INTRODUCTION
Diabetes mellitus (DM) is a chronic metabolic disease characterized by insufficient insulin production or cellular insulin use, currently affecting around 537 million people worldwide. This number is forecasted to grow to 693 million by 2045 if adequate preventive measures are not implemented[1,2]. The projected increase in prevalence is higher in developing countries compared to developed, high-income countries[3]. Diabetes mellitus types 1 and 2, despite having different pathogenic mechanisms, tend to have similar associated complications[4]. One of the most severe complications of diabetes and the most common cause of hospitalization in diabetic patients is diabetic foot disease, which is a term that includes infections, diabetic foot ulcers (DFU), and gangrene[5,6]. The risk of developing DFU in diabetic patients is 25%, and is similar for patients with DM type 1 or type 2[6,7]. Consequently, diabetes is one of the leading causes of limb amputation worldwide, as it accounts for more than 60% of non-traumatic lower extremity amputation, with approximately 80%-85% being preceded by DFU[8,9]. This statistic implies that annually, more than 1 million people with diabetes suffer limb loss. To put this staggering number into perspective, every 20 s, there is a need for amputation somewhere in the world due to diabetes[8,9]. People with diabetes and diabetic foot complications have a higher mortality rate than those without foot complications[10,11]. Furthermore, individuals with diabetes have increased mortality after incidence of DFU compared to people of the same age and duration of diabetes without DFU[12]. This mortality is further increased in diabetic patients with foot disease necessitating amputation[11]. Ischemic heart disease is a significant cause of premature mortality in patients with DFU, and those with neuropathic DFU have even higher mortality[12,13]. Thus, it is evident that diabetes represents a substantial public health and economic burden; more specifically, financial costs related to the treatment of diabetes reach up to 673 million dollars (USD) annually, and 20%-40% of the healthcare budget spent on diabetes is related to foot complications[5,14,15]. Apart from financial burden, diabetic foot disease (particularly DFU) is a major personal tragedy with a significant impact on quality of life for patients and their families, and can represent a major burden for health care professionals and institutions as well. Despite the generally accepted need for a multidisciplinary approach in the prevention, diagnosis, and management of individuals with diabetes, including patients with DFU, data related to financial cost and the sequelae of diabetic foot complications (especially major amputations) is still insufficient[9]. Although guidelines related to diabetic foot management are available, there is only limited high-quality evidence to address remaining critical questions. Therefore, a better understanding of diabetic foot complications is fundamental for further improvement in the care of this group of patients.
PATHOPHYSIOLOGY
Foot ulcers in individuals with diabetes arise via a specific given pathophysiologic mechanism, which can affect clinical presentation and management. Ischemia and neuropathy are two key pathological components that can lead to diabetic foot complications, while infection usually arises as a secondary phenomenon. Nevertheless, all three components often have a synergistic role in the etiologic triad[6]. Peripheral neuropathy is present in around 50% of diabetic patients, who often gradually develop "high-pressure" zones on foot with decreased protective sensation, a phenomenon considered the leading cause of DFU[16]. Although it seems changes are arising with respect to the accepted etiopathology, ischemia has an increasingly prominent role. Recent large-scale studies have shown that in high-income countries, almost half of DFUs are neuroischemic or ischemic in origin, and patients presenting with manifest peripheral arterial disease (PAD) and frank neuropathic ulcers are still more prevalent in low-income countries[3,17-22].
The pathogenesis of diabetic neuropathy is still not fully understood, but it is known that diabetes and associated aberrant glucose metabolism can affect sensory, motor, and autonomic fibers[14]. Two accepted potential mechanisms include ischemic injury to the nerve due to changes to vasa nervorum and oxidative stress in the nerve caused by increased activity of the sorbitol pathway[6]. Sensory neuropathy causes decreased pain and pressure sensation, vibratory perception, proprioception, and altered temperature sensation. Motor neuropathy causes atrophy of foot muscles with secondary foot deformity and impaired gait, leading to high plantar pressure and elevated mechanical stress[23]. Finally, autonomic neuropathy causes anhidrosis, which makes dry skin susceptible to minor fissures and causes impaired microcirculation through arteriovenous shunts[6,24,25].
Microvascular dysfunction in patients with diabetes is also caused by structural and functional changes in endothelial cells, resulting in impaired vasodilatory response, hypercoagulation, and inflammation in the vessel wall[23,26]. Although ischemic complications in diabetes have long been attributed to changes in microcirculation, which mistakenly led to the pervasive opinion that people with diabetes will not benefit from revascularization, today it is known that microvascular dysfunction and PAD are the leading cause of vascular impairment in patients with diabetes[6,27]. Also, PAD and infection impair healing of DFU, and are two main factors leading to amputation in people with diabetes[17,28,29]. PAD in diabetes has some specific charateristics compared to that in the general population. For example, atherosclerotic plaques are usually multisegmental, bilateral, located in infrapopliteal vessels, and involve anterior and posterior tibial arteries with relative sparing of foot arteries. Impaired collateral formation has also been documented[23,27,30].
People with diabetes are more susceptible to infections due to neuropathy, PAD, microcirculation dysfunction, and immunopathy[6,29]. Diabetic foot infections (DFI) can occur in sites of minor skin breaks caused by neuropathy, but most often occur within ulcers[31]. Infection can be uncomplicated and superficial; however, compared to infection in the general population, DFI are more prone to rapid spread to deep structures of the foot, including the fascia, tendons, muscles, joints, and bones. In addition, because of the anatomic compartments of the foot, infection usually spreads along the tendons. At the same time, the ensuing inflammatory response can cause high pressures in these compartments and further impair circulation, leading to a more rapid progression of infection[32-34]. Therefore, DFI can easily become a foot- and life-threatening condition and is a direct cause of amputation in 25%-50% of individuals with diabetes, especially if it arises in the setting of PAD[5,23,31]. Risk factors for developing DFI include neuropathy, limb ischemia, chronic or recurrent deep foot ulcer, traumatic ulcer, chronic renal failure, and poor glycemic control[31,35].
