Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6688
Peer-review started: July 6, 2023
First decision: July 27, 2023
Revised: July 28, 2023
Accepted: August 31, 2023
Article in press: August 31, 2023
Published online: October 6, 2023
Processing time: 81 Days and 2.3 Hours
Antinuclear antibodies (ANAs) are crucial in diagnosing autoimmune diseases, mainly systemic lupus erythematosus (SLE). This study aimed to compare the performance of chemiluminescence assay (CLIA) and line immunoassay (LIA) in detecting ANAs in patients with autoimmune diseases, evaluate their diagnostic accuracy for SLE, and develop a novel diagnostic model using CLIA-detected antibodies for SLE. Specimens from patients with autoimmune diseases and physical examination specimens were collected to parallel detect specific antibodies. Individual antibodies' diagnostic performance and a model combining multiple antibodies were assessed. The findings provide valuable insights into improving the diagnosis of SLE through innovative approaches.
To compare the performance of CLIA and LIA in detecting ANAs in patients with autoimmune diseases, assess their accuracy for SLE, and develop a novel diag
Specimens have been obtained from 270 patients with clinically diagnosed autoimmune disorders, as well as 130 physical examination specimens. After that, parallel detection of anti-double-stranded DNA (dsDNA) antibody, anti-histone (Histone) antibody, anti-nucleosome (Nuc) antibody, anti-Smith (Sm) antibody, anti-ribosomal P protein (Rib-P) antibody, anti-sicca syndrome A (Ro60) antibody, anti-sicca syndrome A (Ro52) antibody, anti-sicca syndrome (SSB) antibody, anti-centromere protein B (Cenp-B) antibody, anti-DNA topoisomerase 1 (Scl-70) antibody, anti-histidyl tRNA synthetase (Jo-1) antibody, and anti-mitochondrial M2 (AMA-M2) antibody was performed using CLIA and LIA. The detection rates, compliance rates, and diagnostic performance for SLE were compared between the two methodologies, followed by developing a novel diagnostic model for SLE.
CLIA and LIA exhibited essentially comparable detection rates for anti-dsDNA antibody, anti-Histone antibody, anti-Nuc antibody, anti-Sm antibody, anti-Rib-P antibody, anti-Ro60 antibody, anti-Ro52 antibody, anti-SSB antibody, anti-Cenp-B antibody, anti-DNAScl-70 antibody, anti-Jo-1 antibody and anti-AMA-M2 antibody (P > 0.05). The two methods displayed identical results for the detection of anti-dsDNA antibody, anti-Histone antibody, anti-Nuc antibody, anti-Sm antibody, anti-Ro60 antibody, anti-Ro52 antibody, anti-SSB antibody, anti-Cenp-B antibody, anti-Scl-70 antibody, and anti-AMA-M2 antibody (Kappa > 0.7, P < 0.05), but showed a moderate agreement for the detection of anti-Rib-P antibody and anti-Jo-1 antibody (Kappa = 0.671 and 0.665; P < 0.05). In addition, the diagnostic performance of these antibodies detected by both methods was similar for SLE. The diagnostic model's area under the curve values, sensitivity, and specificity, including an anti-dsDNA antibody and an anti-Ro60 antibody detected by CLIA, were 0.997, 0.962, and 0.978, respectively. These values were higher than the diagnostic performance of individual antibodies.
CLIA and LIA demonstrated excellent overall consistency in detecting ANA profiles. A diagnostic model based on CLIA-detected antibodies can successfully contribute to developing a novel technique for detecting SLE.
Core Tip: Antinuclear antibodies (ANAs) are important biomarkers for diagnosing autoimmune diseases, with systemic lupus erythematosus (SLE) being one of the most well-known. This study aimed to compare the performance of two commonly used ANA detection methods, chemiluminescence assay (CLIA) and line immunoassay, in patients with autoimmune diseases. The findings demonstrated that for ANAs, particularly those associated with SLE, both techniques had equivalent detection rates and diagnostic precision. Additionally, a diagnostic model for SLE that uses CLIA-detected antibodies has been developed and shown to have better diagnostic accuracy than individual antibodies. Specifically, a combination of anti-dsDNA antibodies and anti-Ro60 antibodies detected by CLIA provided an effective strategy for diagnosing SLE. These results imply that an adequate diagnosis of SLE may benefit from a diagnostic model based on CLIA-detected antibodies, ultimately resulting in more efficient management and treatment of this autoimmune disease.
- Citation: Xiang HY, Xiang XY, Ten TB, Ding X, Liu YW, Luo CH. Clinical value of chemiluminescence method for detection of antinuclear antibody profiles. World J Clin Cases 2023; 11(28): 6688-6697
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6688.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6688
Antinuclear antibodies (ANA) are a general term for organ-specific autoantibodies that essentially use various cellular components of eukaryotic cells as target antigens, and the spectrum of specific antibodies against the multiple substances in the nucleus is regarded as ANA spectrum[1,2]. It is irreplaceable and essential for the clinical diagnosis, disease assessment, and efficacy evaluation of different autoimmune diseases[3]. The main techniques commonly used for ANA detection involve indirect fluorescent assay (IFA), enzyme-linked immune sorbent assay (ELISA), and LIA. IFA is the reference method employed for ANA detection and is widely used to screen autoimmune diseases[4,5]. However, the IFA-ANA test is ineffective in accurately identifying specific ANA target antigens. Therefore, developing a unique approach for verifying ANA target antigens is necessary to enhance the clinical diagnosis of autoimmune disorders. The LIA, was first developed in the 1980s and is presently the predominant method employed in China for confirming ANA target antigens. This method is favored because of its advantageous characteristics, including its user-friendly operation, relatively easy interpretation of results, and absence of reliance on supplementary equipment[6].
However, the lack of quantitative measurements and the inability to establish a sound quality control system for LIA is inconducive to the clinical assessment of autoimmune diseases[7]. For example, in patients with suspected autoimmune disease, LIA, a multi-item combination test, allows for the rapid detection of disease autoantibodies and plays a pivotal role in the clinical differentiation of the different autoimmune diseases. However, it is essential to note that it is sufficient for patients who have already been diagnosed to test for individual positive autoantibodies. Utilizing LIA in such cases may lead to a significant expenditure of medical resources. With the rapid advancement of the medical field and the implementation of a medical insurance billing system based on the payment by disease, hospitals invariably strive to save medical costs as much as possible while meeting treatment needs simultaneously[8,9]. Therefore, there is a pressing need for an alternate technique in ANA spectrometry that can enhance quantitation and allow for a versatile combination of elements in addition to LIA. CLIA is one of the most widely used immunodiagnostic techniques in clinical practice currently, which is often characterized by complete automaticity, quantification, high sensitivity, random loading, fast detection, and flexible combination of items, and commercial detection reagents for CLIA ANA profiles have been recently made available in China[10]. To achieve this objective, the present study assessed the clinical use of CLIA in detecting ANA profiles.
The specimens from 270 patients with clinically confirmed autoimmune diseases and 130 samples from health physical examinations were collected from January 2019 to February 2021 at our hospital. Another 130 healthy individuals (31 males and 99 females) aged 14-79 years were recruited as the normal healthy controls. Diagnosing disorders in the collected specimens was conducted following established diagnostic and treatment recommendations and the standards established[11].
Of 4 mL of fasting blood was collected from all the study participants and centrifuged at 3000 r/min for l0 min. After that, the serum was separated and stored at -80 °C. The CLIA test kit and the accompanying fully automated chemiluminescence assay (CLIA) analyzer (Kaesar 6600) were provided by Guangzhou Kangrun Company. The LIA and IFA assay kits employed the pre-existing reagent brands accessible within the department. A similar control experiment was conducted using the sera obtained from the identical subject. Identical items were tested in both methods, including anti-double-stranded DNA (dsDNA) antibody, anti-histone (Histone) antibody, anti-nucleosome (Nuc) antibody, anti-Smith (Sm) antibody, anti-ribosomal P protein (Rib-P) antibody, anti- sicca syndrome A (Ro60) antibody, anti- sicca syndrome A (Ro52) antibody, anti- sicca syndrome (SSB) antibody, anti-centromere protein B (Cenp-B) antibody, anti-DNA topoisomerase 1 (Scl-70) antibody, anti-histidyl tRNA synthetase (Jo-1) antibody, and anti-mitochondrial M2 (AMA-M2) antibody.
(1) The detection rate of ANA spectra by both CLIA and LIA kits was obtained (through the following formula) and compared.
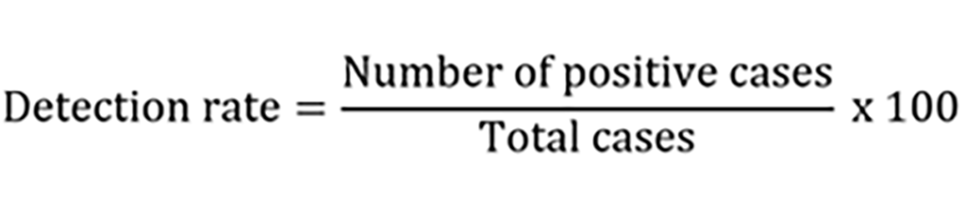
(2) The agreement rate of CLIA and LIA was also calculated as follows:
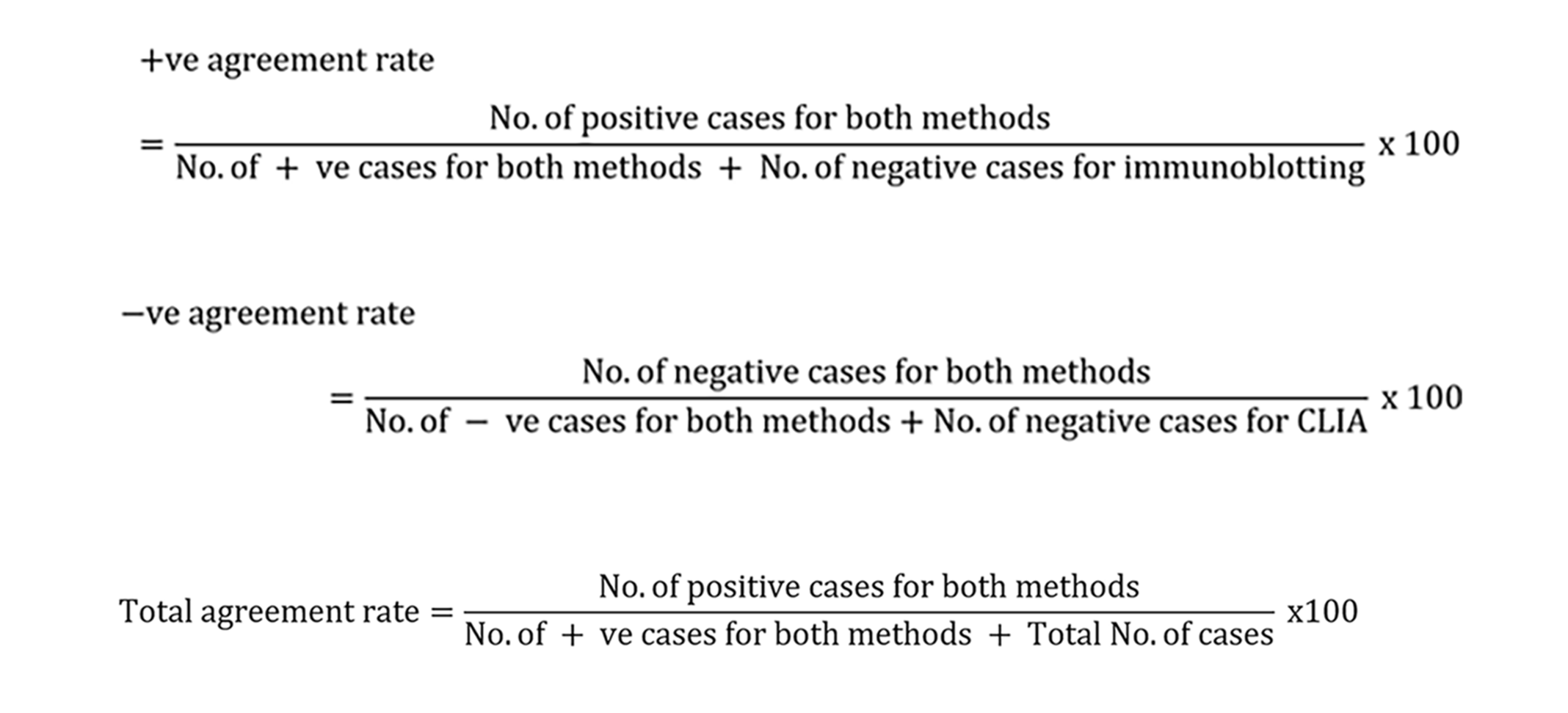
The concordance of the two methods for ANA spectra was also analyzed. Kappa test was employed to analyze the concordance between the two different assays, with Kappa < 0.4 indicating poor agreement, 0.4 ≤ Kappa < 0.7 indicating fair agreement, 0.7 ≤ Kappa < 0.9 indicating good agreement, and Kappa ≥ 0.9 indicating good agreement.
And (3) Due to a large amount of SLE data, receiver operating characteristics (ROC) analysis was performed on these data set individually to compare the potential diagnostic performance of the ANA profiles detected by both methods for SLE. Subsequently, the Boruta algorithm was used to screen the different antibodies detected by the chemiluminescence method for significant variables to establish a novel model for the rapid diagnosis of SLE, which can yield a better diagnostic rate.
The data analysis was performed using SPSS 22.0 software, and GraphPad Prism 9 and R v.4.2.0 were used for ROC curve plotting and analysis. The measurement results were assessed using an independent samples t-test and were expressed as mean ± SD. The chi-square test was used to analyze the count data, which were reported as a rate (%).
There were 138 cases (8 males and 130 females) of systemic lupus erythematosus (SLE), aged 11 to 77 years, 48 cases (2 males and 46 females) of sicca syndrome, aged 18 to 83 years, 50 cases (14 males and 36 females) of rheumatoid arthritis, aged 20 to 85 years, 12 cases (3 males and 9 females) of scleroderma, aged 38 to 69 years, and 22 cases (7 males and 15 females) of dermatomyositis, aged age 9-78 year (Table 1).
| Disease | n | Sex (male/female) | Age (yr) |
| Systemic lupus erythematosus | 138 | 8/130 | 11-77 |
| Sicca syndrome | 48 | 2/46 | 18-83 |
| Rheumatoid arthritis | 50 | 14/36 | 20-85 |
| Scleroderma | 12 | 3/9 | 38-69 |
| Dermatomyositis | 22 | 7/15 | 9-78 |
| Healthy controls | 130 | 31/99 | 14-79 |
CLIA and LIA essentially showed comparable detection rates for anti-dsDNA antibody, anti-Histone antibody, anti-Nuc antibody, anti-Sm antibody, anti-Rib-P antibody, anti-Ro60 antibody, anti-Ro52 antibody, anti-SSB antibody, anti-Cenp-B antibody, anti-DNAScl-70 antibody, anti-Jo-1 antibody, and anti-AMA-M2 antibody (P > 0.05) (Table 2).
| Items | Chemiluminescence method | Immunochemistry | χ2 | P value | ||||||
| SLE | SS | Other | Total | SLE | SS | Other | Total | |||
| Anti-double-stranded DNA (dsDNA) antibody | 69 (50.00) | 5 (10.42) | 3 (3.57) | 77 (28.52) | 61 (44.20) | 4 (8.33) | 3 (3.57) | 68 (25.19) | 0.764 | 0.382 |
| Anti-histone (Histone) antibody | 32 (23.19) | 4 (8.33) | 5 (5.95) | 41 (15.19) | 35 (25.36) | 4 (8.33) | 7 (8.33) | 46 (17.04) | 0.343 | 0.558 |
| Anti-nucleosome (Nuc) antibody | 40 (28.99) | 1 (2.08) | 4 (4.76) | 45 (16.67) | 36 (26.09) | 1 (2.08) | 3 (3.57) | 40 (14.81) | 0.349 | 0.555 |
| Anti-Smith (Sm) antibody | 18 (13.04) | 1 (2.08) | 2 (2.38) | 21 (7.78) | 17 (12.32) | 0 (0.00) | 1 (1.19) | 18 (6.67) | 0.249 | 0.618 |
| Anti-ribosomal P protein (Rib-P) antibody | 38 (27.54) | 2 (4.17) | 0 (0.00) | 40 (14.81) | 49 (35.51) | 2 (4.17) | 0 (0.00) | 51 (18.89) | 1.599 | 0.206 |
| Anti-sicca syndrome A (Ro60) antibody | 82 (59.42) | 40 (83.33) | 14 (16.67) | 136 (50.37) | 85 (61.59) | 36 (75.00) | 13 (15.48) | 134 (49.62) | 0.030 | 0.863 |
| Anti-sicca syndrome A (Ro52) antibody | 77 (55.80) | 37 (77.08) | 19 (23.46) | 133 (49.26) | 79 (57.25) | 36 (75.00) | 21 (25.00) | 136 (50.37) | 0.067 | 0.796 |
| Anti- sicca syndrome (SSB) antibody | 19 (13.77) | 18 (37.50) | 12 (14.29) | 49 (18.15) | 20 (14.49) | 19 (39.58) | 11 (13.10) | 50 (18.52) | 0.012 | 0.911 |
| Anti-centromere protein B (Cenp-B) antibody | 11 (7.97) | 4 (8.33) | 2 (2.38) | 17 (6.30) | 8 (5.80) | 3 (6.25) | 2 (2.38) | 13 (4.81) | 0.565 | 0.452 |
| Anti-DNA topoisomerase 1 (Scl-70) antibody | 5 (3.62) | 0 (0.00) | 6 (7.14) | 11 (4.07) | 4 (2.90) | 0 (0.00) | 6 (7.14) | 10 (3.70) | 0.050 | 0.824 |
| Anti-histidyl tRNA synthetase (Jo-1) antibody | 0 (0.00) | 0 (0.00) | 1 (1.19) | 1 (0.37) | 1 (0.72) | 0 (0.00) | 1 (4.62) | 2 (0.74) | 0.335 | 0.563 |
| Anti-mitochondrial M2 (AMA-M2) antibody | 2 (1.45) | 1 (2.08) | 3 (3.57) | 6 (2.22) | 2 (1.45) | 0 (0.00) | 3 (3.57) | 5 (1.85) | 0.093 | 0.761 |
The two methods displayed good agreement for the detection of anti-dsDNA antibody, anti-Histone antibody, anti-Nuc antibody, anti-Sm antibody, anti-Ro60 antibody, anti-Ro52 antibody, anti-SSB antibody, anti-Cenp-B antibody, anti-Scl-70 antibody, and anti-AMA-M2 antibody (Kappa > 0.7, P < 0.05), but had a moderate agreement for the detection of anti-Rib-P antibody and anti-Jo-1 antibody (Kappa = 0.671 and 0.665; P < 0.05) (Table 3).
| Items | Positive agreement rate | Negative agreement rate | Total agreement rate | Kappa | P value |
| Anti-double-stranded DNA (dsDNA) antibody | 89.71 | 92.08 | 91.48 | 0.783 | < 0.05 |
| Anti-histone (Histone) antibody | 82.61 | 98.66 | 95.93 | 0.849 | < 0.05 |
| Anti-nucleosome (Nuc) antibody | 90.00 | 96.09 | 95.19 | 0.819 | < 0.05 |
| Anti-Smith (Sm) antibody | 83.33 | 97.62 | 96.67 | 0.751 | < 0.05 |
| Anti-ribosomal P protein (Rib-P) antibody | 64.71 | 96.80 | 90.74 | 0.671 | < 0.05 |
| Anti-sicca syndrome A (Ro60) antibody | 93.28 | 91.91 | 92.59 | 0.852 | < 0.05 |
| Anti-sicca syndrome A (Ro52) antibody | 91.18 | 92.59 | 92.22 | 0.844 | < 0.05 |
| Anti- sicca syndrome (SSB) antibody | 88.00 | 97.73 | 95.93 | 0.864 | < 0.05 |
| Anti-centromere protein B (Cenp-B) antibody | 92.31 | 98.05 | 97.78 | 0.788 | < 0.05 |
| Anti-DNA topoisomerase 1 (Scl-70) antibody | 80.00 | 98.85 | 98.15 | 0.752 | < 0.05 |
| Anti-histidyl tRNA synthetase (Jo-1) antibody | 50.00 | 100.00 | 99.63 | 0.665 | < 0.05 |
| Anti-mitochondrial M2 (AMA-M2) antibody | 80.00 | 99.25 | 98.89 | 0.722 | < 0.05 |
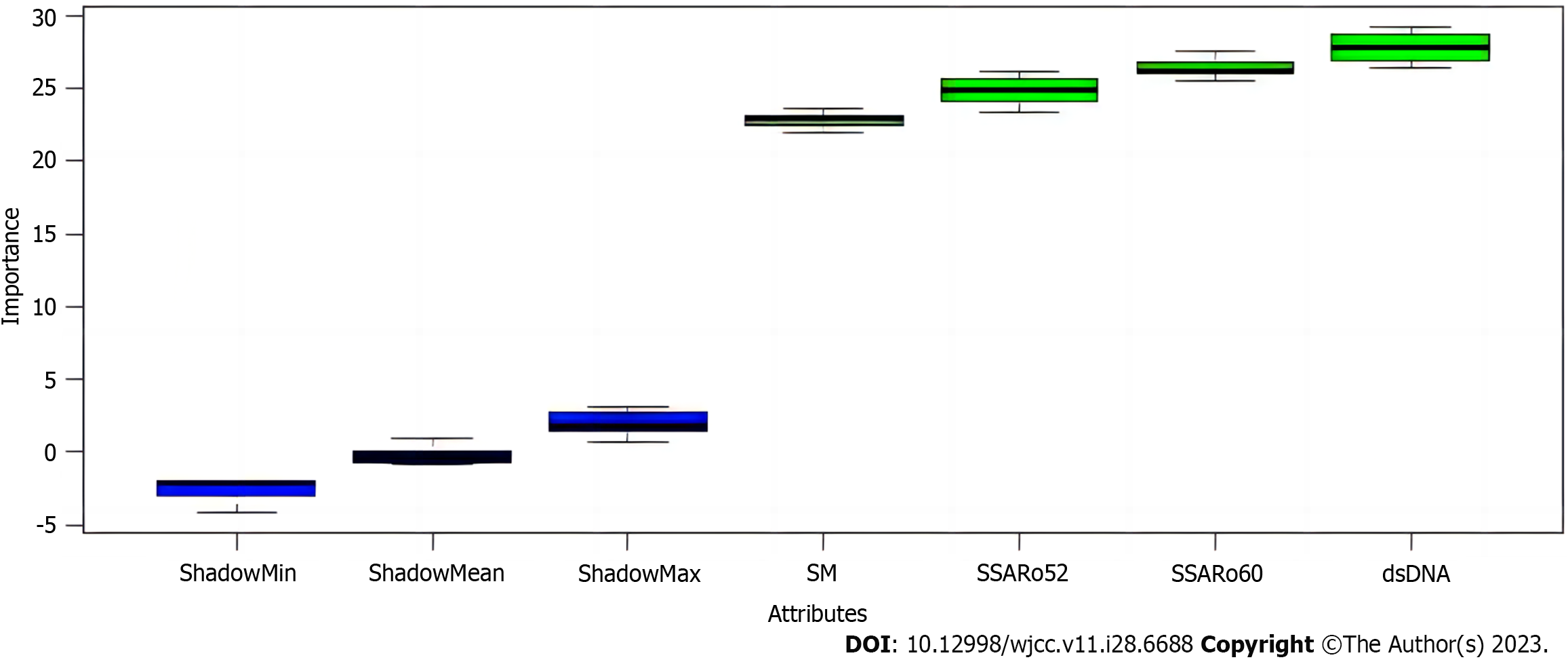
The performance of these 12 antibodies detected by chemiluminescence in independently distinguishing SLE from the healthy controls was similar to those of these 12 antibodies detected by immunoblotting in independently determining SLE from the healthy controls (Table 4). After that, to improve the competence of diagnosing SLE, a variable screening of various antibodies with an area under the curve (AUC) greater than 0.9 detected by chemiluminescence was first performed using the Boruta algorithm, followed by developing a novel diagnostic model. The 4 antibodies, anti-double-stranded DNA (dsDNA) antibody, anti-sicca syndrome A (Ro60) antibody, anti-sicca syndrome A (Ro52) antibody, and anti-Smith (Sm) antibody, were considered as essential variables. Overall, after considering the cost, anti-double-stranded DNA (dsDNA) antibody and anti-sicca syndrome A (Ro60) antibody were used to construct the diagnostic model (Figure 1). ROC analysis showed that the model's AUC values, sensitivity, and specificity were 0.997 (95%CI: 0.994-1.000), 0.962, and 0.978, respectively (Figure 2).
| Antibodies | Chemiluminescence method | Line immunoassay | ||
| AUC | 95%CI | AUC | 95%CI | |
| Anti-double-stranded DNA (dsDNA) antibody | 0.935 | 0.907-0.962 | 0.905 | 0.871-0.940 |
| Anti-histone (Histone) antibody | 0.508 | 0.436-0.580 | 0.513 | 0.441-0.585 |
| Anti-nucleosome (Nuc) antibody | 0.837 | 0.788-0.886 | 0.829 | 0.780-0.879 |
| Anti-Smith (Sm) antibody | 0.941 | 0.914-0.968 | 0.907 | 0.872-0.943 |
| Anti-ribosomal P protein (Rib-P) antibody | 0.776 | 0.721-0.831 | 0.791 | 0.738-0.844 |
| Anti-sicca syndrome A (Ro60) antibody | 0.953 | 0.931-0.975 | 0.945 | 0.921-0.969 |
| Anti-sicca syndrome A (Ro52) antibody | 0.946 | 0.920-0.972 | 0.929 | 0.900-0.958 |
| Anti- sicca syndrome (SSB) antibody | 0.650 | 0.584-0.716 | 0.661 | 0.596-0.727 |
| Anti-centromere protein B (Cenp-B) antibody | 0.811 | 0.761-0.861 | 0.787 | 0.733-0.841 |
| Anti-DNA topoisomerase 1 (Scl-70) antibody | 0.783 | 0.727-0.838 | 0.740 | 0.681-0.800 |
| Anti-histidyl tRNA synthetase (Jo-1) antibody | 0.821 | 0.769-0.874 | 0.781 | 0.722-0.841 |
| Anti-mitochondrial M2 (AMA-M2) antibody | 0.895 | 0.844-0.947 | 0.920 | 0.883-0.957 |
Clinically, autoimmune diseases are considered a group of diseases in which a pathological immune response targeting cell- or organ-specific autoantigens can primarily result from a deficiency in immune tolerance, leading to systemic organ damage[12,13]. The clinical understanding of the origins of various autoimmune disorders remains obscure. However, several prior investigations[14-16] have demonstrated the presence of many autoantibodies in the serum of individuals afflicted with autoimmune conditions. Still, despite the specific role of these antibodies in the pathogenesis and progression of the disease, their direct effects have been scarcely studied. Autoantibodies are considered a vital detection index of autoimmune diseases, and ANA are a general term for autoantibodies. They have a high concentration in patients' serum, so detecting this index is essential for diagnosing disease monitoring and prognosis assessment of AID[17]. In previous decades, most Chinese laboratories utilized the immunoblotting technique to detect the ANA spectrum. This approach is a qualitative assay that has a predetermined set of detection items and can only perform batch detection. Consequently, its effectiveness in identifying the ANA spectrum is constrained[18]. However, in recent years, with the development of immunological detection technology, fully automated chemiluminescence detection technique has been extensively adopted in the detection of clinical samples because of its associated advantages such as those of automation, quantification, random loading, and wide linear range of detection[19,20].
The results of the present study showed that both CLIA and LIA had essentially comparable detection rates for anti-dsDNA antibody, anti-Histone antibody, anti-Nuc antibody, anti-Sm antibody, anti-Rib-P antibody, anti-Ro60 antibody, anti-Ro52 antibody, anti-SSB antibody, anti-Cenp-B antibody, anti-DNAScl-70 antibody, anti-Jo-1 antibody, and anti-AMA-M2 antibody (P > 0.05). The two methods displayed good agreement for the detection of anti-dsDNA antibody, anti-Histone antibody, anti-Nuc antibody, anti-Sm antibody, anti-Ro60 antibody, anti-Ro52 antibody, anti-SSB antibody, anti-Cenp-B antibody, anti-Scl-70 antibody, and anti-AMA-M2 antibody (Kappa > 0.7, P < 0.05), but had a moderate agreement for the detection of anti-Rib-P antibody and anti-Jo-1 antibody (Kappa = 0.671 and 0.665; P < 0.05). The elements that may account for the reasonable agreement are: (1) Disparities in the outcomes arising from variations in the underlying response principles. Although the chemiluminescence method follows a similar principle as the immunoblotting method, the detection sensitivity is significantly improved after the amplification system of biotin-affin[21]; and (2) The detection performance may vary due to differences in the reaction systems employed. The LIA method involves the placement of multiple target antigens on a single nitrocellulose membrane, which may result in inadequate binding between antigens and antibodies within the same reaction system. On the other hand, CLIA utilizes a homogeneous liquid-phase reaction system that offers improved reaction efficiency and a more comprehensive cleaning step. This can lead to a significant enhancement in the specificity of the reaction system[22]. Moreover, the diagnostic performance of these antibodies detected by both methods was found to be similar for SLE, and ROC analysis of the diagnostic model consisting of anti-dsDNA antibody and anti-Ro60 antibody detected by CLIA exhibited that the AUC values, sensitivity, and specificity of the model were 0.997, 0.962, and 0.978. The findings confirmed the reliability of both approaches in the detection of antibodies. The researchers noted that the diagnostic accuracy of the model consisting of numerous antibodies was significantly higher than that of single antibodies in detecting SLE.
While our study provides valuable insights into the detection of specific antibodies used in diagnosing SLE using CLIA and LIA and the development of a diagnostic model, several limitations should be noted. Firstly, our sample size was relatively limited, and all participants were recruited from the same hospital, potentially introducing selection bias. The findings might not be generalizable to a broader population of individuals with SLE. Secondly, the study was conducted over a specific period, and rapid advancements in medical technology could affect the applicability of our results in future settings. Thirdly, while beneficial for our study, our reliance on CLIA and LIA methodologies might not capture the full potential of other diagnostic methods.
In summary, CLIA and LIA displayed good overall agreement in detecting ANA profiles. A diagnostic model consisting of antibodies detected by CLIA can effectively contribute to improving the diagnosis of SLE.
Antinuclear antibodies (ANAs) are essential for diagnosing autoimmune diseases, mainly systemic lupus erythematosus (SLE). This study aimed to compare the performance of two detection methods, chemiluminescence assay (CLIA) and line immunoassay (LIA), in identifying ANAs in patients with autoimmune diseases. The objective was to assess their diagnostic accuracy for SLE and develop a new model using CLIA-detected antibodies specific to SLE.
The motivation behind this research was to improve the diagnosis of SLE, an autoimmune disease, by comparing two detection methods for ANAs. The CLIA and LIA were evaluated for their performance and diagnostic accuracy in detecting ANAs. By developing a novel diagnostic model using CLIA-detected antibodies, the researchers aimed to provide innovative approaches for SLE diagnosis. The study sought to contribute valuable insights into ANA detection and advance strategies for improving the diagnosis of SLE, thus benefiting patients with autoimmune diseases.
The research objectives were to compare the performance of CLIA and LIA in detecting ANAs in patients with autoimmune diseases. The study aimed to assess the diagnostic accuracy of CLIA and LIA for SLE and develop a novel diagnostic model using CLIA-detected antibodies specifically for SLE. Specimens from patients with autoimmune diseases and physical examination specimens were collected to parallel detect specific antibodies. The research focused on evaluating the diagnostic performance of individual antibodies and constructing a diagnostic model combining multiple antibodies. The findings aimed to provide valuable insights into improving SLE diagnosis through innovative approaches and contribute to the development of novel strategies for diagnosing SLE.
In this study, specimens from 270 patients with autoimmune diseases and 130 physical examination specimens were collected. The detection of specific antibodies, including anti-double-stranded DNA (dsDNA) antibody, anti-histone antibody, anti-nucleosome antibody, anti-Smith antibody, anti-ribosomal P protein antibody, anti-sicca syndrome A (Ro60) antibody, anti-sicca syndrome A (Ro52) antibody, anti-sicca syndrome (SSB) antibody, anti-centromere protein B (Cenp-B) antibody, anti-DNA topoisomerase 1 (Scl-70) antibody, anti-histidyl tRNA synthetase (Jo-1) antibody, and anti-mitochondrial M2 (AMA-M2) antibody, was performed using CLIA and LIA. The study compared the detection rates, compliance rates, and diagnostic performance for SLE between CLIA and LIA. Furthermore, a novel diagnostic model for SLE was developed based on the results. The agreement between CLIA and LIA in detecting the ANA profiles was assessed, and the diagnostic performance of individual antibodies and the diagnostic model combining multiple antibodies were evaluated using receiver operating characteristics (ROC) analysis.
The results showed that CLIA and LIA had similar detection rates in detecting ANAs. For a variety of antibodies, including anti-dsDNA antibodies, anti-histone antibodies, anti-Nucleosome antibodies, anti-Smith antibodies, anti-Ribosome P protein antibody, anti-Sjogren's syndrome A (Ro60) antibody, anti-Sjogren's syndrome A (Ro52) antibody, anti-Sjogren's syndrome (SSB) antibody, anti centromeric protein B (Cenp-B) antibody, anti-DNA Topoisomerase 1 (Scl-70) antibody The detection results of anti-Histidine tRNA synthetase (Jo-1) antibody and AMA-M2 antibody are entirely consistent (Kappa > 0.7, P < 0.05). Still, there is moderate consistency in the detection of anti-Ribosome P protein antibody and anti-Histidine tRNA synthetase (Jo-1) antibody (Kappa = 0.671 and 0.665; P < 0.05). In addition, the diagnostic performance of the two methods for SLE is similar. The diagnostic model constructed by chemiluminescence detection of anti-dsDNA antibodies and anti-Ro60 antibodies showed superior diagnostic performance compared to a single antibody after receiving a working ROC analysis, with a curve under area of 0.997, sensitivity of 0.962, and specificity of 0.978. These results indicate that CLIA and the developed diagnostic model can effectively improve the diagnostic level of SLE and provide innovative strategies for improving detection.
CLIA and LIA perform similarly in detecting ANAs in patients with autoimmune diseases. The two methods were consistent for most tested antibodies, indicating their reliability in diagnosing SLE. In addition, diagnostic models constructed using antibodies detected by CLIA, particularly anti-dsDNA antibodies and anti-Ro60 antibodies, showed superior diagnostic performance compared to single antibodies. These results indicate that CLIA and the developed diagnostic models can promote the improvement of the diagnostic level of SLE and provide innovative strategies for improving detection.
This study compared ANAs detection methods for autoimmune diseases and evaluated their accuracy in diagnosing SLE. The results showed that CLIA and LIA had similar effects on detecting most antibodies, especially for anti-dsDNA and anti-Ro60 antibodies. In addition, the diagnostic model constructed using antibodies detected by CLIA outperforms the detection of a single antibody in diagnosing SLE. These findings provide valuable insights for improving the diagnosis of SLE through innovative methods. Overall, this study indicates that CLIA and the developed diagnostic models have the potential to diagnose SLE, providing new ideas for improving SLE detection strategies.
The authors would like to extend their sincere gratitude to all those who contributed to the manuscript.
| 1. | Aringer M, Johnson SR. Systemic Lupus Erythematosus Classification and Diagnosis. Rheum Dis Clin North Am. 2021;47:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Bossuyt X, De Langhe E, Borghi MO, Meroni PL. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat Rev Rheumatol. 2020;16:715-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (2)] |
| 3. | Cha HJ, Hwang J, Lee LE, Park Y, Song JJ. The significance of cytoplasmic antinuclear antibody patterns in autoimmune liver disease. PLoS One. 2021;16:e0244950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, Chan EKL, Miller FW. Increasing Prevalence of Antinuclear Antibodies in the United States. Arthritis Rheumatol. 2020;72:1026-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 5. | Fritzler MJ, Choi MY. Antinuclear Antibody Testing: Gold Standard Revisited. J Appl Lab Med. 2022;7:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Jang J, Kim S, Kim HS, Lee KA, Park J, Park Y. Comparison of antinuclear antibody profiles obtained using line immunoassay and fluorescence enzyme immunoassay. J Int Med Res. 2021;49:3000605211014390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Kwon OC, Kim YG, Park JH, Park MC. Seroconversion to antinuclear antibody negativity and its association with disease flare in patients with systemic lupus erythematosus. Lupus. 2020;29:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Lee AYS, Beroukas D, Roberts-Thomson PJ. Utility of the HEp-2000 antinuclear antibody substrate. Ann Rheum Dis. 2020;79:e67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Lee AYS, Gordon TP. Antinuclear antibody (ANA) monitoring in drug-induced lupus erythematosus (DILE). Rheumatology (Oxford). 2021;60:2022-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ling M, Murali M. Antinuclear Antibody Tests. Clin Lab Med. 2019;39:513-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Lundgren MC, Sapkota S, Peterson DJ, Crosson JT. The antinuclear antibody dense fine speckled pattern and possible clinical associations: An indication of a proinflammatory microenvironment. J Immunol Methods. 2021;488:112904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Nanda R, Gupta P, Patel S, Shah S, Mohapatra E. Uncommon antinuclear antibody patterns as diagnostic indicators. Clin Biochem. 2021;90:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Nashi RA, Shmerling RH. Antinuclear Antibody Testing for the Diagnosis of Systemic Lupus Erythematosus. Med Clin North Am. 2021;105:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Nashi RA, Shmerling RH. Antinuclear Antibody Testing for the Diagnosis of Systemic Lupus Erythematosus. Rheum Dis Clin North Am. 2022;48:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Pisetsky DS. Antinuclear antibody testing - misunderstood or misbegotten? Nat Rev Rheumatol. 2017;13:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 16. | Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16:565-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 220] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 17. | Russell AS. The Variability of Antinuclear Antibody Testing. J Rheumatol. 2021;48:1190. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | de Castro GLC, da Silva Graça Amoras E, Araújo MS, da Silva Conde SRS, Bichara CDA, Queiroz MAF, Vallinoto ACR. High prevalence of antinuclear antibodies in patients with chronic hepatitis C virus infection. Eur J Med Res. 2022;27:180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 19. | Stochmal A, Czuwara J, Trojanowska M, Rudnicka L. Antinuclear Antibodies in Systemic Sclerosis: an Update. Clin Rev Allergy Immunol. 2020;58:40-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 20. | Sur LM, Floca E, Sur DG, Colceriu MC, Samasca G, Sur G. Antinuclear Antibodies: Marker of Diagnosis and Evolution in Autoimmune Diseases. Lab Med. 2018;49:e62-e73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 21. | Tiwary AK, Kumar P. Paradigm shift in antinuclear antibody negative lupus: Current evidence. Indian J Dermatol Venereol Leprol. 2018;84:384-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Cao X, Zhang M, Lu Z, Li C, Zeng Y, Fan J, Yu K. Multiple neurological manifestations in a patient with systemic lupus erythematosus and anti-NXP2-positive myositis: A case report. Medicine (Baltimore). 2021;100:e25063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciorba MA, United States; Clos-Garcia M, Spain S-Editor: Yan JP L-Editor: A P-Editor: Zhao S