Published online Oct 6, 2023. doi: 10.12998/wjcc.v11.i28.6680
Peer-review started: June 21, 2023
First decision: August 16, 2023
Revised: September 2, 2023
Accepted: September 6, 2023
Article in press: September 6, 2023
Published online: October 6, 2023
Processing time: 95 Days and 20.9 Hours
At present, many studies have reported the risk factors for postoperative intracranial reinfection, including age, sex, time to surgery, duration of postoperative catheterization, emergency procedures, type of disease and cerebrospinal fluid leakage, but the academic community has not reached a unified conclusion.
To find factors influencing the surveillance of re-emerging intracranial infections in elective neurosurgical patients.
Ninety-four patients who underwent elective craniotomy from January 1, 2015 to December 31, 2022 in the Department of Neurosurgery, First Hospital of Jilin University, were included in this study. Of those, 45 patients were enrolled in the infection group, and 49 were enrolled in the control group. The clinical data of the patients were collected and divided into three categories, including preoperative baseline conditions, intraoperative characteristics and postoperative infection prevention. The data were analyzed using SPSS 26.0 software.
There were 23 males and 22 females in the infection group with a mean age of 52.8 ± 15.1 years and 17 males and 32 females in the control group with a mean age of 48.9 ± 15.2 years. The univariate analysis showed that the infection group had higher systolic blood pressures and postoperative temperatures, fewer patients who underwent a supratentorial craniotomy, more patients with a history of hypertension and higher initial postoperative white blood cell counts than the control group, with statistically significant differences (P < 0.05). The multifactorial logistic regression analysis showed that a history of hypertension and a high postoperative body temperature were independent risk factors for postoperative infection in neurosurgical patients.
The results obtained in this study indicated that a history of hypertension and a high postoperative body temperature were independent risk factors for postoperative neurological symptoms.
Core Tip: A postoperative intracranial reinfection not only increases the mortality rate, economic burden and length of hospitalization but may even cause permanent sequelae to the patient. The results obtained in our study indicated that a history of hypertension and a high postoperative body temperature were independent risk factors for postoperative neurologic complications that mostly occur within the first 3 d after surgery. The identified risk factors provide a basis for recommending future prevention strategies.
- Citation: Wang JL, Wu XW, Wang SN, Liu X, Xiao B, Wang Y, Yu J. Factors influencing the surveillance of re-emerging intracranial infections in elective neurosurgical patients: A single-center retrospective study. World J Clin Cases 2023; 11(28): 6680-6687
- URL: https://www.wjgnet.com/2307-8960/full/v11/i28/6680.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i28.6680
Postoperative intracranial reinfection can occur in neurosurgical patients who undergo craniotomy, recover well after surgery and are discharged from the hospital despite having inflammation at the original surgical site, thereby requiring another craniotomy for debridement and treatment. These infections include postoperative meningitis, brain abscess, subdural abscess, epidural abscess and more widespread or diffuse infections such as septic meningitis and ventriculitis, which is one of the common serious complications after neurosurgery. The infected patients often present with severe symptoms such as high cranial pressure, cerebral edema and seizures, and the increasing rate of drug resistance of pathogenic bacteria and the decrease in the rate of positive bacterial cultures make clinical treatment difficult. A postoperative intracranial reinfection not only increases the mortality rate, economic burden and length of hospitalization but may even cause permanent sequelae to the patient.
There are many studies on the risk factors for intracranial infection, including age, sex, duration of surgery, emergency procedures, disease type, cerebrospinal fluid leakage, surgical approach, postoperative temperature, artificial implants and leukocyte level[1,2]. However, there is no unified conclusion on whether these risk factors have an effect on the incidence of postoperative intracranial reinfection, and the value of many risk factors is still unknown[3,4]. In this study, 94 patients with neurosurgical diseases who underwent elective surgery at the First Hospital of Jilin University were enrolled to explore the possible risk factors for the occurrence of postoperative intracranial reinfection. In particular, the effect of postoperative fever on the incidence of postoperative intracranial reinfection was evaluated to provide a theoretical reference for the early prevention and control of postoperative intracranial reinfection. The specific reports are as follows.