Finally, it should be emphasized that not all patients with diabetes are at risk for DFU[8]. Established neuropathy, foot deformity, and PAD are the main risk factors. Additional risk factors are history of foot ulceration or any limb amputation[36,37]. Patients who develop DFU are usually those with longstanding diabetes (> 10 years), who are male, have poor glycemic control, and have other diabetes-related comorbidities[38]. An incipient trigger for foot ulcer is often a minor injury caused by repetitive trauma while walking in patients with decreased protective sensation, changed biomechanics, and foot deformity with high-pressure zones resulting from neuropathy. Furthermore, the skin of individuals with diabetes is often dry due to autonomic neuropathy and as such is more prone to breakdown and fissure[5,6]. Although foot ischemia as the causative factor in DFU was once underestimated, it is now known that at least half of DFU is neuroischemic or ischemic in origin[17,19]. Ischemia and infection are the main contributing factors in non-healing ulcer and amputation in patients with DFU[17,28].
IMMUNE RESPONSE TO HYPERGLYCEMIA
The immune system regulates inflammation and works to maintain homeostasis[39]. In addition, the innate and adaptive immune systems have an essential function in promoting all stages of wound healing[40]. The innate immune system consists of different types of cells (e.g., macrophages, monocytes, lymphocytes, basophils, natural killer cells, granulocytes, and mast cells)[40]. It is activated quickly, but with limited specificity[40]. On the other hand, the adaptive immune system includes T- and B-lymphocytes and is activated more slowly with long-term memory and high specificity[40].
The immune system of individuals with diabetes is weaker than in those without diabetes, and hyperglycemia increases the number of macrophages and pro-inflammatory cytokines, directly affecting phagocytosis, chemotaxis, and leukocyte activity[40,41]. The imbalance of immune cells leads to the deterioration of the immune environment of the wound, propagating the inflammatory phase and impairing wound healing[39]. This delayed and incomplete wound healing process leads to impaired healing is thought to underlie development of DFU[42]. Acute wounds typically heal with time, while chronic wounds do not due to continuation of the early inflammatory reaction[40]. Non-healing wounds act as entry points for microorganisms implicated in wound infection[43]. Adequate control of hyperglycemia can accelerate wound healing and help to avoid adverse effects on cellular immunity and infection[41].
Dysfunction of immune cells like neutrophils and monocytes can lead to oxidative stress and inflammation during diabetic wound healing[42]. Diabetic wounds are permanently arrested in the inflammatory phase, which promotes wound infection. Deficits in the innate immune response can also contribute to infection[43]. In addition, hyperglycemia can impair the proliferation and migration of fibroblasts and keratinocytes, resulting in unsuccessful epithelization[43].
Neutrophils participate in the early stages of wound healing[42]. At the wound site, there are high levels of neutrophil elastase, which originates from neutrophil extracellular traps (NETs) and contributes to the degradation of the wound matrix, thus delaying wound healing, especially in diabetic patients[42].
Macrophages comprise two main types, namely inflammatory macrophages (M1) and wound-healing macrophages (M2)[42]. During the normal wound healing process, there is a predominance of M1 macrophages in the initial stages, followed by polarization of these cells into M2 macrophages[39]. Macrophages initiate the inflammatory phase of diabetic wound healing, while the delayed polarization of macrophages from pro-inflammatory (M1) to the anti-inflammatory (M2) type leads to aberrant wound healing and chronic inflammation[42]. M1 macrophages eliminate pathogenic bacteria, cellular debris, and damaged matrix, promoting inflammation[39]. M2 macrophages are anti-inflammatory and have the ability to suppress the inflammatory response by releasing IL-4 and IL-10[39]. Hyperglycemia prevents the polarization of macrophages from the M1 to the M2 phenotype[39]. This is important, as the polarization from M1 to M2 enables the timely restoration of damaged skin[42]. Furthermore, hyperglycemia and an immunosuppressive environment in individuals with diabetes lead to macrophage dysfunction and reduce phagocytic capacity[42]. The proportion of M1 macrophages in diabetic wounds is increased, and impaired polarization to the M2 phenotype in diabetic wounds is associated with reduced angiogenesis, poor collagen deposition, and decreased wound closure[42]. Depletion of anti-inflammatory M2 macrophages in the wound causes further tissue damage[42].
Diabetic patients, especially those with DFU, have a significantly lower number of naïve T-cells and an increased number of memory and effector T-cells[40,42,44]. In addition, the expression of inflammatory chemokine receptors is significantly reduced in individuals with diabetes[40,44]. The decrease in the diversity of T-cell receptors and the proliferation of effector T-cells can be seen as biomarkers of inflammation due to chronic DFU[42]. A subpopulation of regulatory T-cells (Treg) promotes the repair and regeneration of various tissues, including the skin[40]. Treg plays an essential role in angiogenesis and tissue regeneration in diabetic wounds, and people with diabetes have impaired function of these cells[40,42]. Specifically, diabetes sustains a constant pro-inflammatory environment with elevated levels of IL-1, TNF-α, and IL-6 and regulation of regular T cell expression[42].
In a nutshell, wound healing is a complex process that results in the establishment of normal physiological function[43]. The wound healing process involves several overlapping phases (coagulation, inflammation, proliferation, and remodeling)[40,43]. During the normal wound healing process, there is an initial recruitment of platelets that create a fibrin clot, followed by the recruitment of inflammatory cells (monocytes and neutrophils) that release pro-inflammatory cytokines[40,43]. After the inflammation subsides, a proliferative phase follows, characterized by angiogenesis (i.e. the creation of new blood vessels) and the polarization of M1 macrophages into M2 macrophages[43]. M2 macrophages promote the secretion of anti-inflammatory cytokines and angiogenesis, as well as the proliferation of fibroblasts and the formation of extracellular matrix[40]. Consequently, scar tissue formation and remodeling into healed tissue ensues[40].
