Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1467
Peer-review started: December 7, 2022
First decision: January 3, 2023
Revised: January 4, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 6, 2023
Processing time: 85 Days and 1.9 Hours
The incidence rate of cerebral infarction in young people is increasing day by day, the age of onset tends to be younger, and its internal pathogenesis and mechanism are very complicated, which leads to greater difficulties in treatment. Therefore, it is essential to analyze the key pathway that affects the onset of cerebral infarction in young people from the perspective of genetics.
To compare the differentially expressed genes in the brain tissue of young and aged rats with middle cerebral artery occlusion and to analyse their effect on the key signalling pathway involved in the development of cerebral ischaemia in young rats.
The Gene Expression Omnibus 2R online analysis tool was used to analyse the differentially expressed genes in the GSE166162 dataset regarding the devel
Thirty-five differentially expressed genes (such as Igf2, Col1a2, and Sfrp1) were obtained; 73 GO enrichment analysis pathways are mainly involved in biological processes such as drug response, amino acid stimulation response, blood vessel development, various signalling pathways, and enzyme regulation. They are involved in molecular functions such as drug binding, protein binding, dopamine binding, metal ion binding, and dopamine neurotransmitter receptor activity. KEGG pathway enrichment analysis showed a significantly enriched pathway: The cyclic adenosine monophosphate (c-AMP) signalling pathway.
The c-AMP signalling pathway might be the key pathway in the intervention of cerebral infarction in young people.
Core Tip: This study mainly used the analysis technology related to biogenetic informatics to retrieve the samples of young cerebral ischemia rats from the authoritative global Gene Expression Omnibus database, identified the differential genes related to the onset of cerebral ischemia in young rats through the differential gene analysis method, further carried out Gene Ontology function enrichment analysis and Kyoto Encyclopedia of Genes and Genomes function enrichment analysis, and finally obtained the key gene pathway that affects ischaemic stroke in young rats. The findings provide clues for further realizing the transformation of basic medicine into clinical practical application, so as to finally realize the precise target intervention of young people with ischemic stroke and reduce the disability rate and mortality rate.
- Citation: Xia Y, Liu H, Zhu R. Analysis of differentially expressed genes related to cerebral ischaemia in young rats based on the Gene Expression Omnibus database. World J Clin Cases 2023; 11(7): 1467-1476
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1467.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1467
Cerebral infarction has the characteristics of a high disability rate, high mortality rate, and high recurrence rate. It has been the second leading cause of death in the world and seriously endangers human health. With rapid economic and social development, the incidence of cerebral infarction in young people is gradually increasing, and the age of onset tends to be younger. Compared with cerebral infarction in elderly individuals, the aetiology of cerebral infarction in young people is more diversified, which leads to certain difficulties in diagnosis and treatment. Studies have shown that there are approximately 2 million new young people with cerebral infarction every year in the world, but its treatment methods and effects are still limited[1]. Young people are the backbone of the family, society, and even the country. Once disabled, they will cause considerable damage to society and family.
The Gene Expression Omnibus (GEO) database is a public gene expression database created and maintained by National Center for Biotechnology Information in the United States in 2000. It contains a large amount of gene expression data, such as next-generation sequencing data, chip sequencing, and single-cell sequencing (including rich human clinical data and various animal model data). To find suitable intervention targets and more reasonable and effective treatment methods, we analysed the gene chip data of young cerebral infarction rats and old infarcted rats in the GEO database to identify the key gene pathways that distinguish these groups from one another.
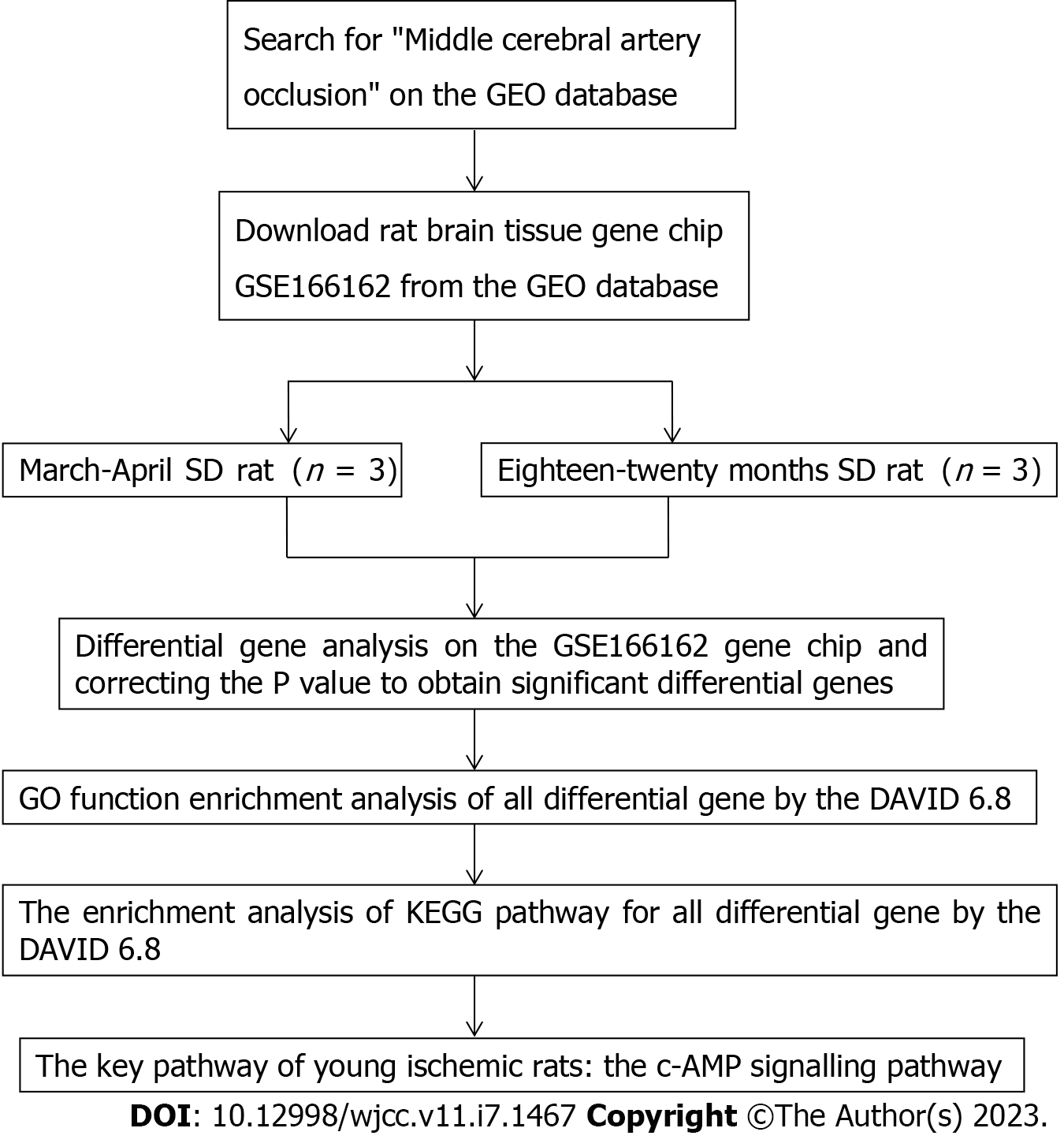
The GEO database (http://www.ncbi.nlm.nih.gov/geo) was searched with the key word "middle cerebral artery occlusion (MCAO)", the deadline was September 2022, and the GSE166162 dataset of the brain tissue of rats in the young MCAO group and the aged MCAO group was downloaded. The GSE166162 dataset included male rats aged 3-4 mo and weighing 340-400 g and male rats aged 18-20 mo and weighing 585-685 g. In this study, the gene chip data of brain tissue was identified according to the principles of rat grouping, age, sampling site, sex, and strain to avoid the influence of differential genes on other factors. Male SD rats aged 3-4 mo and male SD rats aged 18-20 mo were used as the research subjects, and the brain tissue was used as the sampling site. Finally, three rat samples in the young MCAO group were numbered GSM5065037, GSM5065038, and GSM5065039, and three rat samples in the aged MCAO group were numbered GSM5065040, GSM5065041, and GSM5065042 (Table 1).
| Sample number | Strain | Gender | Rat age | Grouping | Sampling site |
| GSM5065037 | SD rat | Male | March-April | Young MCAO | Brain tissue |
| GSM5065038 | SD rat | Male | March-April | Young MCAO | Brain tissue |
| GSM5065039 | SD rat | Male | March-April | Young MCAO | Brain tissue |
| GSM5065040 | SD rat | Male | Eighteen-twenty months | Aged MCAO | Brain tissue |
| GSM5065041 | SD rat | Male | Eighteen-twenty months | Aged MCAO | Brain tissue |
| GSM5065042 | SD rat | Male | Eighteen-twenty months | Aged MCAO | Brain tissue |
The GEO2R (http://www.ncbi.nlm.nih.gov/geo/geo2r) online analysis tool was used to perform differential gene analysis on the GSE166162 dataset. With logFC absolute value ≥ 1 and P < 0.05 as screening conditions, the differentially expressed genes in the brain tissue of the young MCAO group rats and the aged MCAO group rats were selected. In addition, up- and down-regulated genes significantly regulated in young cerebral ischaemia rats were screened out by adjusting P values.
To explain the pathways affected by differential genes in the brain tissue of cerebral infarction rats, all the differentially expressed genes were introduced into DAVID 6.8 (https://david.ncifcrf.gov/) for Gene Ontology (GO) function analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The overall analysis idea and specific analysis steps of this study are shown in a flow chart (Figure 1).
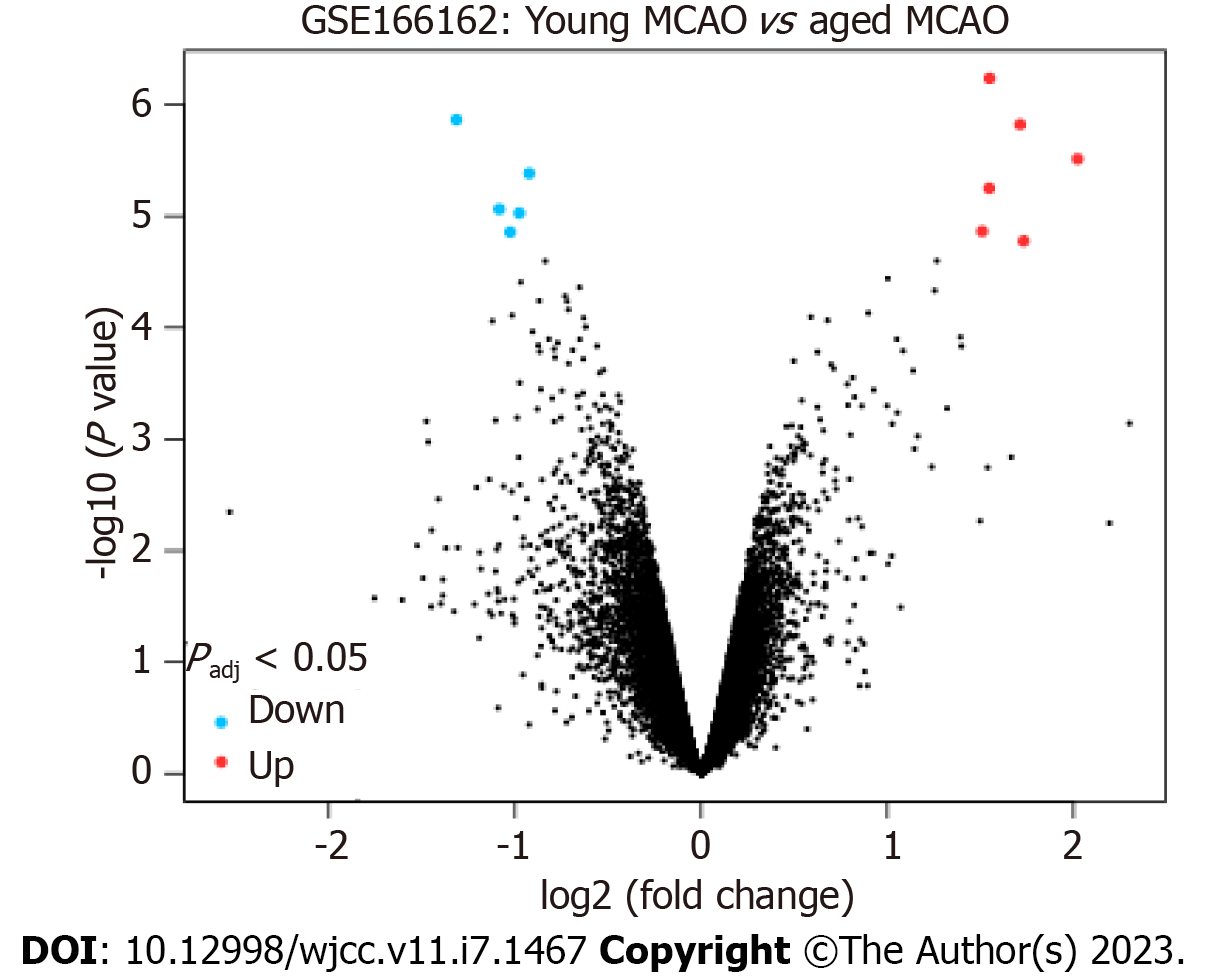
Using GEO2R to conduct differential gene analysis on the GSE166162 dataset, 35 differentially expressed genes were obtained. Among them, 25 were upregulated with positive logFC, and 12 with negative logFC were downregulated (Tables 2 and 3). In addition, the significantly upregulated and downregulated genes were further screened after adjusting the P value with volcano maps (Tables 4 and 5). A volcano map can visually display red upregulated genes and blue downregulated genes (Figure 2). The above methods were used to discern related genes that could play a key role in the pathogenesis of cerebral infarction in young rats. The abnormal expression of these genes might affect biochemical processes, molecular functions, and related gene pathways in rats.
