Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.938
Peer-review started: October 21, 2022
First decision: November 16, 2022
Revised: November 29, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 6, 2023
Processing time: 107 Days and 20.7 Hours
Epithelioid trophoblastic tumor (ETT) is the rarest type of gestational trophoblastic tumor (GTT). It has been reported that more than 50% of ETTs arise in the uterine cervix or the lower uterine segment. Here, we report a case of ETT within the lower uterine segment and cervical canal and discuss its manifestations, possible causes, and related influencing factors.
A 35-year-old woman (gravida 7, miscarriage 3, induction 2 with 1 being twins, para 2 of cesarean section, live 2), who had amenorrhea for 9 mo after breastfeeding for 22 mo after the last cesarean section, was diagnosed with ETT. The lesion was present in the lower uterine segment and endocervical canal with severe involvement of the anterior wall of the lower uterine segment and the front wall of the lower uterine segment where the cesarean incisions were made. Laboratory tests showed slight elevation of serum beta-human chorionic gonadotropin. Intraoperative exploration showed the presence of a normal-sized uterus body with an enlarged tumor in the lower uterine segment. The surface of the lower uterine segment was light blue, the entire lesion was approximately about 8 cm × 8 cm × 9 cm, with compression and displacement of the surrounding tissue. Histological examination diagnosed ETT. Immunohistochemical analysis showed positive expression of p63, with a Ki-67 proliferation index of 40%.
A search of the PubMed database using the search terms "cesarean section" and "epithelioid trophoblastic tumor" retrieved nine articles, including 13 cases of ETT and ETT-related lesions, all 13 cases had a history of cesarean section, and the lesions were all located at the cesarean section incision on the anterior wall of the lower uterine segment. The present case is the 14th reported case of ETT after cesarean section. Therefore, we deduced that cesarean section trauma had an important effect on the occurrence of ETT at this site.
Core Tip: Epithelioid trophoblastic tumor (ETT) is rare clinically. we describe a case of ETT in the lower uterine segment and cervical canal. The lesion was present in the lower uterine segment and endocervical canal where the cesarean incisions were made. A search of the PubMed database using the search terms "cesarean section" and "ETT" retrieved 13 cases of ETT, all of which had a history of cesarean section, and the lesions were located in the cesarean incision of the uterus. Therefore, we deduced that cesarean section trauma had an important effect on the occurrence of ETT at this site.
- Citation: Yuan LQ, Hao T, Pan GY, Guo H, Li DP, Liu NF. Epithelioid trophoblastic tumor of the lower uterine segment and cervical canal: A case report. World J Clin Cases 2023; 11(4): 938-944
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/938.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.938
Epithelioid trophoblastic tumor (ETT) is a rare form of gestational trophoblastic tumor (GTT) and is clinically uncommon. It arises from the malignant transformation of intermediate trophoblastic cells at the chorionic leave. Transformation of placental site nodules to ETT has been observed on histological examination of surgical specimens[1]. ETT tends to be diagnosed after pathological assessment of curettage or surgical specimens. There are no specific symptoms or signs and the reported interval between the antecedent pregnancy and the clinical manifestation ranges from 1 to 15 years, with an average of 6.2 years. Specifically, over 50% of ETTs arise in the cervix or low uterine segment[2]. Here, we report a case of ETT within the lower uterine segment and cervical canal and discuss its manifestations, possible causes, and related influencing factors.
A 35-year-old woman presented to our hospital after 9 mo of amenorrhea and 10 d after hysteroscopy.
She went to the local county hospital because after 22 mo of breastfeeding, she had amenorrhea for 2 mo. She had experienced no discomfort. She was scheduled for an ultrasound that showed a suspected 2 cm uterine fibroid or uterine cyst and was followed up regularly. Seven months later, menstruation had still not commenced. During the follow-up consultation, a 5.0 cm x 4.5 cm x 3.1 cm inhomogeneous echo was observed in the lower uterine cavity and cervix, adjacent to a 2.9 cm x 1.7 cm liquid dark area with poor sound transmission. A hysteroscopy was performed with the removal of a large amount of blood clot-like tissue. The histochemical and immunohistochemical (IHC) pathological diagnosis was ETT.