In diabetic wounds, this process is somewhat different. Diabetic wounds demonstrate impaired recruitment of platelets and increased recruitment of neutrophils and M1 macrophages, resulting in an increased pro-inflammatory response and damage to the surrounding tissue[40]. Due to the increased secretion of inflammatory cytokines, this phase is prolonged in DFU, making it more difficult to progress to the next stage of healing[43]. As the inflammatory phase is prolonged, angiogenesis does not occur. Poor microcirculation then creates a hypoxic environment, leading to oxidative stress, inflammatory polarization of M1 macrophages, and damage to fibroblasts[40]. Polarization to M2 macrophages is very weak, and as a result damaged cells remain in an inflammatory state and re-epithelialization does not occur, ulceration of the skin occurs, and the wound fails to heal[40,43].
MICROBIOLOGICAL PROFILE OF DIABETIC FOOT INFECTIONS
The microbiological causes of diabetic foot infection, which is a frequent complication of DFU, have been increasingly clarified with the pervasive utilization of both classical microbiological and molecular methods. One of the most important challenges is to delineate microorganisms that are purely incidental from those which are true pathogens, as it is known that the infections of diabetic foot may harbor a plethora of different species[45,46]. There may also be a question of contamination, where the microbiological analysis of the diabetic foot is further hindered by the potential presence of commensal bacteria in clinical samples[46]. On the other hand, conventional culturing methods may also give rise to false negative results, as more than 37% of samples are considered culture-negative[45].
In accordance with the guidelines published by the Infectious Diseases Society of America (IDSA), diabetic foot infections can be classified into three distinct categories: Limited superficial infections presenting with mild symptoms; deeper moderate infections leading to more pronounced symptoms; and severe infections with metabolic changes and true systemic response[47,48]. These various clinical presentations warrant different approaches in treatment, as superficial and moderate infections necessitate the administration of narrow-spectrum antimicrobials orally or intravenously for only a short period of time, whereas severe infections require parenteral administration of broad-spectrum antibiotics[49]. However, the exact characterization of a causative pathogen enables a more targeted treatment approach, as antimicrobial sensitivity testing can be pursued in this scenario[49].
A recent meta-analysis has shown that the most frequent aerobic microorganisms isolated from diabetic foot infection are Staphylococcus aureus (23.4%), Escherichia coli (11.5%), Pseudomonas spp. (11.1%), Proteus spp. (8.3%), Klebsiella spp. (6.9%) and Enterococcus spp. (5.4%)[46]. There was also a high frequency of coagulase-negative staphylococci observed in this study, likely indicating a combination of true pathology and sample contamination when commensals are introduced into damaged and dysfunctional tissues. Among those infections caused by S. aureus, a significant prevalence of methicillin-resistant S. aureus (MRSA) was observed (18%), which is in line with previous estimates[46,50,51]. Other studies have shown that harbingers of protracted infections and frequent relapses are usually of two bacterial genera: Acinetobacter spp. and Enterococcus spp.; however, Citrobacter spp. predominate in DFU affecting women[49,52,53]. Interestingly, a higher prevalence of Gram-positive microbial isolates in diabetic foot wounds is generally observed in high-income countries when compared to low- and middle-income countries, where Gram-negative isolates predominate. This may reflect differences in hygiene, sanitation, and footwear usage[46,54,55].
In any case, the monomicrobial vantage point is rather narrow. The use of molecular techniques in recent studies have corroborated the assumption of a polymicrobial nature of chronic wounds including diabetic foot ulcers[56-58]. More specifically, bacteria and other organisms found in foot infections may demonstrate specific non-random polymicrobial patterns that can correlate with clinical factors and wound chronicity[59,60]. This could have direct clinical implications for the optimization of antimicrobial treatment (particularly if the aim is to cover all potential organisms pertinent for such processes) and understanding further evolution of polymicrobial diabetic foot infections (from the perspective of interactions that could have a synergistic or alleviating effect on microbial burden, expression of genes, or pathogenicity)[61-63].
A recent study by Barshes et al[63] suggested three species co-occurrence patterns: (1) The most pervasive S. aureus-dominant pattern (characterized by the absence of Staphylococcus epidermidis and other coagulase-negative or nonspeciated staphylococci); (2) A coagulase-negative staphylococci dominate pattern (i.e. the inverse of the S. aureus-dominant pattern with the absence of S. aureus); and (3) A so-called “pattern C,” characterized by the absence of Bacteroides spp. and Corynebacterium spp. and the presence of two or more of alpha-hemolytic streptococci, E. coli, Klebsiella spp., Enterobacter spp., Proteus spp., or Enterococcus faecalis. Simply put, these co-occurrence patterns show that S. aureus is rarely seen with coagulase-negative staphylococci, while Proteobacteria can be seen with enterococci and alpha-hemolytic streptococci, but not with corynebacteria. In addition, patients that have a polymicrobial diabetic foot infection belonging to the aforementioned “pattern C” group have substantially higher rates of treatment-resistant osteomyelitis[63].
Similar patterns have been reported in other studies. Gardner et al[59] discerned a Staphylococcus-dominated pattern, a streptococci-dominated pattern, and a high-diversity pattern, the latter typically including members of the phylum Proteobacteria. This means that diabetic foot infections are more commonly polymicrobial in nature when compared to bone and soft tissue infections in other locations, and that the spectrum of microorganisms isolated from foot bone and soft tissue can be comparable among different individuals but distinct from that isolated from other locations in the body[63,64]. This is not simply an academic exercise, as the recognition of specific microbial profiles and patterns has important clinical implications. For example, recognizing either polymicrobial “pattern C” or isolating P. aeruginosa can be considered high risk for treatment failure, and a recent study showed that 15% of patients with infection caused by P. aeruginosa ultimately required amputation[49]. Understanding these nuanced interactions might prompt specific intervention; for example, probiotics may be used to fill the niche with beneficial bacteria and eliminate hazardous ones[65,66].