| ID | P value | t | B | logFC | Gene symbol | |
| 1367571_at | 0.00000058 | 13.77 | 4.3557 | 1.55 | Igf2 | |
| 1387854_at | 0.0000015 | 12.2 | 3.9423 | 1.714 | Col1a2 | |
| 1370155_at | 0.00000306 | 11.14 | 3.5959 | 2.022 | Col1a2 | |
| 1371861_at | 0.00000562 | 10.29 | 3.2741 | 1.547 | Sfrp1 | |
| 1374172_at | 0.00001368 | 9.16 | 2.7581 | 1.51 | Col8a2 | |
| 1367592_at | 0.00001669 | 8.92 | 2.6355 | 1.732 | Tnnt2 | |
| 1390450_at | 0.00002515 | 8.45 | 2.3752 | 1.266 | LOC100910855Ogn | |
| 1372299_at | 0.00003639 | 8.03 | 2.1313 | 1.003 | Cdkn1c | |
| 1392510_at | 0.00004648 | 7.77 | 1.9652 | 1.255 | Fam180a | |
| 1370391_at | 0.0001211 | 6.81 | 1.2813 | 1.393 | LOC100911902Crabp2 | |
| 1370956_at | 0.00012686 | 6.76 | 1.2468 | 1.051 | Dcn | |
| 1390532_at | 0.00014662 | 6.62 | 1.1385 | 1.399 | Slc13a4 | |
| 1388116_at | 0.00016115 | 6.54 | 1.0672 | 1.085 | Col1a1 | |
| 1387197_at | 0.00024297 | 6.16 | 0.7521 | 1.139 | Omd | |
| 1370959_at | 0.00052678 | 5.49 | 0.1359 | 1.321 | Col3a1 | |
| 1367700_at | 0.00057972 | 5.42 | 0.0577 | 1.054 | Fmod | |
| 1385248_at | 0.00072166 | 5.24 | -0.1224 | 2.301 | LOC100910855Ogn | |
| 1385486_at | 0.00072866 | 5.23 | -0.1304 | 1.026 | Bnc2 | |
| 1367847_at | 0.00093931 | 5.03 | -0.3419 | 1.163 | Nupr1 | |
| 1373148_at | 0.00122143 | 4.82 | -0.5632 | 1.146 | Cpxm2 | |
| 1387306_at | 0.00144695 | 4.69 | -0.7073 | 1.666 | Egr2 | |
| 1390835_at | 0.00176986 | 4.54 | -0.8798 | 1.239 | Slc47a1 | |
| 1383263_at | 0.00179702 | 4.53 | -0.8929 | 1.539 | LOC100910855Ogn | |
| ID | P value | t | B | logFC | Gene symbol | |
| 1374503_at | 0.00000136 | -12.35 | 3.9877 | -1.314 | Pbx3 | |
| 1395359_at | 0.00000867 | -9.73 | 3.0291 | -1.084 | - | |
| 1390783_at | 0.00001389 | -9.14 | 2.7485 | -1.025 | Abca8a | |
| 1390052_at | 0.00007757 | -7.24 | 1.606 | -1.015 | LOC100912140 | |
| 1373250_at | 0.00008716 | -7.13 | 1.5221 | -1.12 | Anln/Anlnl1 | |
| 1396833_at | 0.00067817 | -5.29 | -0.0711 | -1.105 | - | |
| 1368708_at | 0.00068945 | -5.27 | -0.0847 | -1.475 | Drd2 | |
| 1386931_at | 0.00105762 | -4.93 | -0.4415 | -1.465 | Tnni3 | |
| 1383516_at | 0.00229766 | -4.35 | -1.1053 | -1.138 | Fgl2 | |
| 1368478_at | 0.00264936 | -4.25 | -1.2292 | -1.061 | Drd1 | |
| 1368300_at | 0.00271339 | -4.23 | -1.25 | -1.206 | Adora2a | |
| 1383578_at | 0.00295369 | -4.17 | -1.3241 | -1.017 | LOC100911267Rad51 | |
| ID | Adjusted P value | t | B | logFC | Gene symbol |
| 1367571_at | 0.0156 | 13.77 | 4.3557 | 1.55 | Igf2 |
| 1387854_at | 0.0156 | 12.2 | 3.9423 | 1.714 | Col1a2 |
| 1370155_at | 0.0238 | 11.14 | 3.5959 | 2.022 | Col1a2 |
| 1371861_at | 0.0291 | 10.29 | 3.2741 | 1.547 | Sfrp1 |
| 1374172_at | 0.0432 | 9.16 | 2.7581 | 1.51 | Col8a2 |
| 1367592_at | 0.0472 | 8.92 | 2.6355 | 1.732 | Tnnt2 |
| ID | Adjusted P value | t | B | logFC | Gene symbol |
| 1374503_at | 0.0156 | -12.35 | 3.9877 | -1.314 | Pbx3 |
| 1387450_at | 0.0257 | -10.71 | 3.4396 | -0.922 | Tgfa |
| 1395359_at | 0.0365 | -9.73 | 3.0291 | -1.084 | - |
| 1367695_at | 0.0365 | -9.63 | 2.9826 | -0.976 | Qdpr |
| 1390783_at | 0.0432 | -9.14 | 2.7485 | -1.025 | Abca8 |