The patient had no past illness. In terms of her pregnancy history, she had a total of seven pregnancies, of which three were miscarriages, two midterm induction, one resulted in twins, and two full-term cesarean sections for the first and last pregnancies.
No other special circumstances.
Specialist examination on admission showed a small amount of dark red bloody fluid in the vagina and no purplish-blue nodules in the vulva, vagina, or cervix. The appearance of the cervix was normal. The uterus was the size of a uterus at 12 wk of pregnancy and was irregular in shape, slightly soft in texture, movable, and slightly tender. No obvious mass was found in the bilateral adnexal areas.
The laboratory investigations showed beta-human chorionic gonadotropin (βHCG) levels of 64.30 mIU/mL [normal range (NR): 0-3] and cytokeratin 19 fragment levels of 3.94 ng/mL (NR: 0-3.3). No abnormalities in other tumor markers were seen: Squamous-cell carcinoma, 0.33 ng/mL (NR: 0-2.7); carcinoembryonic antigen, 0.61 ng/mL (NR: 0-3.4); carbohydrate antigen (CA) 125, 22.60 U/mL (NR: 0-35); CA 19-9, 13.70 U/mL (NR: 0-39); CA 15-3, 11.10 U/mL (NR: 0-25); CA 72-4, 3.0 U/mL (NR: 0-6.9); neuron-specific enolase, 12.3 ng/mL (NR: 0-17); human epididymal protein 4, 28.8 pmol/L (NR: 0-140).
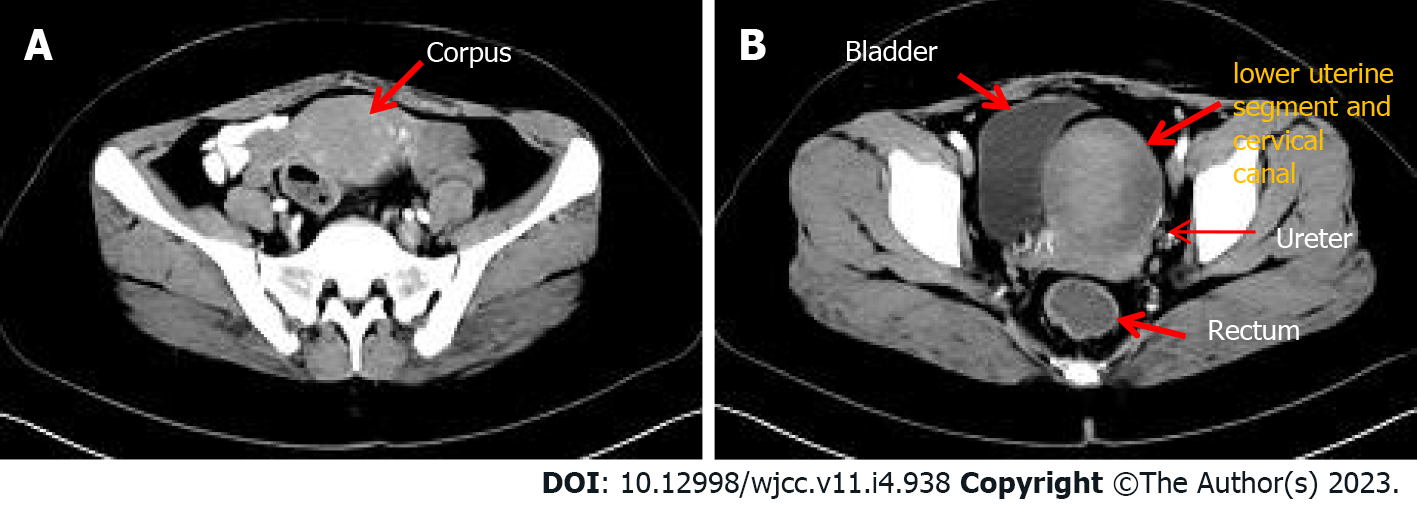
On admission, 16 d after her previous hysteroscopy, ultrasonography revealed a 7.1 cm × 6.3 cm anechoic mass in the lower segment of the uterus, with blurred margins, solid cyst-like appearance, and reduced blood flow signals. Computerized tomography (CT) imaging of the chest, abdomen, and pelvis showed significant enlargement of the uterine cavity, with a large-scale high-density shadow with punctate gas and unclear edges visible in the plain scan. The enhancement was not significant after application of contrast agent, and the muscle layer was compressed and thinned. No other abnormalities were seen. The CT imaging findings indicated that, after hysteroscopy, part of the hematoma should be considered in the operation area (Figure 1).