Finally, the frequency of antimicrobial resistance is becoming a true public health concern and is essential to consider in the treatment of diabetic foot infections[67,68]. Even studies published 10 years ago have demonstrated a high prevalence (up to 33%) of resistant bacteria present in diabetic ulcers[69,70]. The fact that diabetic patients are prone to developing foot infections and that PAD and cigarette smoking may increase the risk of diabetic foot infection[47,49,53], the control of these infections may become increasingly difficult with the continued rise of resistant microorganisms. As a result, there will likely be many negative downstream effects for the healthcare system.
Bacterial biofilm formation also plays a major role in the development of chronic DFU. Biofilms form when commensal and pathogenic bacteria symbiotically merge, perpetuating chronic infection[71]. The presence of biofilms often necessitates complex treatment, which contributes to antibiotic resistance[71]. The most common reason for unsuccessful treatment of biofilm infections with conventional antibiotics is the inability of drugs to pass through the exopolysaccharide matrix formed by sessile cells[72,73]. The seriousness of such infections is demonstrated by the fact that 80% of lower limb amputations in patients with diabetes and foot ulceration are caused by biofilm-forming species. Overall, biofilm is present in 60% of chronic wounds and 6% of acute wounds associated with DFU[74]. For the detection of biofilm in deep tissues, the appropriate sample is a tissue biopsy. Techniques for quantifying biofilm in biopsy specimens include fluorescence in situ hybridization, scanning electron microscopy, and confocal laser scanning microscopy[75-79]. Due to the presence of biofilm, treatment of DFU with conventional antibiotics is problematic, as resistance to these drugs quickly develops[14,71]. Accordingly, new strategies must be developed to solve this problem. Phage, silver nanoparticle, and antimicrobial peptide therapies are being introduced as alternatives to antibiotic drugs[72,80-82].
Fungal pathogens may also compound the issue of diabetic foot infection. Their prevalence varies between 5% and 27%, with Candida spp. being the most frequently isolated organisms[83-85]. Mold infections are much less prevalent, but can be more menacing in comparison to infections in which only Candida is implicated. However, there are just a handful of case reports documenting infections with Aspergillus ochraceus or species of the genera Blastomyces or Fusarium, with skin and nails being the main entry portals[84,86,87]. In any case, consideration of fungal or mold infection must be included in the approach to diabetic foot infection in order obtain a full microbiological profile to guide treatment.
CLINICAL PRESENTATION
The diabetic foot at first glance may have a seemingly normal appearance, and symptoms and signs of complications can easily be missed. However, the clinical presentation of the diabetic foot has some specific characteristics that are important for every clinician to recognize, especially those actively caring for the diabetic patient population.
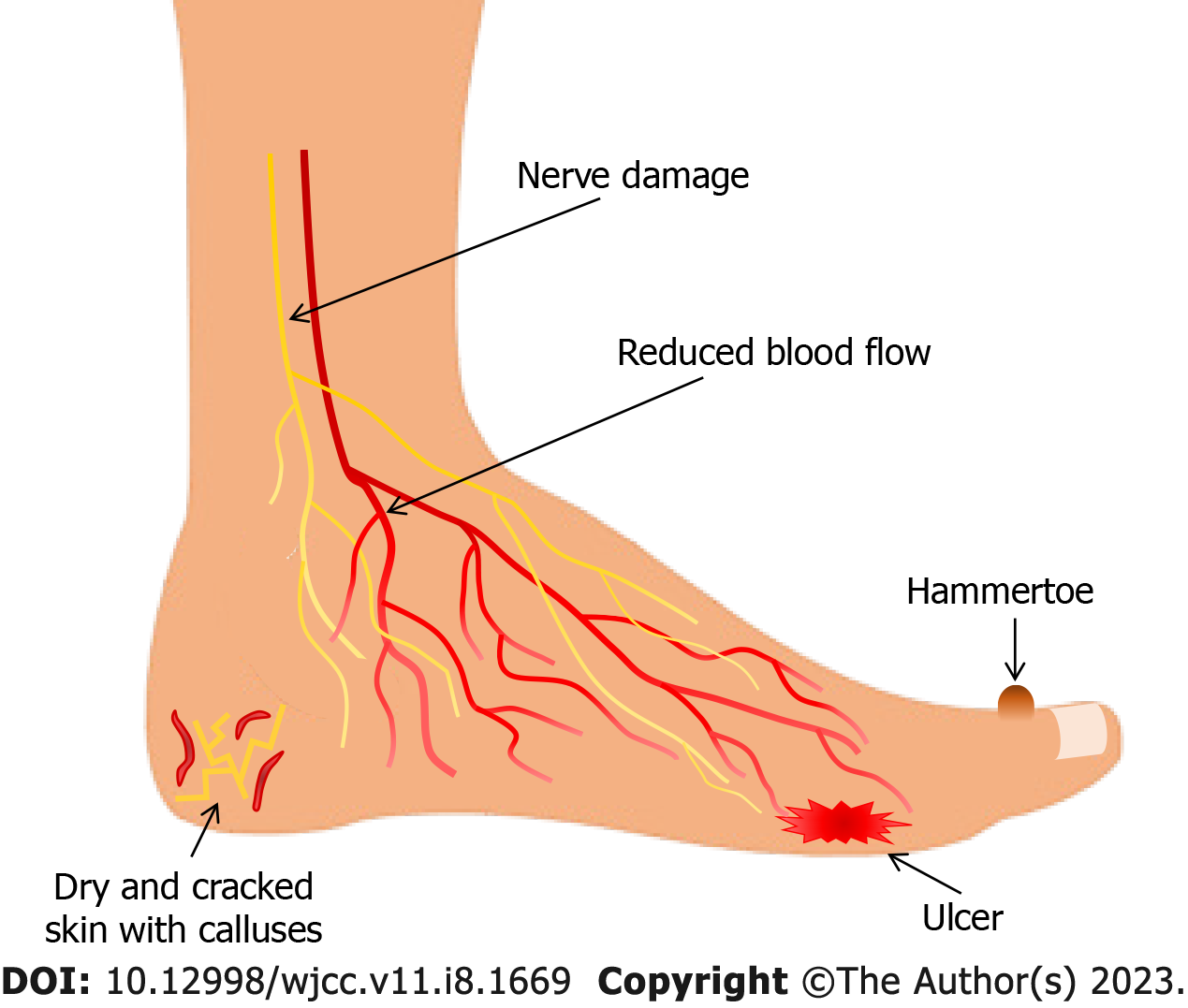