The DAVID 6.8 online analysis tool was used to perform GO functional enrichment analysis on the above 35 differentially expressed genes, and 111 GO items were obtained. With P < 0.05 as a significant enrichment criterion, 73 pathways were obtained. Among them, 54 (48.65%) were enriched in biological processes, including drug response, amino acid stimulation response, vascular development, various signalling pathways, enzyme regulation, and other biological processes; 10 (9.01%) were enriched in cell composition, mainly including the extracellular matrix, collagen trimer, and troponin complex; and 9 (8.11%) were enriched in molecular functions, mainly including drug binding, protein binding, dopamine binding, metal ion binding, and dopamine neurotransmitter receptor activity (Table 6).
| GO | GO entry | GO description | Number of genes | P value |
| Biological process | GO:0042493 | Drug reaction | 6 | 5.21E-04 |
| GO:0001975 | Response to amphetamine | 3 | 0.001644593 | |
| GO:0001568 | Vascular development | 3 | 0.003764749 | |
| GO:0071230 | Cellular response to amino acid stimulation | 3 | 0.00430878 | |
| GO:0006469 | Negative regulation of protein kinase activity | 3 | 0.007534713 | |
| GO:0010579 | Positive regulation of adenylate cyclase activity involved in G protein-coupled receptor signalling | 2 | 0.01174588 | |
| GO:0060158 | Phospholipase C activates the dopamine receptor signalling pathway | 2 | 0.01174588 | |
| GO:0007212 | Dopamine receptor signalling pathway | 2 | 0.022075774 | |
| GO:1900273 | Positive regulation of long-term synaptic potentiation | 2 | 0.023359725 | |
| GO:0070208 | Protein heterotrimer | 2 | 0.025922792 | |
| GO:0007190 | Activation of adenylate cyclase activity | 2 | 0.028479424 | |
| GO:0051968 | Positive regulation of glutamatergic synaptic transmission | 2 | 0.033573445 | |
| GO:0051482 | Positive regulation of G protein-coupled cytosolic calcium concentration by phospholipase C activation | 2 | 0.039905051 | |
| GO:0048147 | Negative regulation of fibroblast proliferation | 2 | 0.043684948 | |
| Cellular composition | GO:0031012 | Extracellular matrix | 9 | 4.60E-10 |
| GO:0005578 | Protein, extracellular matrix | 7 | 5.09E-07 | |
| GO:0005581 | Collagen trimer | 4 | 5.47E-05 | |
| GO:0005584 | Type I collagen trimer | 2 | 0.002482326 | |
| GO:0005861 | Troponin complex | 2 | 0.009893989 | |
| Molecular function | GO:0004952 | Dopamine neurotransmitter receptor activity | 2 | 0.006291172 |
| GO:0008144 | Drug combination | 3 | 0.00881302 | |
| GO:0005515 | Protein binding | 7 | 0.011558726 | |
| GO:0035240 | Dopamine binding | 2 | 0.013790827 | |
| GO:0046872 | Metal ion binding | 6 | 0.025540723 |
Using the DAVID 6.8 online analysis tool, the KEGG pathway enrichment analysis was performed on the 35 differentially expressed genes, and a total of seven pathways were enriched. With P < 0.01 as the significant enrichment criterion, a significant enrichment pathway was obtained. This pathway is the cyclic adenosine monophosphate (c-AMP) signalling pathway (Table 7).
| Entry | Patheway name | Number of genes | P value |
| rno04024 | c-AMP signalling pathway | 4 | 0.005710593 |
| rno04512 | ECM receptor interactions | 3 | 0.012428441 |
| rno04974 | protein digestion and absorption | 3 | 0.012428441 |
| rno05146 | amebiasis | 3 | 0.019228955 |
| rno04611 | platelet activation | 3 | 0.027273273 |
| rno05012 | Parkinson's disease | 3 | 0.033955639 |
| rno05034 | alcoholism | 3 | 0.044367858 |
Cerebral infarction is an important cause of disability and death worldwide. Although traditionally considered a disease of elderly individuals, infarction in young people is increasingly a global public health problem, and the average age of stroke onset is gradually decreasing. The incidence of infarction and hospitalization rates are increasing among young people in some countries[1]. The infarction in young people is generally considered to be different from that in older patients in terms of genetic pathogenesis, and its onset is a complex process of multigene and multipathway interactions, which is an important reason for the difficulty in treating the disease[2,3]. Therefore, revealing the pathogenesis of ischaemic infarction in young people at the molecular level has become a research hotspot in the treatment of this disease. In this study, suitable young rats with ischaemic infarction were selected from the Gene Expression Omnibus database as the research subjects, and biological gene chip technology was used to identify important genetic pathways to intervene and reduce the incidence of ischaemic infarction in young people to the greatest extent.
Studies have found that the collagen type I alpha 2 (COL1A2) gene, which is a susceptibility gene of collagen-related diseases such as systemic sclerosis, contains a specific combination of two dinucleotide repeats and is involved in the regulation of gene expression. The COL1A2 gene overaccumulates in affected organs or tissues, eventually leading to a systemic inflammatory immune response that causes damage to blood vessels in infarcts in young patients[4,5]. The above findings suggest that COL1A2 may be closely related to ischaemic cerebrovascular diseases, such as vascular inflammation caused by systemic immune system abnormalities. This study is the first to find that COL1A2 is associated with ischaemic stroke in young adults, which suggests that COL1A2 could be modulated to reduce vascular inflammation and stabilize the vascular intima, thereby avoiding thrombus aggregation caused by vascular intimal injury. From the perspective of the c-AMP pathway, the increase in the c-AMP concentration could inhibit the migration and intimal hyperplasia of vascular smooth muscle cells (VSMCs), promote the degradation of type I collagen by lysosomes, and reduce the content of type I collagen secreted into and out of VSMCs to protect them[6]. Collagen type VIII alpha 2 (COL8A2) is a protein-coding gene that encodes the α2 chain of type VIII collagen, a component of the vascular endothelium, which is responsible for the migration of VSMCs. It is necessary for proliferation and thus plays an important role in maintaining vessel wall integrity and structure[7]. This indicates that COL8A2 is similar to COL1A2, and regulation of COL8A2 can also reduce the incidence of stroke in young adults. It has been reported in the past that tumour necrosis factor-α (TNF-α) is an important proinflammatory cytokine secreted by vascular endothelial cells that mediates the production of many inflammatory mediators. Increased TNF-α is an independent risk factor for acute cerebral infarction, which is consistent with the conclusion of this study[8]. The systemic inflammatory immune response may play a very important role in the pathogenesis of stroke in young people, and it is worthy of further exploration.
Previous studies have confirmed that the secreted frizzled related protein 1 gene is a genetic predisposition gene for large atherosclerotic cerebral infarction, which is consistent with the results of this study[9]. Therefore, young people with high risk for ischaemic stroke (such as long-term hypertension, heavy smoking, and severe abdominal obesity) are genetically tested to screen out high-risk stroke patients who carry this susceptibility gene to minimize stroke morbidity. Studies have shown that insulin-like growth factor 2 (IGF2) is abundantly expressed in the central nervous system, and its deficiency is closely related to neuropsychiatric diseases such as vascular dementia[10]. This suggests that severe deficiency of IGF2 may cause the repeated occurrence of strokes in such patients, which in turn leads to a stepwise aggravation of the condition of patients with vascular cognitive impairment and eventually leads to the occurrence of diseases such as vascular dementia. This is also a scientific proposition worthy of in-depth exploration. Studies have shown that elevated serum troponin T is a predictor of poor prognosis in acute stroke, and elevated troponin T concentrations are associated with an increased risk of death in hospitalized patients, which is consistent with the findings of this study[11]. Therefore, in addition to paying attention to the common haematological risk factors for stroke in young people, clinicians also need to pay attention to troponin T, an indicator of cardiac injury. Preleukaemia transcription factor 3 (PBX3) and ATP-binding cassette transporter subfamily A member 8 (ABCA8) have been reported in malignant tumours. They play a key role in the pathogenesis of cancer, and high levels of PBX3 and ABCA8 are closely associated with poor prognosis in cancer patients. Their association with tumorigenesis indirectly suggests that they may contribute to a hypercoagulable state in normal young patients, leading to thrombosis and eventual stroke[12,13]. This indicates that genes regulating tumorigenesis may be involved in the pathophysiological process of cerebral infarction, which is worthy of further exploration. Quinoid dihydrobiopterin is reduced to tetrahydrobiopterin under the action of quinoid dihydropteridine reductase (QDPR). Tetrahydrobiopterin can affect the vascular endothelium in various ways to promote the occurrence and development of cardiovascular and cerebrovascular diseases. For example, tetrahydrobiopterin deficiency can promote atherosclerosis and the production of oxygen free radicals[14]. This suggests that QDPR may play an important role in the pathogenesis of young infarcts. Based on the above findings, we speculate that atherosclerosis may also play an important role in the incidence of stroke in young adults, especially those after the age of 35 with poor diet and living habits (because they may have a higher proportion of diseases such as hypertension).
In addition, we obtained 73 significantly enriched pathways after GO functional enrichment analysis, of which 54 were significantly enriched in biological processes including drug reactions, various signalling pathways, enzyme regulation, and other biological processes; ten were significantly enriched with a focus on cell composition, mainly including the extracellular matrix, collagen trimer, troponin complex, etc.; nine were significantly enriched in molecular functionds, mainly including drug binding, protein binding, dopamine binding, and dopamine neurotransmitter receptors. Finally, KEGG pathway enrichment analysis was performed on 35 differentially expressed genes to obtain a significantly enriched pathway: The c-AMP signalling pathway. Studies have shown that the main target of c-AMP was the c-AMP-dependent protein kinase A and exchange protein directly activated by c-AMP. We might reduce the incidence of ischaemia in young rats by interfering with corresponding targets, thereby reducing their disability and mortality. The c-AMP signalling pathway can promote vascular endothelial repair and inhibit vascular intimal proliferation and platelet aggregation by regulating downstream effector molecules, thereby inhibiting vascular remodelling. In addition, activation of the c-AMP pathway can also alleviate ischaemic stroke-reperfusion injury, promote neuronal repair, and improve poststroke cognitive function[15,16]. The c-AMP signalling pathway may be a key pathway for the intervention of stroke-related diseases such as cerebral infarction and vascular cognitive impairment in young people and has extremely important clinical significance and research value.
To date, there have been no relevant research papers at home and abroad reporting the key signalling pathway involved in the development ischemic stroke in young rats. In this paper, we identified that the c-AMP signalling pathway is the key signalling pathway for young rats developing acute ischemic infarction through the methods of related gene analysis, which has extremely significant clinical significance. However, the subjects of this study are rats, and the sample size is relatively small. If more gene expression profile data are published in the future, further in-depth analysis could be conducted to verify the research results. Finally, we need to collect a certain number of clinical cases in the future to verify whether the research results are suitable for human beings, so as to realize the clinical transformation of major basic scientific discoveries.
Our study demonstrated that the c-AMP signalling pathway may be the key pathway in the intervention of cerebral infarction in young people. Further fundamental studies are needed to comprehensively explore the impact of the key pathway response on infarction in young people to finally achieve clinical transformation.
The incidence rate of cerebral infarction in young people is increasing day by day, the age of onset tends to be younger, and its internal pathogenesis and mechanism are very complicated, which leads to greater difficulties in treatment. Therefore, it is essential to analyze the key pathway that affects the onset of cerebral infarction in young people from the perspective of genetics.
To compare the differentially expressed genes in the brain tissue of young and aged rats with middle cerebral artery occlusion and to analyse their effect on the key signalling pathway involved in the development of cerebral ischaemia in young rats.
To compare the differentially expressed genes in the brain tissue of young and aged rats with middle cerebral artery occlusion and to analyse their effect on the key signalling pathway involved in the development of cerebral ischaemia in young rats.
The Gene Expression Omnibus 2R online analysis tool was used to analyse the differentially expressed genes in the GSE166162 dataset regarding the development of cerebral ischaemia in young and aged groups of rats. DAVID 6.8 software was further used to filter the differentially expressed genes. These genes were subjected to Gene Ontology (GO) function analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis to determine the key gene pathway that affects the occurrence of cerebral ischaemia in young rats.
Thirty-five differentially expressed genes (such as Igf2, Col1a2, and Sfrp1) were obtained; 73 GO enrichment analysis pathways are mainly involved in biological processes such as drug response, amino acid stimulation response, blood vessel development, various signalling pathways, and enzyme regulation. They are involved in molecular functions such as drug binding, protein binding, dopamine binding, metal ion binding, and dopamine neurotransmitter receptor activity. KEGG pathway enrichment analysis showed a significantly enriched pathway: The cyclic adenosine monophosphate (c-AMP) signalling pathway.
The c-AMP signalling pathway might be the key pathway in the intervention of cerebral infarction in young people.
Further fundamental studies are needed to comprehensively explore the impact of the key pathway response on infarction in young people to finally achieve clinical transformation.
| 1. | Ampbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM. Ischaemic stroke. Nature reviews Disease primers. 2019;5:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Quan Z, Quan Y, Wei B, Fang D, Yu W, Jia H, Quan W, Liu Y, Wang Q. Protein-protein interaction network and mechanism analysis in ischemic stroke. Mol Med Rep. 2015;11:29-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Hagrass AI, Almaghary BK, Mostafa MA, Elfil M, Elsayed SM, Aboali AA, Hamdallah A, Hasan MT, Al-Kafarna M, Ragab KM, Doheim MF. Antiplatelets Versus Anticoagulation in Cervical Artery Dissection: A Systematic Review and Meta-analysis of 2064 Patients. Drugs R D. 2022;22:187-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Hata R, Akai J, Kimura A, Ishikawa O, Kuwana M, Shinkai H. Association of functional microsatellites in the human type I collagen alpha2 chain (COL1A2) gene with systemic sclerosis. Biochem Biophys Res Commun. 2000;272:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Wenzel D, Haddadi NS, Afshari K, Richmond JM, Rashighi M. Upcoming treatments for morphea. Immun Inflamm Dis. 2021;9:1101-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Cai Y, Miller CL, Nagel DJ, Jeon KI, Lim S, Gao P, Knight PA, Yan C. Cyclic nucleotide phosphodiesterase 1 regulates lysosome-dependent type I collagen protein degradation in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2011;31:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Hwang JS, Ma DJ, Choi J, Shin YJ. COL8A2 Regulates the Fate of Corneal Endothelial Cells. Invest Ophthalmol Vis Sci. 2020;61:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Li X, Ma D, Sun G. Effects of Edaravone on Neurological Function and Tumor Necrosis Factor Alpha and Interleukin 8 Levels in Patients with Cerebral Infarction. Eur Neurol. 2020;83:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Kim TH, Chang JS, Park KS, Park J, Kim N, Lee JI, Kong ID. Effects of exercise training on circulating levels of Dickkpof-1 and secreted frizzled-related protein-1 in breast cancer survivors: A pilot single-blind randomized controlled trial. PLoS One. 2017;12:e0171771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Pardo M, Cheng Y, Sitbon YH, Lowell JA, Grieco SF, Worthen RJ, Desse S, Barreda-Diaz A. Insulin growth factor 2 (IGF2) as an emergent target in psychiatric and neurological disorders. Review. Neurosci Res. 2019;149:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Vafaie M, Giannitsis E, Mueller-Hennessen M, Biener M, Makarenko E, Yueksel B, Katus HA, Stoyanov KM. High-sensitivity cardiac troponin T as an independent predictor of stroke in patients admitted to an emergency department with atrial fibrillation. PLoS One. 2019;14:e0212278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Morgan R, Pandha HS. PBX3 in Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Trigueros-Motos L, van Capelleveen JC, Torta F, Castaño D, Zhang LH, Chai EC, Kang M, Dimova LG, Schimmel AWM, Tietjen I, Radomski C, Tan LJ, Thiam CH, Narayanaswamy P, Wu DH, Dorninger F, Yakala GK, Barhdadi A, Angeli V, Dubé MP, Berger J, Dallinga-Thie GM, Tietge UJF, Wenk MR, Hayden MR, Hovingh GK, Singaraja RR. ABCA8 Regulates Cholesterol Efflux and High-Density Lipoprotein Cholesterol Levels. Arterioscler Thromb Vasc Biol. 2017;37:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Chandrashekaran S, Karthikeyan S, Balakrishnan A, Nair S, Satheesh Kumar MK, Vattathara JJ, Menon KN. Expression and Purification of Quinine Dihydro Pteridine Reductase from astrocytes and its significance in the astrocyte pathology. Int J Biol Macromol. 2018;110:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Jing FQ, Qin H, Liu H, Wang ZL. [Influence of acupuncture combined with rehabilitation therapy on limb motor function and serum cAMP and cGMP in patients with hemiplegia of cerebral ischemic stroke]. Zhongguo Zhen Jiu. 2020;40:581-585. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Lind L. The metabolomic profile of carotid artery intima-media thickness and echogenicity. Atherosclerosis. 2021;335:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hakim GD, Turkey; Thongon N, Thailand S-Editor: Wang LL L-Editor: Wang TQ P-Editor: Wang LL