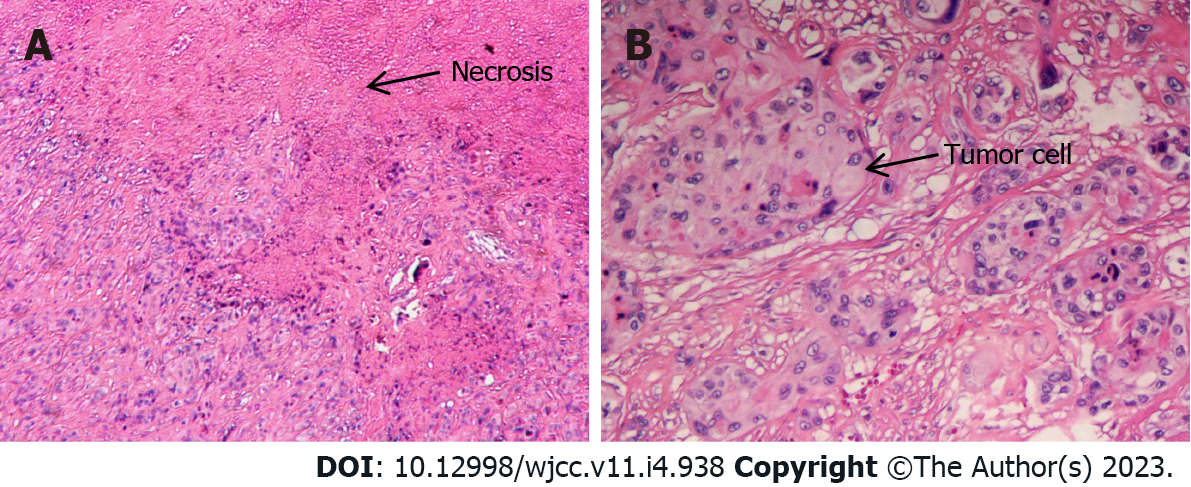
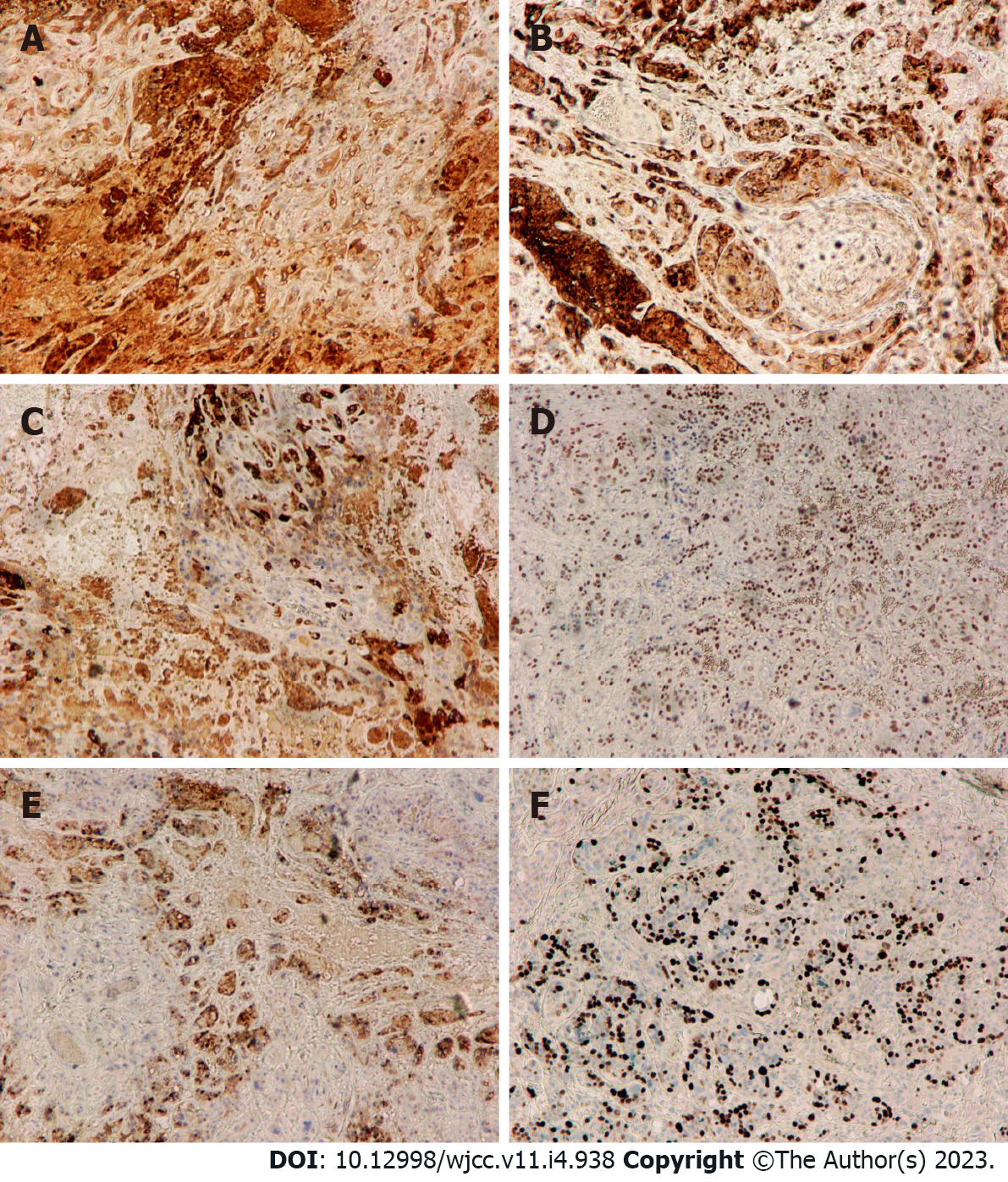
Postoperative histological examination of the tumor showed that it was composed of mononuclear intermediate trophoblast cells with vacuoles and prominent nucleoli and “geographic” necrosis (Figure 2A and B). IHC analysis showed positive expression of HCG, epithelial membrane antigen (EMA), inhibin-alpha, p63, and human placental alkaline phosphatase (PLAP), with a Ki-67 proliferation index of 40% (Figure 3A-F). The final diagnosis was lower uterine segment and cervical canal ETT.