Due to atrophy of lumbrical and interosseus muscles caused by motor neuropathy, the anatomy of the diabetic foot changes such that the arch and toes are pulled in a "claw" position with prominent metatarsal heads, hammertoe contracture of digits, and other bony prominences. These deformations can create focal areas of high pressure at which susceptibility for ulcer development is increased. Charcot neuroarthropathy (CN) is a severe diabetic foot complication that significantly increases morbidity and mortality, and primarily is seen in patients with concomitant DFU. Patients with CN have a life expectancy reduced by an average of 14 years. CN is characterized by bone and joint destruction, and can be asymptomatic or can mimic other more common conditions such as cellulitis, osteomyelitis, deep vein thrombosis, inflammatory arthritis, or ankle sprain. Due to variability in the presence of symptoms and the variety of potential mimickers, CN is often overlooked in the evaluation of the diabetic foot. In the acute phase, this condition presents as a warm, swollen red joint, often in the absence of pain. In the early stages of CN, there are no clinical signs of bone fracture, but radiological examination usually shows microfractures. Up to 58% of patients with CN also have DFU on initial presentation. Additionally, CN can lead to mid-foot collapse, rocker-bottom foot (collapse and inversion of the plantar arch), acute fracture, and joint dislocation if left untreated[6,23]. With or without extreme deformities such as those seen in CN, the skin of the diabetic foot is usually dry and cracked and often has calluses indicative of increased pressure. In addition, pre-ulcerative signs such as localized redness, blisters, fissures, or hemorrhage may be present[36,37] (Figure 1).
Figure 1 Clinical presentation of the diabetic foot.
If concomitant with PAD, the diabetic foot appears pale and cold. However, in an ischemic setting, the foot may conversely be warm and pink due to the presence of arteriovenous shunts, the early stages of CN, or fracture. Moreover, tissue ischemia can manifest as pain at rest, claudication, gangrene, or ulceration. However, classical signs and symptoms of PAD may not always be present in diabetic patients with PAD[20]. Therefore, the assessment of vascularization in diabetes should not be performed by clinical examination only. Moreover, clinical signs and symptoms of DFI are often diminished due to PAD, neuropathy, and immunopathy[31]. These signs and symptoms include redness, warmth, swelling, pain, and purulent secretion. Furthermore, even in the presence of deep infection, usual systemic signs of infection (e.g., fever, elevated white blood cell count) may or may not be apparent. C-reactive protein (CRP) concentrations can be absent or diminished, also contributing to delayed diagnosis. If CRP levels are elevated, this could indicate severe infection, potentially limb- or life-threatening[5,31,88]. Sometimes, the only sign of infection in the setting of DFU can be unexplained hyperglycemia[6]. If a foot ulcer is present, the most common infection symptom is excessive exudate. However, an accurate clinical assessment of an ulcer can be hampered by the presence of a superficial eschar, which must be removed to reveal possible abscess and involvement of deep structures[6,89,90].
DIAGNOSTIC EVALUATION
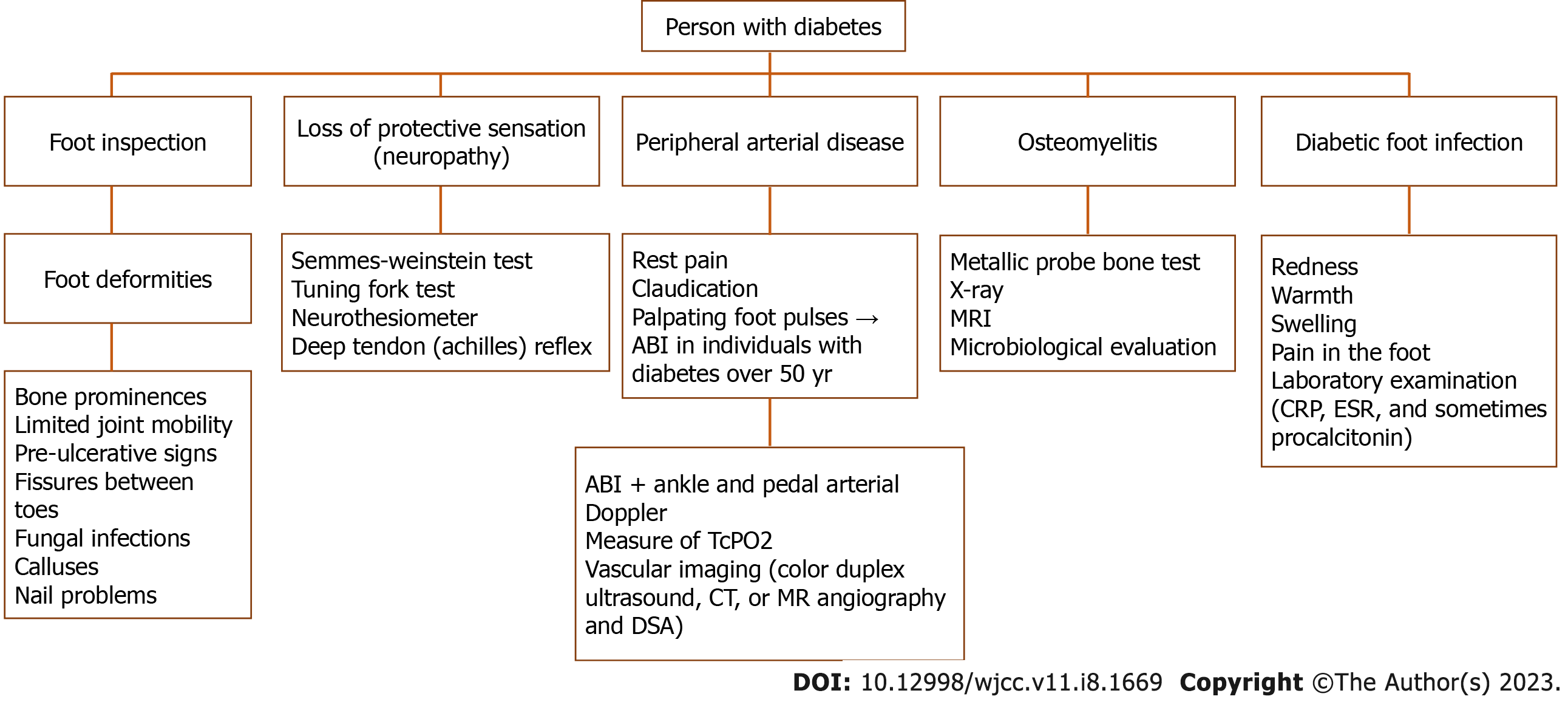
All patients with diabetes should be screened annually to assess the risk of foot complications (Figure 2). These patients should be stratified into high-risk and no-risk using the International Working Group on the Diabetic Foot (IWGDF) risk stratification system to establish the necessary frequency of further visits and examinations. This basic evaluation is part of the prevention strategy and includes foot inspection, evaluation of loss of protective sensation (i.e. neuropathy), and assessment of PAD. For full risk assessment, the primary evaluation should also include taking of a general medical history as well as a foot-specific history to include instances of previous foot ulceration or amputation. Special attention should be given to assessment of pertinent comorbidities, especially end-stage renal disease[90].