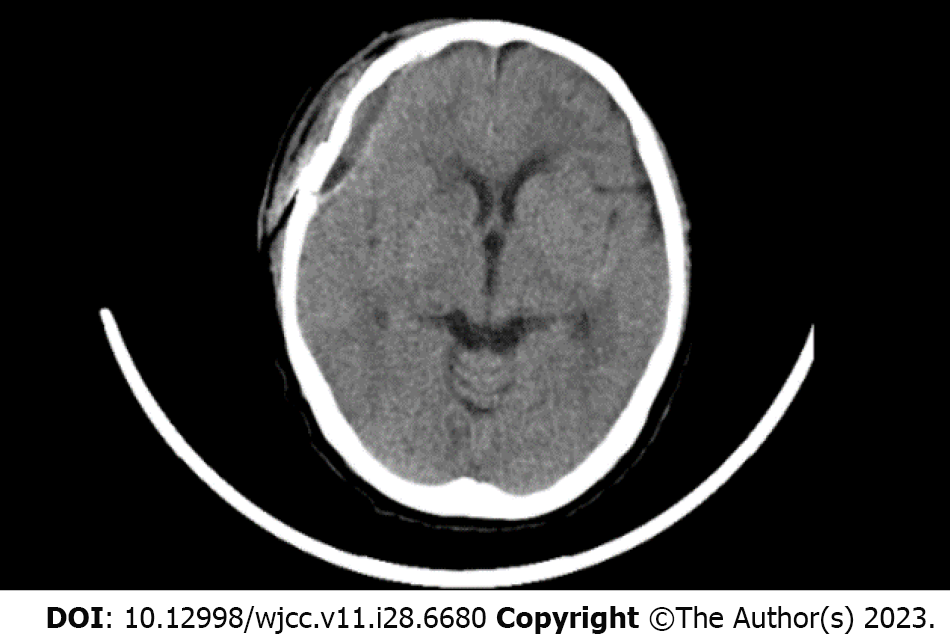
Inclusion criteria: Infection group: (1) Patients who underwent a craniotomy for the treatment of a neurological tumor, cerebrovascular disease, intracranial hemorrhage, etc. recovered well perioperatively and were discharged; and (2) Patients with a late-onset postoperative central nervous system infection who met the following criteria: (a) Patients with unhealed scalp wounds and intermittent subcutaneous sensations, and computed tomography images showed a biconvex intracranial epidural lesion (Figure 1); and (b) Patients who suffered a preoperative scalp infection or subcutaneous rupture that required a repeat craniotomy for debridement treatment but recovered well after surgery and were discharged. Control group: Patients who underwent a craniotomy for the treatment of a neurological tumor, cerebrovascular disease, intracranial hemorrhage, etc, recovered well perioperatively and were discharged but did not have a postoperative infection during the same period (from January 1, 2015 to December 31, 2022) following neurosurgery at the First Hospital of Jilin University.
Exclusion criteria: (1) Patients with a postoperative diagnosis of septic meningitis, which improved after anti-inflammatory treatment; and (2) Patients with a high suspicion of having an intracranial infection who refused to undergo the debridement procedure.
This study was approved by the Ethics Committee of the First Hospital of Jilin University (Grant No. 2023-293), and informed consent was obtained from all patients or their families.
The data of the patients who met the above criteria were collected in the clinical electronic case database in this study and divided into three categories. The first category was preoperative baseline conditions, including age, sex, weight, operation time, operation table, blood pressure and admission white blood cell (WBC) count. The second category was intraoperative characteristics, including surgical access, intraoperative blood transfusion, operation duration and intraoperative implants (cranial mesh plate). The third category consisted of information related to postoperative infection prevention, including postoperative temperature, first postoperative WBC and discharge WBC count.
In this study, based on previous literature and routine clinical practice, factors such as age, sex, weight, duration of operation, operation table, blood pressure, operation access, intraoperative blood transfusion, operation duration, intraoperative implants (cranial mesh, artificial dura, etc), bone flap return and postoperative body temperature were included. These factors affect the incidence of postoperative intracranial reinfection. A single-factor analysis and a multifactor logistics regression were performed. The risk factors affecting the incidence of postoperative intracranial reinfection were investigated.
The patients who met the above criteria were prepared preoperatively, and surgical debridement was performed. After the operation, the specimens that were retrieved from the operative area were sent for pathological examination, and antibiotics were routinely administered to prevent postoperative infection. Patients who suffered a postoperative intracranial reinfection were followed up to observe the postoperative recovery effect.
SPSS 26.0 software (IBM Corp., Armonk, NY, United States) was used for data analysis. The t test was used for comparisons between groups for data meeting a normal distribution; the Mann-Whitney U test was used for comparisons between groups for data not meeting a normal distribution. The Pearson χ2 test was used for comparisons among groups for categorical variables. The variables that were statistically significant in the univariate analysis were further included in the logistic regression equation for multivariate analysis to screen patients for risk factors for postoperative intracranial reinfection. P < 0.05 indicated that the difference was statistically significant.
A total of 94 patients were included in this study. There were 23 males and 22 females in the infected group with a mean age of 52.8 ± 15.1 years and 17 males and 32 females in the control group with a mean age of 48.9 ± 15.2 years.
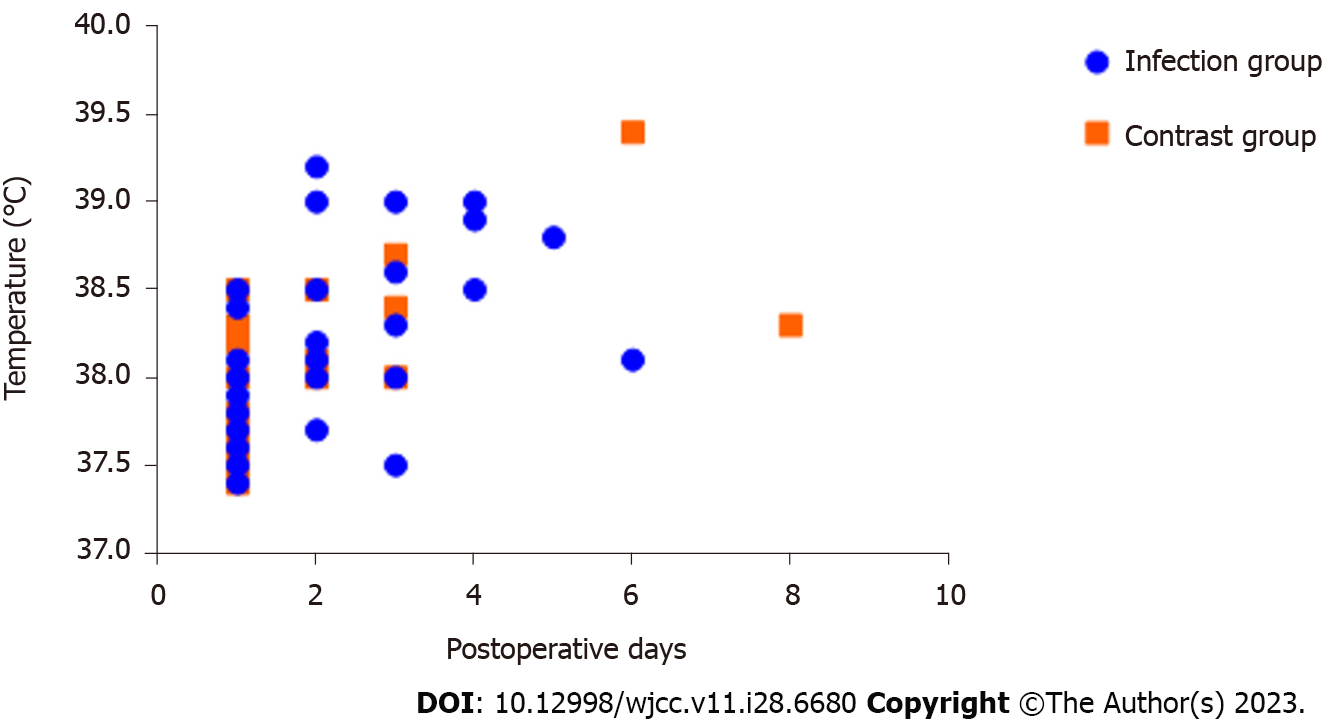
The postoperative body temperature in the infected group was 38.1 °C (37.7 °C, 38.5°C) with a normal range from 36.9 °C to 39.2 °C. The body temperature in the control group was 37.40 °C (36.8 °C, 37.8 °C) with a normal range from 36.0 °C to 38.5 °C. Forty-one patients in the infected group had a postoperative fever, 36 of which mostly occurred within 3 d after surgery; 25 patients in the control group had an elevated postoperative body temperature.
