Published online Oct 16, 2023. doi: 10.12998/wjcc.v11.i29.7253
Peer-review started: August 28, 2023
First decision: September 4, 2023
Revised: September 11, 2023
Accepted: September 26, 2023
Article in press: September 26, 2023
Published online: October 16, 2023
Processing time: 46 Days and 0.3 Hours
Occult thyroid papillary carcinoma (OTPC) is typically characterized by initial presentation with cervical lymph node metastasis and can be detected through ultrasound. However, the initial and sole manifestation was a submandibular solid-cystic mass. High-frequency ultrasound, enhanced multislice computed tomography (CT) scan, and thyroid function tests revealed no abnormalities, which is relatively uncommon.
A 24-year-old Chinese female, who studied at a university in Shandong Province, presented to the clinic in June 2019 with a right submandibular mass that she had noticed 2 mo earlier. Clinical examination revealed a 2-cm, nontender, movable solid-cystic mass in the submandibular region, with no palpable thyroid mass observed. Ultrasonography revealed a 2.0 cm × 1.1 cm solid-cystic mass in the right submandibular region, and the thyroid gland showed no abnormalities. CT scan and 131I whole body follow-up scan showed that there were no abnor
The presentation of a submandibular solid-cystic mass as the primary and solitary indication of OTPC is relatively uncommon. Fine needle aspiration is advised for evaluating neck masses.
Core Tip: Occult thyroid papillary carcinoma is rare. However, submandibular solid-cystic mass was the initial and sole manifestation, ultrasound and computed tomography scan showed no abnormalities in the thyroid gland, which is relatively rare. We report the case with submandibular solid-cystic mass as the first and sole manifestation was diagnosed with papillary thyroid carcinoma after surgery.
- Citation: Chen GY, Li T. Submandibular solid-cystic mass as the first and sole manifestation of occult thyroid papillary carcinoma: A case report. World J Clin Cases 2023; 11(29): 7253-7257
- URL: https://www.wjgnet.com/2307-8960/full/v11/i29/7253.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i29.7253
Thyroid carcinoma is a prevalent endocrine malignancy in clinical practice, with papillary thyroid carcinoma (PTC) being the most common subtype, accounting for approximately 90%[1,2]. Despite the favorable prognosis of PTC, early lymph node metastasis occurs in 20% to 90% of cases[3-6]. Lymph node metastasis in PTC predominantly involves multiple regions, while single-region metastasis is rare. Metastatic involvement commonly affects the central VI level and lateral groups II, III, and IV; however, it is infrequent in levels V and I[7]. Occult thyroid papillary carcinoma (OTPC) refers to any PTC with a diameter less than 1 cm. OTPC typically presents initially as cervical lymph node metastasis but rarely manifests as a submandibular solid-cystic mass. Palpation alone may not effectively detect OTPC. Most thyroid nodules or ectopic thyroids can be identified using high-frequency ultrasound imaging, enhanced multislice computed tomography (CT) scanning, or 131I whole-body follow-up scan (131I WBS)[8-10]. However, some cases of OTPC remain undetected[11]. Therefore, determining the necessity for regular follow-up and whether thyroid surgery should be performed are crucial topics, along with defining the extent of lesion resection and cervical lymph node dissection scope. In this particular case study, an initial presentation solely consisting of a submandibular solid-cystic mass was observed without any abnormalities detected through high-frequency ultrasound imaging, enhanced multislice CT scanning, or thyroid function tests-a relatively uncommon occurrence.
A right submandibular mass that she had noticed 2 mo earlier.
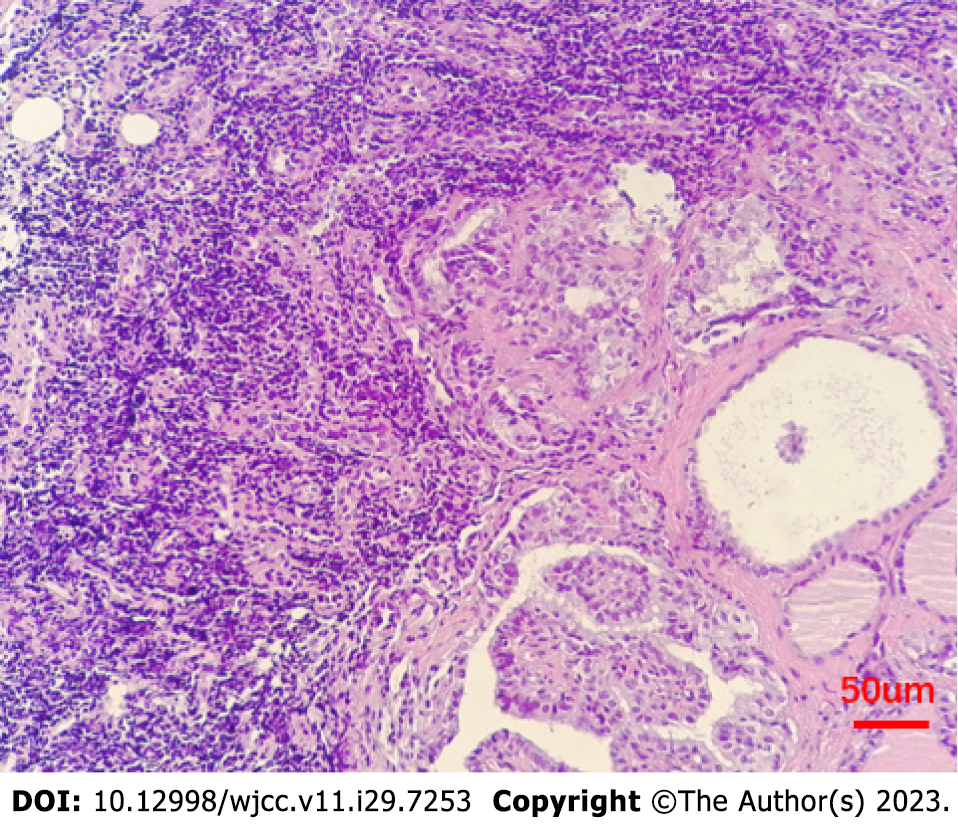
A 24-year-old Chinese female, who studied at a university in Shandong Province, presented to the clinic in June 2019 with a right submandibular mass that she had noticed 2 mo earlier. Clinical examination revealed a 2-cm, nontender, movable solid-cystic mass in the submandibular region, with no palpable thyroid mass observed. Ultrasonography revealed a 2.0 cm × 1.1 cm solid-cystic mass in the right submandibular region, and the thyroid gland showed no abnormalities. CT scan and 131I WBS showed that there were no abnormalities in the thyroid. However, cytology and pathology showed papillary tumor cell clusters, consistent with PTC. The pathology revealed metastatic carcinoma in lymphoid tissue with morphology consistent with thyroid papillary carcinoma metastasis (Figure 1).
Without special past illness.
Without special personal and family history.
Clinical examination revealed a 2-cm, nontender, movable solid-cystic mass in the submandibular region, with no palpable thyroid mass observed.
Laboratory examinations were all at normal levels.
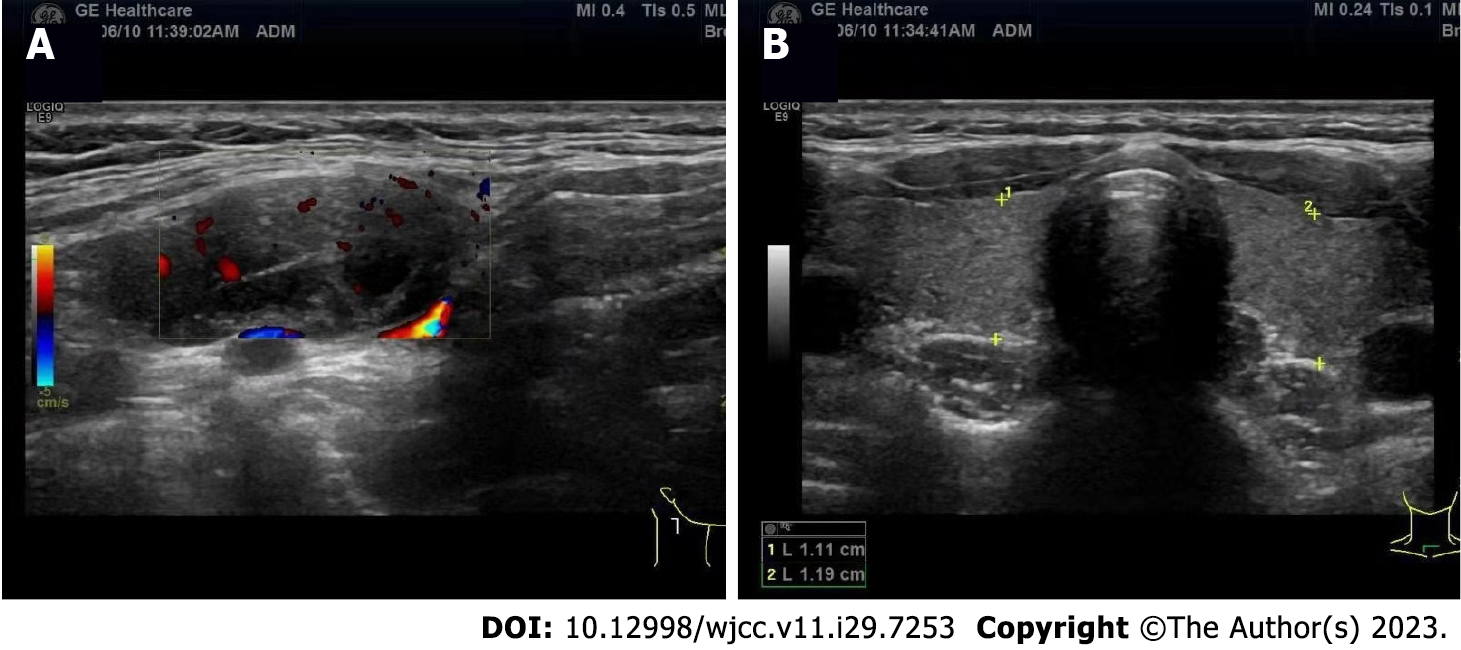
Ultrasonography demonstrated a 2.0 cm × 1.1 cm solid-cystic mass in the right submandibular region while revealing no abnormalities in the thyroid gland (Figure 2). US-guided fine-needle aspiration (FNA) exhibited papillary tumor cell clusters consistent with PTC.
Pathology identified a 0.3 cm nodule adjacent to the capsule as classical thyroid micropapillary carcinoma that invaded the capsule in the left lobe of the thyroid gland. Additionally, a 0.1 cm micropapillary carcinoma was found in the left lobe of the thyroid gland. Fortunately, cervical lymph nodes at level VI central and levels II, III, IV, V on the right side showed no tumor metastasis but chronic inflammation.
The patient was treated by total thyroidectomy and right neck lymph node dissection. Postoperative radioiodine therapy and thyrotropin (TSH) inhibition therapy were administered after surgery.
The patient has been followed up for 2 years without significant recurrence.
Regarding the progressive enlargement of masses in the submandibular region, especially in young patients, we strongly recommend heightened vigilance. Although most are benign lumps, such as submandibular lymphadenitis, submandibular cysts, and hypognathadenitis, reported cases of malignant or metastatic lymph nodes have been documented. Ethar reported a case where a right submandibular mass was diagnosed as mammary analogue secretory carcinoma through fine needle aspiration[12]. Chen et al[13] reported two cases of nodal diffuse large B-cell lymphoma with morphologic features resembling primary mediastinal large B-cell lymphoma, presenting in the submandibular lymph nodes. Miles demonstrated that a right submandibular mass, confirmed on histological analysis following excision of the right submandibular gland, was multinodular adenomatous oncocytic hyperplasia[14]. Performing high-frequency ultrasound to evaluate submandibular masses is necessary to differentiate between benign and malignant nodules. Sonographic features suggestive of malignancy or metastatic lymph nodes include enlargement, irregular borders, round shape, ill-defined contours, absence of an echogenic hilum, microcalcification within lymph nodes, cystic areas within lymph nodes, and color Doppler abnormalities such as hypervascularity[15,16]. If ultrasound fails to distinguish between benign and malignant lesions, further cytology is recommended. In cases where ultrasound or cytology indicates metastasis, excisional biopsy should be performed for definitive diagnosis. In our present case study, ultrasound revealed a solid-cystic mass with separate vascularity and clear border indicating potential malignancy. To establish a definitive diagnosis, US-guided FNA was performed which showed papillary tumor cell clusters consistent with PTC. Further surgical intervention indicated lymph node metastasis from PTC. Some studies have shown that PTCs frequently undergo cystic transformation, both in the primary tumor and in the metastatic lymph node[17], which is consistent with this case. OTPC is relatively infrequent in clinical practice. It commonly presents with neck lymph node metastasis as an initial symptom but can occasionally manifest as lung or bone metastasis[11,18]. High-frequency ultrasound serves as the gold standard for diagnosing thyroid cancer by enabling detection of lesions as small as 3 mm[19]. Thyroid cancer exhibits irregular margins, hypoechogenicity, and microcalcifications. Malignant nodules demonstrate significantly greater hardness than benign ones. Real-time shear wave elastography plays a crucial role in differentiating between benign and malignant nodules[20]. Additional diagnostic tools include CT scans, magnetic resonance imaging studies, thyroglobulin (TG), and carcinoembryonic antigen measurements. In this specific case study, the patient's physical examination findings related to the thyroid gland were normal, along with unremarkable results from a thyroid scan test evaluating both function and TG levels. There is ongoing debate regarding the necessity of surgery for this patient, the extent of the operation, and whether cervical lymph node dissection is warranted. Although OTPC is generally associated with low invasiveness and a benign biological behavior, clinical practice has revealed cases of lymph node metastasis, distant metastasis, and even mortality[21]. It is commonly accepted that unilateral thyroid gland and isthmus resection can be considered for solitary masses in OTPC. However, if there is tumor invasion into the capsule or multiple lesions are present, total thyroidectomy along with prophylactic cervical lymph node dissection may be performed. In this case, the patient underwent total thyroidectomy and prophylactic cervical lymph node dissection. Postoperative pathology confirmed PTC as a multifocal lesion with capsular invasion. Nevertheless, no evidence of cervical lymph node metastasis was observed. Subsequent postoperative radioiodine therapy and TSH inhibition therapy were administered. Currently, there have been no significant signs of recurrence or metastasis in the patient.
The occurrence of a submandibular solid-cystic mass as the initial and sole manifestation of OTPC is relatively uncommon. Fine needle aspiration is necessary for evaluating neck masses.
| 1. | Hanley JP, Jackson E, Morrissey LA, Rizzo DM, Sprague BL, Sarkar IN, Carr FE. Geospatial and Temporal Analysis of Thyroid Cancer Incidence in a Rural Population. Thyroid. 2015;25:812-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Li J, Yang T, Zhao T, Liang J, Lin YS. Clinical Outcome of Radioiodine Therapy in Low-intermediate Risk Papillary Thyroid Carcinoma with BRAF(V600E) Mutation. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 3. | Ito Y, Fukushima M, Kihara M, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Investigation of the prognosis of patients with papillary thyroid carcinoma by tumor size. Endocr J. 2012;59:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 4. | Shindo M, Wu JC, Park EE, Tanzella F. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2006;132:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Qubain SW, Nakano S, Baba M, Takao S, Aikou T. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Chow TL, Lim BH, Kwok SP. Sentinel lymph node dissection in papillary thyroid carcinoma. ANZ J Surg. 2004;74:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Caron NR, Tan YY, Ogilvie JB, Triponez F, Reiff ES, Kebebew E, Duh QY, Clark OH. Selective modified radical neck dissection for papillary thyroid cancer-is level I, II and V dissection always necessary? World J Surg. 2006;30:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 8. | Ito Y, Hirokawa M, Fukushima M, Inoue H, Yabuta T, Uruno T, Kihara M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Miyauchi A. Occult papillary thyroid carcinoma: diagnostic and clinical implications in the era of routine ultrasonography. World J Surg. 2008;32:1955-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Chang YC, Lo WC, Lo CY, Liao LJ. Occult Papillary Thyroid Carcinoma Initially Presenting as Cervical Cystic Lymph Node Metastasis: Report of Two Cases. Journal of Medical Ultrasound. 2013;21:92-96. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yasuda M, Osaki T, Fukuich Y, Kobayashi K, Iwata T, So T. Anterior mediastinal tumor as a solitary lymph node metastasis of occult thyroid carcinoma. J Surg Case Rep. 2019;2019:rjz029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Sasaki H, Sirakusa T, Suzuki K, Eimoto T, Okumura M. [Occult papillary carcinoma of the thyroid presenting as a solitary pulmonary metastasis]. Nihon Naibunpi Gakkai Zasshi. 1991;67:655-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Al-Husseinawi E, Hamidpour S, Omoscharka E. Mammary analogue secretory carcinoma of salivary gland diagnosed on submandibular gland cytology: A case report and review of the literature. Cytopathology. 2019;30:318-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Chen BJ, Ruminy P, Roth CG, Bisig B, Mankel B, Steinhilber J, Bohers E, Jardin F, Fend F, Swerdlow SH, Copie-Bergman C, de Leval L, Quintanilla-Martinez L. Cyclin D1-positive Mediastinal Large B-Cell Lymphoma With Copy Number Gains of CCND1 Gene: A Study of 3 Cases With Nonmediastinal Disease. Am J Surg Pathol. 2019;43:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Bannister M, Thompson CSG, Conn B. Bilateral submandibular gland nodular oncocytic hyperplasia with papillary cystadenoma-like areas. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Rosário PW, de Faria S, Bicalho L, Alves MF, Borges MA, Purisch S, Padrão EL, Rezende LL, Barroso AL. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005;24:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, Hartl DM, Lassau N, Baudin E, Schlumberger M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590-3594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 17. | Kessler A, Rappaport Y, Blank A, Marmor S, Weiss J, Graif M. Cystic appearance of cervical lymph nodes is characteristic of metastatic papillary thyroid carcinoma. J Clin Ultrasound. 2003;31:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Pittas AG, Adler M, Fazzari M, Tickoo S, Rosai J, Larson SM, Robbins RJ. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 131] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Boucek J, Kastner J, Skrivan J, Grosso E, Gibelli B, Giugliano G, Betka J. Occult thyroid carcinoma. Acta Otorhinolaryngol Ital. 2009;29:296-304. [PubMed] |
| 20. | Sriram U, Patacsil LM. Thyroid nodule. ScienceDirect. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Piana S, Ragazzi M, Tallini G, de Biase D, Ciarrocchi A, Frasoldati A, Rosai J. Papillary thyroid microcarcinoma with fatal outcome: evidence of tumor progression in lymph node metastases: report of 3 cases, with morphological and molecular analysis. Hum Pathol. 2013;44:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nambi G, Saudi Arabia S-Editor: Qu XL L-Editor: A P-Editor: Qu XL