Figure 2 Flow diagram for screening for foot complications in persons with diabetes.
ABI: Ankle-brachial index; CRP: C-reactive protein; DSA: Digital subtraction angiography; ESR: Erythrocyte sedimentation rate; TcPO2: Transcutaneous oxygen pressure.
Foot inspection should establish any presence of foot deformities, bony prominences, limited joint mobility, and pre-ulcerative signs. Also, the clinician should actively and thoroughly search for other signs of ulceration or infection, which often can be hidden (e.g., between the toes). The clinician should also inspect for and document any fissures, fungal infections, calluses, or nail problems[90].
Almost all working groups agree that the Semmes-Weinstein test is the most important in the evaluation of neuropathy, as studies have shown that it has high sensitivity for identifying patients at risk and is predictive of foot ulceration[5,9,91]. This test is performed by applying pressure stimulation to defined areas on the foot with nylon monofilaments[6,9]. Other tests (e.g., tuning fork and neurothesiometer tests) can also assess for the presence of neuropathy, but studies have shown that these are less predictive of foot ulceration[6]. The deep tendon (Achilles) reflex should be also examined[5,23].
In all patients with diabetes, vascularization should be assessed annually by taking the relevant history (i.e. rest pain, claudication) and palpating foot pulses. The most valuable noninvasive diagnostic method for diagnosing PAD is the ankle-brachial index (ABI)[20]. ABI should be performed in all individuals with diabetes aged 50 or older, regardless of the presence of other risk factors. If other risk factors are present, this test should be performed annually. This approach should also be used for all patients with diabetic foot ulcers[9], as it will not only aid in the diagnosis of PAD but also in evaluating the potential for ulcer healing, the need for revascularization, and the risk for limb amputation[92]. ABI is usually combined with ankle and pedal arterial Doppler assessment[20]. Because of frequent advanced arterial calcification in people with diabetes, ABI can be falsely elevated. Therefore, it is often recommended to measure toe or transcutaneous oxygen pressure, both of which have been shown to be good predictors of ulcer healing potential or the need for revascularization[5,20]. If the ulcer is not healing, when considering revascularization or when critical ABI is present, vascular imaging should be ordered. Useful imaging modalities include color duplex ultrasound, computed tomography, methicillin-resistant angiography and, in some instances, digital subtraction angiography[20].
For the diagnosis of diabetic foot infection, including infection of DFU, it is recommended to start with appraising classical clinical signs of infection such as redness, warmth, swelling, pain in the foot, and possible entry of pathogens through minor skin fissures[31]. Additionally, the most common sign of an infected ulcer is increased exudate. However, the severity of infection should be assessed after wound debridement and evaluation of the extent and depth of infection with consideration of any masking superficial eschar[5,88]. If clinical findings are unclear, laboratory examination with CRP, ESR, and sometimes procalcitonin should be performed. However, diagnosis of infection in individuals with diabetes can be challenging as signs and symptoms are often subtle.
Further difficulties include diagnosing deep tissue infection, osteomyelitis, and CN, which can mimic infection. If osteomyelitis is suspected, a metallic probe bone test should be performed[31]. If a sterile probe hits the bone, the diagnosis of osteomyelitis is highly likely, with a positive predictive value of 89%[93]. A positive probe test, elevated CRP or procalcitonin, and plain X-ray findings compatible with osteomyelitis are sufficient for diagnosis. However, further diagnostic procedures should be performed in the case of equivocal findings. The plain X-ray has low sensitivity and specificity for osteomyelitis. Still, it should always be performed in patients with new DFI to detect possible foreign bodies, soft tissue gas, bone destruction, fracture, or deformity[9,94]. To diagnose osteomyelitis, it is better to perform two X-ray studies with an interval of at least 2 wk to detect changes in radiologic appearance[48]. At the moment, magnetic resonance is considered the best imaging modality, with 90% sensitivity and 79% specificity for osteomyelitis[94]. It is rarely necessary to obtain a bone specimen by debridement or biopsy for diagnosis. Still, an appropriate sample of the infected wound should always be collected for culture and microbiological evaluation before starting empirical therapy. Wound swabs should be avoided; the preferred method for sample collection is curettage or biopsy of the ulcer[31].
If a DFU is present, not only potential limb ischemia and infection should be assessed, but the characteristics of the ulcer should also be described. The most significant are the localization, size, and depth of the ulcer, as well as any presence of gangrene. Following reduction in the size of the ulcer, it is possible to predict treatment outcomes[95,96].
MANAGEMENT
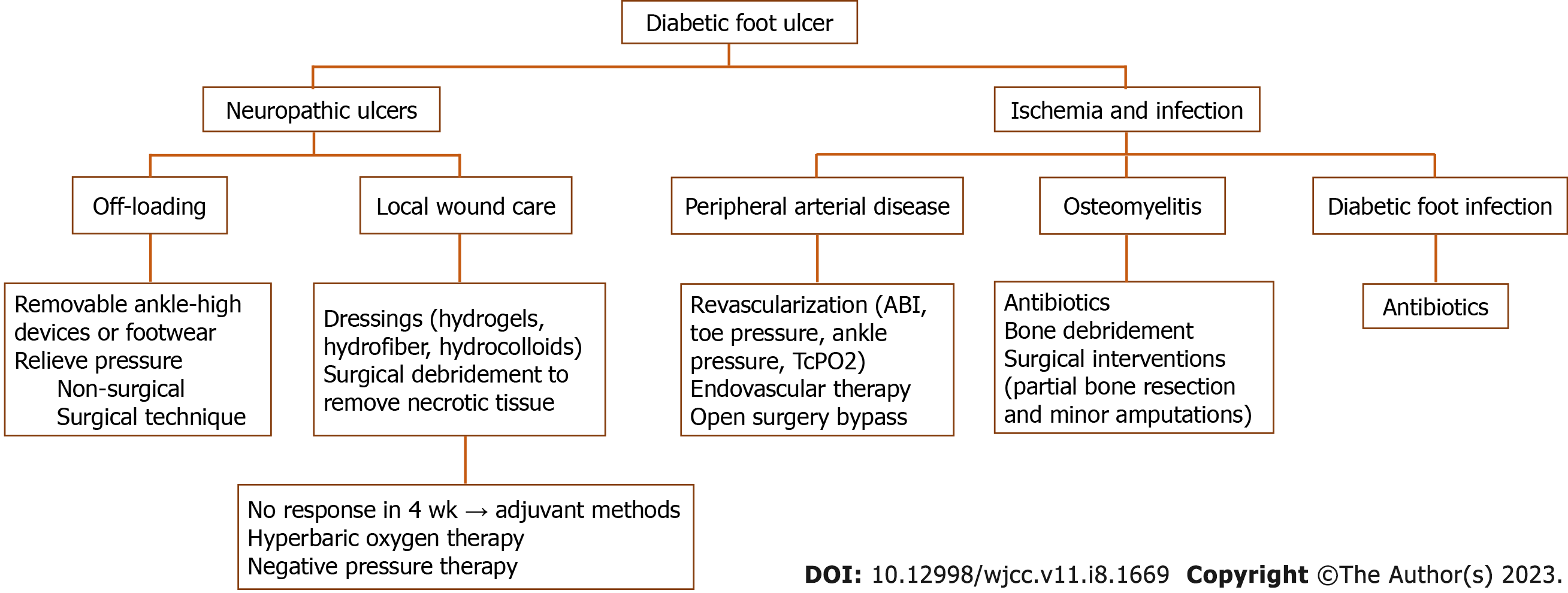
In consideration of the complexity of diabetic foot pathophysiology, the necessity of a multidisciplinary approach to treating its complications is very evident (Figure 3). Studies have shown that adequate glycemic control with a glycosylated hemoglobin (HbA1c) goal of less than 6.5%-7% is important in preventing DFU and other complications, and can also significantly decrease the risk of amputation and improve the rate of wound healing[9,97,98]. Management should be directed towards all established contributing etiological factors to achieve adequate healing of the DFU. In the case of purely neuropathic ulcers, offloading and local wound care is most likely sufficient. Still, in the case of concomitant ischemia and infection, treatment may be more complicated.
Figure 3 Algorithm for management of diabetic foot ulcer.
ABI: Ankle-brachial index; TcPO2: Transcutaneous oxygen pressure.
Offloading strategies aim to relieve pressure on the extremity and prevent high-pressure focal zones, the most common site of ulcer development. A simple debridement of nonviable tissue or callus around the ulcer is often the best starting point for distributing the pressure. There are many offloading techniques available. If non-surgical offloading fails to promote healing of the ulcer, even with appropriate standard wound care, surgical offloading must be considered. The site of the ulcer determines the selection of the appropriate offloading device. For example, if the ulcer is located on the plantar side of the foot, a non-removable knee-high device like a total contact cast or non-removable knee-high walker are usually recommended. Removable knee- or ankle-high devices are recommended in case of contraindication or patient intolerance. Additionally, the offloading strategy should be determined accordingly if moderate or severe infection or ischemia are present[16]. Plantar heel ulcers are less prevalent than plantar forefoot or mid-foot ulcers. These ulcers are characterized by higher pressures, longer healing time, and more cumbersome pressure decrease via offloading[16,17,99]. If the ulcer is localized on the dorsal foot, removable ankle-high devices or footwear modifications, orthoses, or toe spacers can be used. It is extremely important to address patient compliance, as patients frequently forgo wearing recommended offloading devices[9,100]. Surgical techniques can help offload pressure from the ulcer and promote healing in selected patients. In patients with plantar metatarsal head ulcer, Achilles tendon lengthening, metatarsal head resection, or joint arthroplasty, surgery should be considered. Moreover, in patients with apex or digital plantar ulcers, digital flexor tenotomy can help decrease pressure to the ulcer site[16]. Also, surgical correction should be considered if there are foot deformities that cannot be managed with therapeutic footwear[5].
In patients with DFU and PAD, revascularization should always be considered, especially in those with a severe degree of ischemia as established by one or more appropriate test (i.e. ABI, toe pressure, ankle pressure, TcPO2) or in patients with non-healing ulcer and PAD regardless of test results. An ABI of 0.9-1.3 suggests that PAD is less likely, and an ABI less than 0.8 is associated with an increased risk of limb amputation[92]. Usually, it is recommended to use wound, ischemia, and foot infection classification to predict which patients with diabetes and PAD are more likely to require and benefit from revascularization[9,20]. There are two approaches to revascularization: Endovascular therapy and open surgical bypass. Randomized clinical trials providing evidence favoring one technique over the other are lacking[5,101]. However, there has been significant progress in endovascular medicine, and there are emerging endovascular techniques such as drug-eluting technologies for PAD management. Nevertheless, high-quality randomized studies to evaluate the efficacy of these techniques in diabetic foot patients are still lacking[102]. Furthermore, recent reports suggest an innovative treatment alternative for patients without endovascular or surgical options. Intravascular ultrasound (IVUS), a guided percutaneous deep vein arterialization with the creation of an arteriovenous fistula between the posterior tibial artery and its satellite deep vein, has shown promising results in such patients with critical limb ischemia[103]. Special consideration should be given to patients with DFU and PAD who are candidates for revascularization but have invasive foot infections, as they are at exceptionally high risk of amputation. In that case, the infection should be controlled before revascularization is pursued to avoid sepsis. Appropriate and aggressive therapy (i.e. surgical interventions and antibiotics) usually takes a few days to stabilize the patient. After stabilization, prompt revascularization should be considered to help resolve infection and improve circulation to avoid limb amputation[20].
Antibiotics are key to treating DFI, but they are often insufficient in controlling the infection, especially in the presence of polymicrobial infection, as described above. Hence, surgical treatment in managing DFI is often required due to the special features of DFI. Indications for surgical interventions include the involvement of deep tissues (especially bone), abscess formation, the presence of necrotic tissue, compartment syndrome, and extensive gangrene. In such cases, prompt surgical incision allowing abscess drainage and debridement of necrotic tissue must be performed. Uncomplicated osteomyelitis can be initially treated with antibiotics for no longer than 6 wk. However, his strategy has limited long-term results in controlling infection[104,105]. Thus, surgical intervention such as partial bone resection or minor amputation is often necessary to manage infection. Selection, duration, and the route of antibiotic administration in treating DFI should be based on the likely causative pathogen and the clinical severity of infection. It is always preferable to prove a causative pathogen by tissue culture[31]. Using IDSA/IWGDF infection classification is also recommended to guide the management of DFI[9,106]. Finally, amputation below the knee may be necessary if minor amputation is insufficient to control infection or in the case of extensive tissue loss or severe tissue ischemia after failed revascularization[5,107]. Generally, surgeons should try to preserve the knee joint if possible as ambulation is significant in successful rehabilitation[6].
Finally, appropriate standards of local wound care should be followed to promote DFU healing. This involves frequent clinical evaluation with irrigation and debridement and use of modern dressings. There are different debridement methods available, but sharp surgical debridement is usually recommended to remove necrotic tissue in DFU[9,108]. Other possible techniques are hydrogels, occlusive dressings, larval therapy, enzymes, ultrasound, and hydrotherapy, but studies have yet to prove that any method is better than the others[109]. Dressings should provide optimal conditions for wound healing, including maintenance of a moist wound bed, exudate control with prevention of maceration of the surrounding skin, prevention of infection, and promotion of granulation tissue. They should also be comfortable for the patients and enable atraumatic dressing changes. Modern (advanced) non-adherent dressings usually meet these requirements. The most commonly used dressings are hydrogels, hydrofiber dressings, hydrocolloids, foam dressings, and alginates. Still, studies showed that none of these is superior to the others in promoting wound healing, and the choice is usually made based on the assessment of wound characteristics, comfort, and cost[9,108].
If improvement in DFU healing is not seen after a minimum of 4 wk of treatment with the standard of care, adjuvant methods should be considered. Hyperbaric oxygen therapy and negative pressure therapy are most often recommended[110]. However, before any other treatment modality is used, reevaluation of vascular status, the presence of infection, and high-pressure zones should be pursued[9,108].
CONCLUSION
Although knowledge of diabetic foot problems has grown tremendously in recent years, there are still unmet therapeutic needs. DFU, often associated with infection or ischemia, is thought to precede the majority of diabetes-related lower extremity amputations and is the leading cause of non-traumatic lower extremity amputation worldwide. In patients with peripheral neuropathy or PAD, DFUs mainly result from mild or recurrent trauma to the foot. The pathophysiology of diabetic wound healing is complex, multidimensional, and remains to be fully understood. In addition, more than half of DFUs become infected, which impairs wound healing and increases the likelihood of foot amputation. The management of the diabetic foot is complex for any healthcare provider; however, as the number of people with diabetes increases, so does the need for DFU treatment. Hence, more targeted research endeavors are needed for establishment of an evidence-based approach to diabetic wound healing and improving patient quality of life.