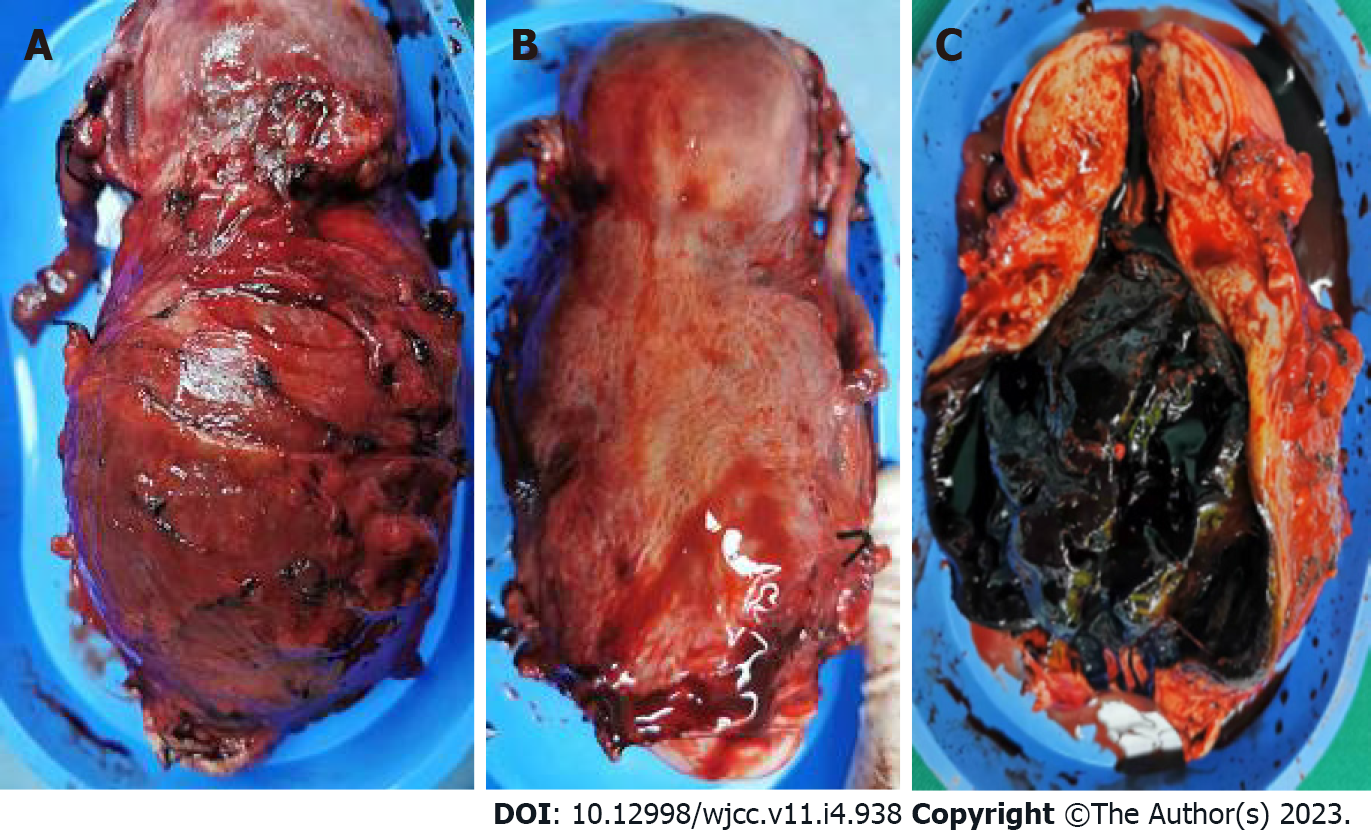
On March 11, a total abdominal hysterectomy and bilateral salpingectomy were performed. During the operation, a normal-sized uterus was seen with an enlarged tumor in the lower uterine segment, together with severe involvement of the anterior wall of the lower uterine segment. The surface of the anterior wall of the lower uterine segment was light blue and thin, the entire enlarged lower uterine segment with the cervical canal was about 8 cm × 8 cm × 9 cm in size, with compression and displacement of the surrounding tissues (Figure 4A and B). Other organs and tissues were normal. Postoperatively, the uterus was dissected through an anterior wall incision, showing that the walls of the lower uterine segment and cervical canal were 2 mm in thickness, with black blood clot-like tissue visible inside (Figure 4C).
The patient recovered smoothly after surgery. After three days, the βHCG level had decreased to 5.53 mIU/mL and the cytokeratin 19 fragments had returned to the normal range of 1.22 ng/mL, while after another seven days, the βHCG level returned to the normal range of 0.48 mIU/mL.
ETT is the rarest form of GTT and was previously known as atypical choriocarcinoma. It was first described by Mazur in 1989, and the term “ETT” has become widely used since the detailed documentation of its clinicopathological and IHC features by Shih and Kurman in 1998[3-6]. It primarily affects women of reproductive age, although there have been reported cases of postmenopausal patients, with a reported age range from 15 to 66 years[7,8].