All 45 patients in the infected group received artificial implants. In the control group, 43 of 49 patients received artificial implants, while 6 patients did not receive artificial grafts. All 45 patients in the infection group had intraoperative pus accumulation under the bone flap, of which 38 patients were evaluated and the bone flap was returned. Patients with postoperative intracranial reinfection were followed up for 3 years. Among them, 34 patients recovered well without reinfection, and 11 patients were lost to follow-up.
The first postoperative WBC count in the infected group was 13.9 × 109/L (11.0 × 109/L, 16.6 × 109/L) ranging from 3.7-30.4 × 109/L. The first postoperative WBC count in the control group was 12.8 × 109/L (10.1 × 109/L, 15.2 × 109/L) ranging from 4.2-22.5 × 109/L. The remaining information is detailed in Table 1.
| Projects | Infection group, n = 45 | Control group, n = 49 | Test value | P value |
| Age (mean ± SD) | 47.867 ± 15.286 | 52.816 ± 15.073 | 1.580a | 0.118 |
| Male | 23 (57.5) | 17 (42.5) | 2.586b | 0.108 |
| Weight [kg, M (P25, P75)] | 65 (58, 75) | 64 (57, 68.5) | -1.251c | 0.211 |
| Time of surgery [mo, M (P25, P75)] | 7 (4, 10) | 7 (3, 9) | -0.951c | 0.341 |
| First unit | 24 (53.3) | 24 (49.0) | 0.178b | 0.673 |
| Systolic pressure [mmHG, M (P25, P75)] | 144 (126, 160) | 126 (120, 137) | -3.526c | < 0.001 |
| Diastolic pressure [mmHG, M (P25, P75)] | 80.0 (70.0, 83.0) | 80.0 (72.5, 90.0) | -1.345c | 0.179 |
| History of hypertension | 28 (57.1) | 8 (17.8) | 15.383b | < 0.001 |
| Supratentorial craniotomy | 32 (71.1) | 44 (89.8) | 5.290b | 0.021 |
| Postoperative body temperature [C, M (P25, P75)] | 38.10 (37.72, 38.50) | 37.40 (36.75, 37.80) | -5.471c | < 0.001 |
| Admission WBC [109, M (P25, P75)] | 7.98 (6.67, 10.88) | 7.52 (5.19, 9.60) | -1.557c | 0.119 |
| First postoperative WBC [109, M (P25, P75)] | 14.22 (11.08, 16.70) | 12.78 (10.13, 15.17) | -2.030c | 0.042 |
| Discharge WBC [× 109/L, M (P25, P75)] | 8.67 (6.59, 11.30) | 8.36 (6.89, 9.91) | -0.701c | 0.483 |
| Intraoperative blood loss [mL, M (P25, P75)] | 30 (30, 350) | 100 (30, 250) | -0.090c | 0.929 |
| Intraoperative blood transfusion | 12.0 (26.6) | 6.0 (12.2) | 2.525b | 0.112 |
| Duration of surgery [h, M (P25, P75)] | 3.90 (3.00, 5.42) | 3.40 (2.45, 5.00) | -1.261 | 0.207 |
| With artificial implants | 45(100) | 43(87.8) | 3.419 b | 0.064 |
| Reduction of bone flap | 39 (86.7) | 47 (95.9) | 2.579b | 0.108 |
The univariate analysis showed that compared with the control group, the infection group had higher systolic blood pressures and postoperative body temperatures (Figure 2), fewer patients who underwent a craniotomy, more patients with a history of hypertension and higher initial postoperative WBC counts, with statistically significant differences (P < 0.05). In contrast, age, sex, weight, time to surgery, admission WBC count, discharge WBC count, intraoperative blood loss, intraoperative bleeding volume, surgery duration, artificial implants and bone flap return were not significantly different between the two groups (P > 0.05). The indicators that were statistically significant in the univariate analysis were included in the multifactorial logistic regression analysis, which showed that a history of hypertension and a high postoperative temperature were independent factors influencing the incidence of postoperative infection in neurosurgical patients. The remaining information is detailed in Table 2.
| Projects | Β value | Standard error | Wald value | OR value | 95%CI | P value |
| History of hypertension | 1.827 | 0.630 | 8.403 | 6.214 | 1.807-21.370 | 0.004 |
| Supratentorial craniotomy | 0.679 | 0.767 | 0.784 | 1.972 | 0.439-8.869 | 0.367 |
| Postoperative body temperature | 2.536 | 0.598 | 17.986 | 0.078 | 3.913-40.795 | < 0.001 |
| First postoperative WBC | 0.061 | 0.061 | 1.004 | 1.063 | 0.943-1.119 | 0.316 |
Neurosurgical craniotomy has a high incidence of postoperative infections due to the difficulty of the procedure and the long hospital stay[5]. Postoperative infections usually manifest as meningitis, brain abscesses, subdural pustules and/or epidural abscesses[6], representing the most common complications in patients undergoing neurosurgical craniotomy[7]. Postoperative intracranial reinfections are highly prevalent during the 3-7 d postoperative period, especially in patients with open craniocerebral trauma, postoperative cerebrospinal fluid leakage, subcutaneous effusion from the incision, ventricular drainage and reoperation for postoperative emergencies. Patients with severe infections complicated by epidural abscesses must undergo debridement again. Related studies have found that the duration of surgery and intraoperative blood transfusion and other indicators have an impact on the incidence of postoperative intracranial reinfection because craniotomy severely damages the protective tissues of the brain. The results of the present study cannot support this view, which may be related to the development of new surgical techniques and improvements in surgical equipment and medical standards, strict perioperative management, timely intraoperative management of bleeding and the patients’ nutritional statuses[8].
In the present study, an elevated postoperative body temperature was found to be an independent risk factor for postoperative intracranial reinfection in neurosurgical patients, with most fevers occurring within 3 d after surgery, consistent with the findings of Raviv et al[9]. A meta-analysis by Chen et al[10] showed that titanium alloy artificial implant material is a risk factor for postoperative infection in neurosurgery patients, which may be related to the body’s intolerance to titanium alloy material. In this study, all 45 patients in the infected group received artificial implants, and the increase in their postoperative body temperatures may have been related to the application of artificial implants or differences in patient factors. Therefore, the specific mechanism needs further exploration.
Postoperative fever may also be related to inflammation in the operative area. Measures such as strengthening nutrition, increasing attention, avoiding low protein in the postoperative period and physical therapy of the operative area can be taken to prevent or reduce the risk of infection. Most cases of an elevated postoperative body temperature are associated with an infection, as supported by previous studies[11,12]. Therefore, measures such as bacterial culture should be performed promptly for patients with a postoperative fever to clarify the etiology and provide symptomatic treatment. Until the results of bacterial culture and drug sensitivity are available, it is important to keep track of bacterial resistance, select sensitive drugs for treatment and adjust the medication regimen once the report is available. Luo et al[13] found that a postoperative fever could contribute to the early neurological deterioration of patients and that the higher the temperature, the worse their prognosis. The implementation of a more individualized temperature management strategy for neurosurgical patients with an early fever to reduce the peak postoperative body temperature and shorten the duration of fever may help to reduce the risk of postoperative intracranial reinfection.
A meta-analysis showed that the intraoperative use of powdered vancomycin in neurosurgical patients prevented postoperative intracranial reinfection to some degree[14]. Some studies have also shown that the duration of surgery and the time to reoperation have important effects on the incidence of postoperative intracranial reinfection in patients, suggesting that good surgical management practices can effectively reduce the incidence of postoperative infection[15]. Therefore, the prevention of postoperative intracranial reinfection should be based on various aspects, such as strengthening perioperative management and prophylactic application of antibiotics.
In this study, we found that an increased postoperative body temperature was an early warning factor for postoperative intracranial reinfection in neurosurgical patients and can be used as a follow-up tool to prevent postoperative infection in neurosurgical patients. Postoperative temperature should be closely monitored in neurosurgical craniotomy patients, and timely intervention should be implemented to reduce a high temperature and ultimately reduce the risk of postoperative intracranial reinfection.
In this study, it was found that the initial postoperative WBC count was higher in patients with a history of hypertension, and the difference was statistically significant (P < 0.05), indicating a higher risk of postoperative intracranial reinfection. Studies on hypertension as a factor influencing the incidence of postoperative intracranial reinfection are scarce, but Saeedinia et al[16] and Yao et al[17] found a strong association between hypertension and the incidence of postoperative intracranial reinfection. Few studies have reported the relationship between hypertension history and postoperative intracranial bacterial infection, possibly because hypertension patients often have other cardiovascular and cerebrovascular diseases, vascular stenosis, thin vascular walls, malnutrition and other factors. There are few hypotheses regarding this, and AlGamdi et al[5] suggested that the higher incidence of postoperative intracranial reinfection in patients may be related to inadequate perfusion of skin and subcutaneous tissues. Therefore, the influence of hypertension history on postoperative intracranial infection needs further study. In the future, we can study the changes in microbial cerebrospinal fluid in patients with intracranial infection after the application of different antihypertensive drugs to further verify our speculation.
This was a single-center retrospective study, and its findings must be validated in a multicenter, large-sample randomized controlled trial. However, our findings are consistent with other studies on postneurosurgical fever and are applicable to a larger population of postneurosurgical patients.
The results obtained in this study indicated that a history of hypertension and a high postoperative body temperature were independent risk factors for postoperative neurologic complications that mostly occurred within the first 3 d after surgery. Therefore, clinical staff should pay close attention to patients’ postoperative body temperatures after surgery and promptly treat patients with an elevated body temperature. Patients with a history of high blood pressure should also be particularly concerned.
Neurosurgical craniotomy has a high incidence of postoperative infections due to the difficulty of the procedure and the long hospital stay.
There are many studies on the risk factors for intracranial infection. However, there is no unified conclusion on whether these risk factors influence the incidence of postoperative intracranial reinfection, and the value of many risk factors is still unknown.
Ninety-four patients who underwent elective craniotomy from January 1, 2015 to December 31, 2022 in the Department of Neurosurgery, First Hospital of Jilin University, were included in this study. Of those, 45 patients were enrolled in the infection group, and 49 were enrolled in the control group.
The clinical data of the patients were collected and divided into three categories, including preoperative baseline conditions, intraoperative characteristics and postoperative infection prevention. The data were analyzed using SPSS 26.0 software.
A history of hypertension and a high postoperative body temperature were independent risk factors for postoperative neurologic complications.
The results obtained in this study indicated that a history of hypertension and a high postoperative body temperature were independent risk factors for postoperative neurologic complications that mostly occur within the first 3 d after surgery.
Clinical staff should pay close attention to patients’ postoperative body temperatures and promptly treat patients with an elevated body temperature. Patients with a history of high blood pressure should also be particularly concerned.
| 1. | Huang X, Zhang X, Zhou J, Li G, Zheng G, Peng L, Yan Z, Chen S. Analysis of risk factors and preventive strategies for intracranial infection after neuroendoscopic transnasal pituitary adenoma resection. BMC Neurosci. 2022;23:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Wang J, Ji Y, Jiang L, Zhao X, Guan S, Yang P, Yu J, Liu Y, Zhang H. Analysis of factors influencing hospital-acquired infection in postoperative patients with intracranial aneurysm. BMC Neurol. 2019;19:332. [DOI] [Full Text] |
| 3. | Liu WJ, Duan YC, Chen MJ, Tu L, Yu AP, Jiang XL. Effectiveness of preoperative shaving and postoperative shampooing on the infection rate in neurosurgery patients: A meta-analysis. Int J Nurs Stud. 2022;131:104240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Chidambaram S, Nair MN, Krishnan SS, Cai L, Gu W, Vasudevan MC. Postoperative Central Nervous System Infection After Neurosurgery in a Modernized, Resource-Limited Tertiary Neurosurgical Center in South Asia. World Neurosurg. 2015;84:1668-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | AlGamdi SS, Alawi M, Bokhari R, Bajunaid K, Mukhtar A, Baeesa SS. Risk factors for surgical site infection following spinal surgery in Saudi Arabia: A retrospective case-control study. Medicine (Baltimore). 2021;100:e25567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | McClelland S 3rd. Postoperative intracranial neurosurgery infection rates in North America versus Europe: a systematic analysis. Am J Infect Control. 2008;36:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Yang ZJ, Zhong HL, Wang ZM, Zhao F, Liu PN. Prevention of postoperative intracranial infection in patients with cerebrospinal fluid rhinorrhea. Chin Med J (Engl). 2011;124:4189-4192. [PubMed] |
| 8. | Wolkewitz M, Schumacher M, Rücker G, Harbarth S, Beyersmann J. Estimands to quantify prolonged hospital stay associated with nosocomial infections. BMC Med Res Methodol. 2019;19:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Raviv N, Field N, Adamo MA. Postoperative fever workup in pediatric neurosurgery patients. J Neurosurg Pediatr. 2020;26:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Chen Y, Zhang L, Qin T, Wang Z, Li Y, Gu B. Evaluation of neurosurgical implant infection rates and associated pathogens: evidence from 1118 postoperative infections. Neurosurg Focus. 2019;47:E6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Campos F, Blanco M, Barral D, Agulla J, Ramos-Cabrer P, Castillo J. Influence of temperature on ischemic brain: basic and clinical principles. Neurochem Int. 2012;60:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Castillo J, Dávalos A, Marrugat J, Noya M. Timing for fever-related brain damage in acute ischemic stroke. Stroke. 1998;29:2455-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 227] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Luo Y, Chen M, Fang J, Dong S, Ma M, Bao J, Feng L, He L. Correction to: Relationship Between Body Temperature and Early Neurological Deterioration after Endovascular Thrombectomy for Acute Ischemic Stroke with Large Vessel Occlusion. Neurocrit Care. 2022;36:690. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Texakalidis P, Lu VM, Yolcu Y, Kerezoudis P, Alvi MA, Parney IF, Fogelson JL, Bydon M. Impact of Powdered Vancomycin on Preventing Surgical Site Infections in Neurosurgery: A Systematic Review and Meta-analysis. Neurosurgery. 2019;84:569-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhan R, Zhu Y, Shen Y, Shen J, Tong Y, Yu H, Wen L. Post-operative central nervous system infections after cranial surgery in China: incidence, causative agents, and risk factors in 1,470 patients. Eur J Clin Microbiol Infect Dis. 2014;33:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Saeedinia S, Nouri M, Azarhomayoun A, Hanif H, Mortazavi A, Bahramian P, Yarandi KK, Amirjamshidi A. The incidence and risk factors for surgical site infection after clean spinal operations: A prospective cohort study and review of the literature. Surg Neurol Int. 2015;6:154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Yao R, Zhou H, Choma TJ, Kwon BK, Street J. Surgical Site Infection in Spine Surgery: Who Is at Risk? Global Spine J. 2018;8:5S-30S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Neurosciences
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dioguardi M, Italy; Ewers A, Austria S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH