tissue replacement when used in conjunction with standard treatment led to an increase in the healing rate of DFU and slightly lowered the chance of amputation. However, evidence of long-term effectiveness is uncertain[60].
Amputation represents the final management option when treating DFU and is reserved for the most chronic levels of infection or deformity that render the foot nonfunctional. Amputation can be classified as either minor or major, with minor amputation being the removal of a smaller area (e.g., removal of a toe or a part of the foot). Major amputation, however, can be performed above or below a major joint such as the knee or elbow. In a clinical trial, minor amputation was performed for 38.4% and major amputation was performed for 6.8% of patients with DFU[61].
DFU results in substantial morbidity and mortality in patients with diabetes. It also often leads to longer hospitalizations and associated increases in health care spending. Thus, prompt diagnosis and catered management is essential to management of this prevalent consequence of diabetes. Standard local and invasive care along with novel approaches like stem cell therapy pave the way to reduce morbidity, decrease the need for amputation, and prevent mortality due to DFU (Table 3). Further research into newer modalities that aid in prompt and effective management will further help alleviate the healthcare burden of DFU.
| Treatment modality | Level of evidence | Strength of recommendation |
| Non-invasive modalities | ||
| Wound dressing | High | Strong recommendation |
| Antibiotics | Low to moderate | Strong recommendation |
| Total-contact casting and pressure offloading techniques | High | Strong recommendation |
| Maggot therapy | Low | Weak recommendation |
| Hyperbaric oxygen | Low | Weak recommendation |
| Topical growth factors | Moderate | Could be beneficial |
| Cell therapy | Low (more studies required) | Weak recommendation |
| Invasive modalities | ||
| Debridement | Moderate to high | Strong recommendation |
| Skin grafting | Moderate | Could be beneficial |
| Revascularization | Moderate | Strong recommendation |
| 1. | GBD 2019 Diabetes in the Americas Collaborators. Burden of diabetes and hyperglycaemia in adults in the Americas, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol. 2022;10:655-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Centers for Disease Control and Prevention. National Diabetes Statistics Report. Center for Disease Control and Prevention. 2020. [cited 22 October, 2022]. Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. |
| 3. | University of California San Francisco. Vascular & Endovascular Surgery - Diabetic Foot Ulcers. [cited 22 October, 2022]. Available from: https://vascularsurgery.ucsf.edu/conditions--procedures/diabetic-foot-ulcers.aspx. |
| 4. | Boulton AJ, Kirsner RS, Vileikyte L. Clinical practice. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 342] [Article Influence: 15.5] [Reference Citation Analysis (2)] |
| 5. | Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, Flores-Morales V, Martinez-Fierro ML. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics (Basel). 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (3)] |
| 6. | Brings S, Fleming T, Freichel M, Muckenthaler MU, Herzig S, Nawroth PP. Dicarbonyls and Advanced Glycation End-Products in the Development of Diabetic Complications and Targets for Intervention. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22:1036-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 420] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Tesfaye S. Recent advances in the management of diabetic distal symmetrical polyneuropathy. J Diabetes Investig. 2011;2:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 9. | Skopljak A, Sukalo A, Batic-Mujanovic O, Muftic M, Tiric-Campara M, Zunic L. Assessment of diabetic polyneuropathy and plantar pressure in patients with diabetes mellitus in prevention of diabetic foot. Med Arch. 2014;68:389-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 10. | Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1739] [Article Influence: 82.8] [Reference Citation Analysis (1)] |
| 11. | Rubitschung K, Sherwood A, Crisologo AP, Bhavan K, Haley RW, Wukich DK, Castellino L, Hwang H, La Fontaine J, Chhabra A, Lavery L, Öz OK. Pathophysiology and Molecular Imaging of Diabetic Foot Infections. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (3)] |
| 12. | Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5773] [Cited by in RCA: 7615] [Article Influence: 346.1] [Reference Citation Analysis (0)] |
| 13. | Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam BH, Malabanan A, Trackman PC, Badwey JA, Van Dyke TE. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005;78:862-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, Albiero M, Vigili de Kreutzenberg S, Avogaro A, Fadini GP. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015;52:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Byun K, Yoo Y, Son M, Lee J, Jeong GB, Park YM, Salekdeh GH, Lee B. Advanced glycation end-products produced systemically and by macrophages: A common contributor to inflammation and degenerative diseases. Pharmacol Ther. 2017;177:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 16. | Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 408] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 17. | Mahali S, Raviprakash N, Raghavendra PB, Manna SK. Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: involvement of Ca2+ mediated by interleukin-8 protein. J Biol Chem. 2011;286:34903-34913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Apte SS, Parks WC. Metalloproteinases: A parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015;44-46:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 19. | Hopkinson I. Molecular components of the extracellular matrix. J Wound Care. 1992;1:52-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Ferroni L, Gardin C, Dalla Paola L, Campo G, Cimaglia P, Bellin G, Pinton P, Zavan B. Characterization of Dermal Stem Cells of Diabetic Patients. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Alikhani Z, Alikhani M, Boyd CM, Nagao K, Trackman PC, Graves DT. Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem. 2005;280:12087-12095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Patel S, Srivastava S, Singh MR, Singh D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112:108615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 650] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 23. | Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60:107-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 512] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Kulwas A, Drela E, Jundziłł W, Góralczyk B, Ruszkowska-Ciastek B, Rość D. Circulating endothelial progenitor cells and angiogenic factors in diabetes complicated diabetic foot and without foot complications. J Diabetes Complications. 2015;29:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 25. | Li X. The association between MCP-1, VEGF polymorphisms and their serum levels in patients with diabetic foot ulcer. Medicine (Baltimore). 2018;97:e10959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 26. | Kajdaniuk D, Marek B, Borgiel-Marek H, Kos-Kudła B. Vascular endothelial growth factor (VEGF) - part 1: in physiology and pathophysiology. Endokrynol Pol. 2011;62:444-455. [PubMed] |
| 27. | Mizia-Malarz A, Sobol G, Woś H. [Proangiogenic factors: vascular-endothelial growth factor (VEGF) and basic fibroblast growth factor--the characteristics and function]. Przegl Lek. 2008;65:353-357. [PubMed] |
| 28. | Tecilazich F, Dinh T, Pradhan-Nabzdyk L, Leal E, Tellechea A, Kafanas A, Gnardellis C, Magargee ML, Dejam A, Toxavidis V, Tigges JC, Carvalho E, Lyons TE, Veves A. Role of endothelial progenitor cells and inflammatory cytokines in healing of diabetic foot ulcers. PLoS One. 2013;8:e83314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 29. | Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781-2786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1117] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 30. | Chen MC, Sheu JJ, Wang PW, Chen CY, Kuo MC, Hsieh CJ, Chen JF, Chang HW. Complications impaired endothelial progenitor cell function in Type 2 diabetic patients with or without critical leg ischaemia: implication for impaired neovascularization in diabetes. Diabet Med. 2009;26:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Boström L, Linder LE, Bergström J. Influence of smoking on the outcome of periodontal surgery. A 5-year follow-up. J Clin Periodontol. 1998;25:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 32. | Lim JZ, Ng NS, Thomas C. Prevention and treatment of diabetic foot ulcers. J R Soc Med. 2017;110:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 287] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 33. | Veves A, Falanga V, Armstrong DG, Sabolinski ML; Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 501] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 34. | Wu SC, Jensen JL, Weber AK, Robinson DE, Armstrong DG. Use of pressure offloading devices in diabetic foot ulcers: do we practice what we preach? Diabetes Care. 2008;31:2118-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Mueller MJ, Diamond JE, Sinacore DR, Delitto A, Blair VP 3rd, Drury DA, Rose SJ. Total contact casting in treatment of diabetic plantar ulcers. Controlled clinical trial. Diabetes Care. 1989;12:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 192] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Roeckl-Wiedmann I, Bennett M, Kranke P. Systematic review of hyperbaric oxygen in the management of chronic wounds. Br J Surg. 2005;92:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 37. | Mohd Zubir MZ, Holloway S, Mohd Noor N. Maggot Therapy in Wound Healing: A Systematic Review. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Sridharan K, Sivaramakrishnan G. Growth factors for diabetic foot ulcers: Mixed treatment comparison analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84:434-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Galiano R, Snyder R, Mayer P, Rogers LC, Alvarez O; Sanuwave Trial Investigators. Focused shockwave therapy in diabetic foot ulcers: secondary endpoints of two multicentre randomised controlled trials. J Wound Care. 2019;28:383-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31:817-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 460] [Article Influence: 38.3] [Reference Citation Analysis (1)] |
| 41. | Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care. 2012;25:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 477] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 42. | Lopes L, Setia O, Aurshina A, Liu S, Hu H, Isaji T, Liu H, Wang T, Ono S, Guo X, Yatsula B, Guo J, Gu Y, Navarro T, Dardik A. Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res Ther. 2018;9:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 43. | Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581-2587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 722] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 44. | Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 620] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 45. | Chiang KJ, Chiu LC, Kang YN, Chen C. Autologous Stem Cell Therapy for Chronic Lower Extremity Wounds: A Meta-Analysis of Randomized Controlled Trials. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 46. | Wu Q, Lei X, Chen L, Zheng Y, Huang H, Qian C, Liang Z. Autologous platelet-rich gel combined with in vitro amplification of bone marrow mesenchymal stem cell transplantation to treat the diabetic foot ulcer: a case report. Ann Transl Med. 2018;6:307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 48. | Qin HL, Zhu XH, Zhang B, Zhou L, Wang WY. Clinical Evaluation of Human Umbilical Cord Mesenchymal Stem Cell Transplantation After Angioplasty for Diabetic Foot. Exp Clin Endocrinol Diabetes. 2016;124:497-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 49. | Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, Shimamoto S, Imamura T, Nakashima K, Saitou M, Hayashi K. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 411] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 50. | Jiang XY, Lu DB, Chen B. Progress in stem cell therapy for the diabetic foot. Diabetes Res Clin Pract. 2012;97:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Lee KB, Choi J, Cho SB, Chung JY, Moon ES, Kim NS, Han HJ. Topical embryonic stem cells enhance wound healing in diabetic rats. J Orthop Res. 2011;29:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 52. | Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1205] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 53. | Kwon KT, Armstrong DG. Microbiology and Antimicrobial Therapy for Diabetic Foot Infections. Infect Chemother. 2018;50:11-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 54. | Borys S, Hohendorff J, Frankfurter C, Kiec-Wilk B, Malecki MT. Negative pressure wound therapy use in diabetic foot syndrome-from mechanisms of action to clinical practice. Eur J Clin Invest. 2019;49:e13067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 55. | Lebrun E, Tomic-Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound Repair Regen. 2010;18:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Vouillarmet J, Bourron O, Gaudric J, Lermusiaux P, Millon A, Hartemann A. Lower-extremity arterial revascularization: Is there any evidence for diabetic foot ulcer-healing? Diabetes Metab. 2016;42:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 57. | Tepe G, Brodmann M, Werner M, Bachinsky W, Holden A, Zeller T, Mangalmurti S, Nolte-Ernsting C, Bertolet B, Scheinert D, Gray WA; Disrupt PAD III Investigators. Intravascular Lithotripsy for Peripheral Artery Calcification: 30-Day Outcomes From the Randomized Disrupt PAD III Trial. JACC Cardiovasc Interv. 2021;14:1352-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 58. | Meloni M, Morosetti D, Giurato L, Stefanini M, Loreni G, Doddi M, Panunzi A, Bellia A, Gandini R, Brocco E, Lazaro-Martinez JL, Lauro D, Uccioli L. Foot Revascularization Avoids Major Amputation in Persons with Diabetes and Ischaemic Foot Ulcers. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 59. | Pandey A, Banerjee S, Ngo C, Mody P, Marso SP, Brilakis ES, Armstrong EJ, Giri J, Bonaca MP, Pradhan A, Bavry AA, Kumbhani DJ. Comparative Efficacy of Endovascular Revascularization Versus Supervised Exercise Training in Patients With Intermittent Claudication: Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc Interv. 2017;10:712-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Santema TB, Poyck PP, Ubbink DT. Skin grafting and tissue replacement for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev. 2016;2:CD011255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 61. | Kim SY, Kim TH, Choi JY, Kwon YJ, Choi DH, Kim KC, Kim MJ, Hwang HK, Lee KB. Predictors for Amputation in Patients with Diabetic Foot Wound. Vasc Specialist Int. 2018;34:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Liao Z, Singapore; Moreno-Gómez-Toledano R, Spain; Shalaby MN, Egypt; Sutkowska E, Poland S-Editor: Wang DM L-Editor: Filipodia P-Editor: Wang DM