ETT mostly arises within the uterine cavity, although this may vary. Stănculescu et al[9] reported cervical occurrence of ETT in 31% of cases, while Hui et al[2] reported that 50% of ETTs arise within the uterine cervix or lower uterine segment. The lower uterine segment is derived from the extension of the uterine isthmus during pregnancy, that is, the junction between the uterus and the cervix. The incision for cesarean section is in the lower uterine segment. The present case had had seven pregnancies, three miscarriages: Two midterm inductions, one for twins, and two term cesarean sections. The final diagnosis was lower uterine segment and cervical canal ETT. Her last pregnancy had resulted in a full-term cesarean section 31 mo previously. The tumor made a large, tree stump-like swelling in the lower uterine segment and cervical canal, while the uterus was normal-sized and rested on top of the swelling (Figure 4A). Trauma to the continuity and integrity of the uterine tissue after surgical incision and wound healing in the lower uterine segment may be associated with the development of ETT, and this may be part of the reason why ETTs appear in the lower uterine segment and cervical canal. There have been reports of ETT occurring at and around the uterine incision scar[10,11]. A search of the PubMed database using the search terms "cesarean section" and "epithelioid trophoblastic tumor" retrieved nine articles, including 13 cases of ETT and ETT-related lesions[12]. All 13 cases had a history of cesarean section, and the lesions were all located at the cesarean section incision on the anterior wall of the lower uterine segment. The present case is the 14th reported case of ETT after cesarean section. Therefore, we deduced that cesarean section trauma was an important influencing factor of ETT at this site.
ETT is usually associated with a prior gestational event. The antecedent gestations can be term pregnancy, abortion, hydatidiform mole, and ectopic pregnancy. Phippen et al[13] reported a case of ETT that occurred in the cervix after an ectopic pregnancy. There are even reported cases of isolated lung ETT, where microsatellite genotyping of the tumor cells at informative microsatellite loci revealed the biparental origin of the tumors, with the presence of paternal alleles at each locus, and confirmed their placental origin[14]. Thus, a history of pregnancy is a prerequisite for ETT, although the preceding gestation may be sometimes remote. Vaginal bleeding or menometrorrhagia is the most common symptom but amenorrhea can also occur, and in our case, amenorrhea was the only presenting complaint. The patient had experienced amenorrhea for nine months before her second visit to the local hospital for hysteroscopy. It was after the hysteroscopy that the patient developed vaginal bleeding. ETT tumor cells express both HCG and keratin, and laboratory tests showed that the serum βHCG levels were slightly elevated to 64.30 mIU/mL, while the cytokeratin 19 fragment was 3.94 ng/mL, which is consistent with literature reports[2,4,6]. The cytokeratin level decreased to the normal range three days postoperatively, and the βHCG returned to the normal range 10 d postoperatively.
Immunohistochemistry, in this case, showed that the ETT tumor cells typically expressed HCG, inhibin-alpha, EMA, and p63, with locally expressed PLAP and a Ki-67 nuclear labeling index of 40%; this is consistent with literature reports. The expression of p63 is helpful for the differential diagnosis of ETT and placental site reaction and placental site trophoblastic tumor, as the latter two are negative and ETT is positive[15]. Most reports have found a Ki-67 nuclear labeling index of approximately 17%, with a few reports describing significant elevation[2,14,16].
ETT is a relatively indolent malignancy that, in our case, persisted for seven months without extrauterine invasion and metastasis. Clinicians tend to be unaware of the existence of this lesion. It can occur many years after the previous pregnancy; Keser et al[4] reported a case of ETT that occurred 16 years after the previous pregnancy, and Hsiue et al[17] reported a case of ETT that occurred 23 years after the previous pregnancy. For uncertain uterine and extrauterine lesions that do not conform to the general rules, low blood βHCG levels combined with histopathology, immunohistochemistry, and genetic testing of the diseased tissue are of great significance for the diagnosis and treatment of the disease.
ETT is not benign, but it is an indolent tumor. ETT patients do not present any specific symptoms or signs. A commonly used treatment strategy is surgical resection, which is effective when there is no extrauterine spread or metastasis of the tumor cells. The ETT case discussed in the present report would help enhance the understanding of the disease from a clinician’s perspective. Importantly, the present report identified cesarean section trauma was an important influencing factor of ETT that occurred in the patient’s cervical canal and lower uterine segment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aniţei MG, Romania; Zhang X, United States S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Tsai HW, Lin CP, Chou CY, Li CF, Chow NH, Shih IM, Ho CL. Placental site nodule transformed into a malignant epithelioid trophoblastic tumour with pelvic lymph node and lung metastasis. Histopathology. 2008;53:601-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Hui P. Gestational Trophoblastic Tumors: A Timely Review of Diagnostic Pathology. Arch Pathol Lab Med. 2019;143:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Bell SG, Uppal S, Sakala MD, Sciallis AP, Rolston A. An extrauterine extensively metastatic epithelioid trophoblastic tumor responsive to pembrolizumab. Gynecol Oncol Rep. 2021;37:100819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Keser SH, Kokten SC, Cakir C, Sensu S, Buyukbayrak EE, Karadayi N. Epithelioid trophoblastic tumor. Taiwan J Obstet Gynecol. 2015;54:621-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Mazur MT. Metastatic gestational choriocarcinoma. Unusual pathologic variant following therapy. Cancer. 1989;63:1370-1377. [PubMed] [DOI] [Full Text] |
| 6. | Shih IM, Kurman RJ. Epithelioid trophoblastic tumor: a neoplasm distinct from choriocarcinoma and placental site trophoblastic tumor simulating carcinoma. Am J Surg Pathol. 1998;22:1393-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 215] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Zhang X, Shi H, Chen X. Epithelioid trophoblastic tumor after induced abortion with previous broad choriocarcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2014;7:8245-8250. [PubMed] |
| 8. | Yigit S, Gun E, Yilmaz B, Kolsuz Z. Epithelioid trophoblastic tumor in a postmenopausal woman: A case report and review of the literature in the postmenopausal group. Indian J Pathol Microbiol. 2020;63:S98-S101. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Stănculescu RV, Bauşic V, Vlădescu TC, Vasilescu F, Brătilă E. Epithelioid trophoblastic tumor: a case report and literature review. Rom J Morphol Embryol. 2016;57:1365-1370. [PubMed] |
| 10. | Black KA, Simone K, Hirt-Walsh C, Sabourin J. Epithelioid trophoblastic tumor presenting as a Caesarean scar defect: A case report. Gynecol Oncol Rep. 2021;36:100715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Yang C, Li J, Zhang Y, Xiong H, Sheng X. Epithelioid trophoblastic tumor coexisting with choriocarcinoma around an abdominal wall cesarean scar: a case report and review of the literature. J Med Case Rep. 2020;14:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Zhou F, Lin K, Shi H, Qin J, Lu B, Huang L. Atypical postcesarean epithelioid trophoblastic lesion with cyst formation: a case report and literature review. Hum Pathol. 2015;46:1036-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Phippen NT, Lowery WJ, Leath CA 3rd, Kost ER. Epithelioid trophoblastic tumor masquerading as invasive squamous cell carcinoma of the cervix after an ectopic pregnancy. Gynecol Oncol. 2010;117:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Fénichel P, Rouzier C, Butori C, Chevallier P, Poullot AG, Thyss A, Mouroux J. Extragestational βHCG secretion due to an isolated lung epithelioid trophoblastic tumor: microsatellite genotyping of tumoral cells confirmed their placental origin and oriented specific chemotherapy. J Clin Endocrinol Metab. 2014;99:3515-3520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Houghton O, McCluggage WG. The expression and diagnostic utility of p63 in the female genital tract. Adv Anat Pathol. 2009;16:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Zhang T, Zeng X, Xu H, Gao L, Xiong L, An R, Xue Y. Clinical characteristics and outcomes of extrauterine epithelioid trophoblastic tumors. Arch Gynecol Obstet. 2019;300:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Hsiue EH, Hsu C, Tseng LH, Lu TP, Kuo KT. Epithelioid Trophoblastic Tumor Around an Abdominal Cesarean Scar: A Pathologic and Molecular Genetic Analysis. Int J Gynecol Pathol. 2017;36:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |