Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5804
Peer-review started: June 1, 2023
First decision: July 19, 2023
Revised: July 29, 2023
Accepted: August 3, 2023
Article in press: August 3, 2023
Published online: August 26, 2023
Processing time: 84 Days and 19.5 Hours
This paper presents a case of malignant hidroacanthoma simplex (HAS) and review the literature of previous cases to summarize the histopathological and immunohistochemical features and display the dermoscopic features of malignant HAS.
We present an 88-year-old Asian female with malignant HAS. The diagnosis was made according to the histopathological and immunohistochemical results after biopsy. Previous case reports of malignant HAS were retrieved from PubMed to characterize the histopathological and immunohistochemical features. We also display the dermoscopic features of malignant HAS that have not been reported.
Our findings demonstrate that prompt surgical treatment is an effective strategy for malignant HAS. Histopathology and immunohistochemistry are valuable diagnostic tools. This is the first case report to display the dermoscopic features of malignant HAS, and we speculate that dermoscopy may contribute to the diagnosis of malignant HAS.
Core Tip: Malignant hidroacanthoma simplex (HAS) is a clinically uncommon malignant cutaneous tumour with only a few case reports. Herein, we present an additional case of malignant HAS. By combining this case with a literature review of previous cases retrieved in PubMed, we summarize the histopathological and immunohistochemical features of malignant HAS and found that timely surgical operation is an effective treatment. Furthermore, we first display dermoscopic features of malignant HAS and speculate that dermoscopy may be a valuable tool for the early diagnosis of malignant HAS.
- Citation: Yang YF, Wang R, Xu H, Long WG, Zhao XH, Li YM. Malignant form of hidroacanthoma simplex: A case report. World J Clin Cases 2023; 11(24): 5804-5810
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5804.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5804
Hidroacanthoma simplex (HAS), a rare tumour arising from terminal sweat ducts, was initially characterized in 1956[1]. As an intraepidermal variant of eccrine poroma, the vast majority of HAS cases are benign[2,3]. Malignant transformation is an infrequent occurrence and no dermoscopic features of malignant HAS have yet been reported. Here, we present a case of malignant HAS and review the literature of previous cases to summarize the histopathological and immunohistochemical features and display the dermoscopic features of malignant HAS.
An 88-year-old Asian female presented to the Department of Dermatology with a complaint of a cutaneous mass on the right thigh for 30 mo.
The lesion first appeared as a slightly elevated papule 30 mo prior and then gradually enlarged to become a brown-coloured verrucous lump.
The patient had a history of hypertension for more than 10 years and the patient had undergone surgery for squamous cell carcinoma of the tongue 21 years prior.
The patient denied any family history of autoimmune disease, malignant tumours or other genetic conditions.
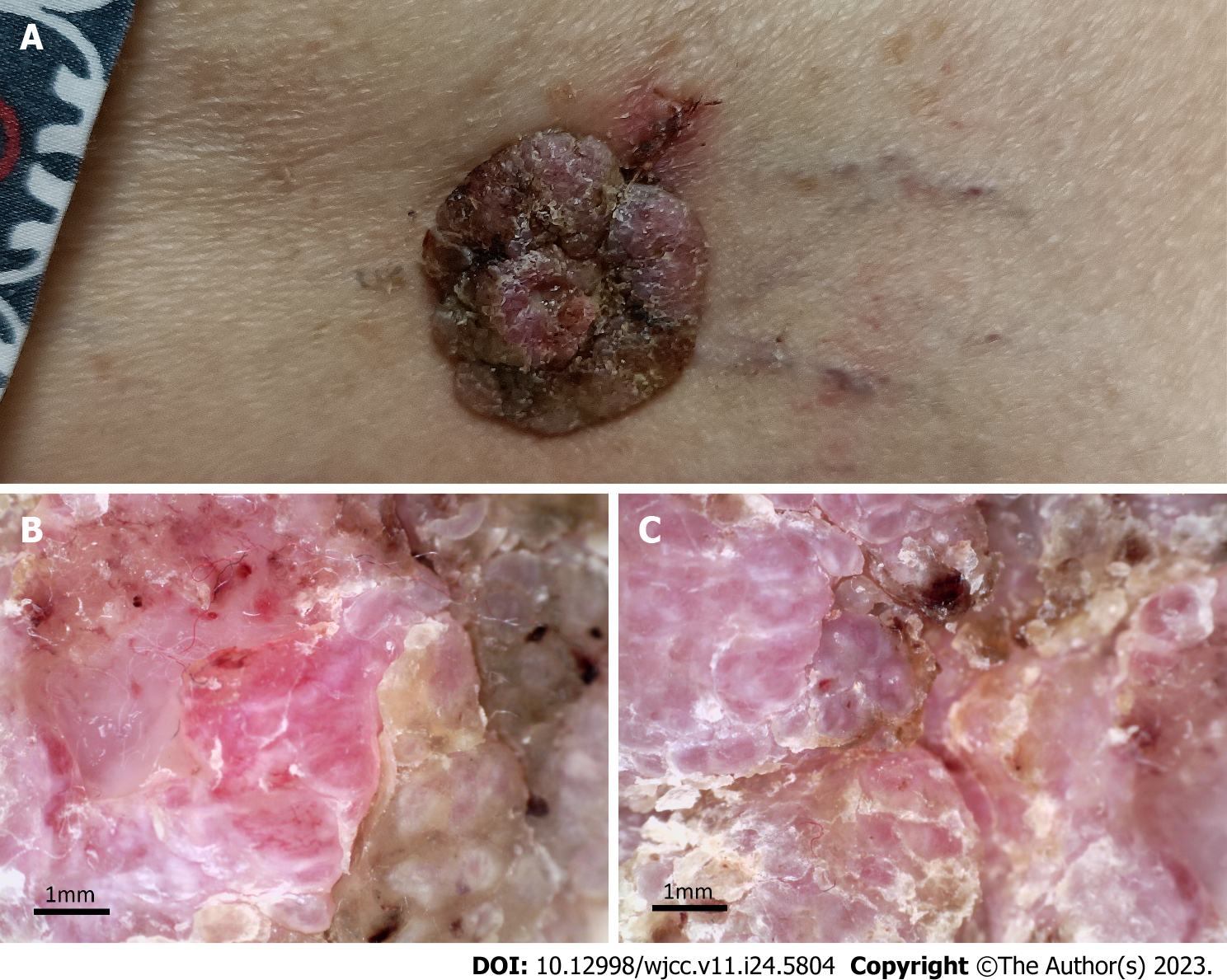
The vital signs of the patient were as follows: body temperature, 36.8℃; heart rate, 82 beats per min; blood pressure, 130/80 mmHg; and respiratory rate, 19 breaths per min. Furthermore, a well-demarcated tumour (3.0 cm × 2.8 cm) associated with exulceration was notted on the right thigh (Figure 1A), and no palpable lymph nodes were detected.
The glomerular filtration rate was 59 mL/min; uric acid was 456 µmol/L; triglyceride was 2.74 mmol/L; and total cholesterol was 6.30 mmol/L. Routine examination of the patient’s stools, blood and urine did not indicate any abnormalities.
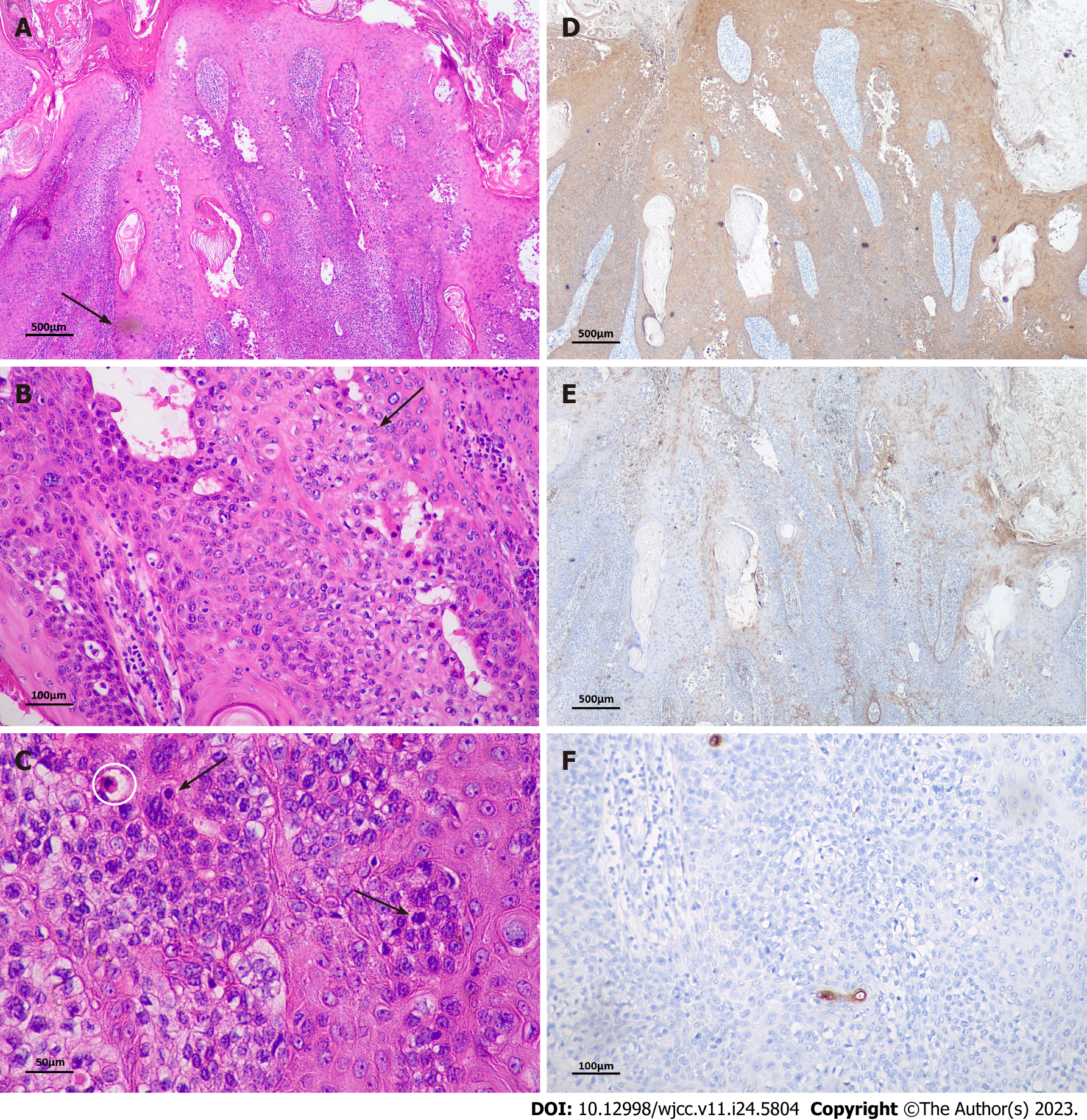
Haematoxylin-eosin staining was used for histopathological examination, and immunohistochemistry was performed according to the instructions of the Maxvision 2 HRP-Polymer anti-Mouse/Rabbit Immunohistochemistry (IHC) Kit. Histopathological examination revealed irregularly thickened epidermis with hyperkeratosis and parakeratosis, papillary formation in local areas, a multinodular pattern of tumour nests, and widened, blunted epithelial feet within the epidermis (Figure 2A). There was moderate or abundant cytoplasm, which was slightly less stained than the surrounding residual squamous epithelium (Figure 2B). The neoplastic cells exhibited pleomorphism with nuclear atypia and mitotic figures, and scattered dyskeratotic cells were observed within the epidermis (Figure 2C). No invasive growth was observed. Immunohistochemical staining was positive for cytokeratin 5/6 and epithelial membrane antigen (Figure 2D and E). Carcinoembryonic antigen expression was absent in neoplastic cells, but it highlighted the presence of ductal structures (Figure 2F).
The final diagnosis was established as malignant hidroacanthoma simplex.
Radical resection and flap transplantation were performed under general anaesthesia. Vacuum sealing drainage was used to promote wound recovery after surgery.
There was no recurrence in the six-month postoperative follow-up.
HAS is a rare form of the four subtypes of eccrine poroma (EP) and seldom undergoes malignant transformation. We searched PubMed using the keyword ‘malignant hidroacanthoma simplex’ and reviewed 10 case reports of malignant HAS (Tables 1 and 2)[2-11]. Malignant HAS primarily affects the extremities, and the majority of patients are over 70 years old. Although malignant HAS has the potential to regionally and distantly metastasize[10], prompt surgery, including moth micrographic surgery[11] has been demonstrated as an effective treatment strategy with no instances of recurrence.
| Case | Ref. | Age | Gender | Duration (yr) | Location | Size (cm) | Color | Ulceration | Clinical feature | Recurrence |
| 1 | Sun Kim et al[2], 2012 | 69 | F | 1 | Suprapubic area | 2 × 2 | Pigmented | (-) | Verrucous nodule | No Recurrence |
| 2 | Lee et al[3], 2006 | 71 | F | NR | Right knee | 1.8 × 2.0 | Pigmented | (-) | Hyperkeratotic tumor | NR |
| 3 | Ishida et al[10], 2009 | 72 | M | 3 | Right thigh | 1.7 × 1.2 | Brown to black | (-) | Flat plaque | Liver and bone metastases before surgery |
| 4 | Bardach et al[4], 1978 | 70 | F | 15-20 | Right leg | Not mentioned | Red | (-) | Irregularly shaped and crusted | NR |
| 5 | Yang et al[11], 2022 | 80 | M | 5 | Left foot | 1.2 × 0.8 | Black | (-) | Elevated nodule | No Recurrence |
| 6 | Kohli et al[5], 2015 | 79 | M | NR | Scalp | 0.6 × 0.5 | Pink | (+) | Papule | No Recurrence |
| 7 | Piqué et al[6], 1995 | 73 | F | 15 | Right leg | 3 × 3 | Pigmented | (-) | Verrucous lesion | NR |
| 8 | Ansai et al[7], 1994 | 75 | M | 2 | Right ankle | 2.5 × 3.3 | Pigmented | (-) | Verrucous plaque | No Recurrence |
| 9 | Lee et al[8], 2000 | 67 | F | 16 | Right thigh | 5 × 7 | Pigmented | (-) | Verrucous lesion | NR |
| 10 | Takano et al[9], 1989 | 74 | F | NR | Left thigh | 1.5 × 1.5 | Light brown | (+) | Elevated nodule | Died of cardiac and respiratory failure |
| Case | Intraepidermal nests | Sharp delineation | Hyperkeratosis or acanthotic epidermis | Invasive growth | Nuclear and cytoplasmic pleomorphisms | Mitotic figure | Ductal structure | Immunohistochemistry |
| 1 | (+) | (-) | (+) | (+) | (-) | (+) | (+) | EMA (+), CK10 (+), CK14(+) |
| 2 | (+) | (+) | (-) | (-) | (-) | (+) | (-) | EMA (+), CEA (+) |
| 3 | (+) | (+) | (+) | (+) | (+) | (+) | (+) | NR |
| 4 | (+) | (+) | (+) | (-) | (-) | (-) | (+) | NR |
| 5 | (+) | (+) | (+) | (-) | (+) | (+) | (+) | CK5/6 (+), P63 (+), CEA (-) CK7 (-) |
| 6 | (+) | (+) | (+) | (-) | (+) | (+) | (+) | CytokeratinAE1/3 (+), EMA (+), CEA (+), CK7 (+) |
| 7 | (+) | (+) | (+) | (-) | (+) | (+) | (+) | CAM5.2 (+), CEA (+), S100 (+), EMA (+) |
| 8 | (+) | (+) | (+) | (+) | (+) | (+) | (-) | NR |
| 9 | (+) | (+) | (+) | (+) | (+) | (-) | (-) | EMA (+), CEA (+), S100 (+) |
| 10 | (+) | (+) | (-) | (-) | (+) | (-) | (-) | NR |
Malignant HAS lacks specific clinical manifestations and usually presents as pigmented wart-like lumps. Malignant HAS is often mistaken for other cutaneous neoplasms such as Bowen’s disease (BD) or seborrheic keratosis (SK). Histopathology is an indispensable tool for diagnosing malignant HAS. Through the analysis of 10 cases, we aimed to identify the pathological features of malignant hidroacanthoma simplex: (1) Tumour nests are well-demarcated, and the epidermis often exhibits irregular acanthosis; (2) Most tumour cells are characterized by vacuolated nuclei and small nucleoli; (3) Some tumour nests show invasive growth, whereas neoplastic cells exhibit nuclear and cytoplasmic pleomorphisms and mitotic figures; and (4) Ductal differentiation can be observed. IHC has emerged as a powerful diagnostic examination. As a subtype of EP, HAS can arise from the ductal part of either the large or small sweat glands. Previous research has revealed that the majority of EP tumour cells express cytokeratin 5 (CK5) and cytokeratin 14, and squamous epithelial-like sections express cytokeratin 1 and cytokeratin 10, whereas ductal areas express cytokeratin 77 and cytokeratin 6[12]. Epithelial membrane antigen (EMA) has been reported to be positive in the cytoplasm of neoplastic cells of HAS and negative in SK and BD[13]. CEA was found to highlight the ductal structures and intracytoplasmic lumina[3]. In this case, IHC staining revealed positivity for CK5/6 and EMA, and CEA was only expressed in ductal structures.
Furthermore, dermoscopy, an emerging dermatological examination tool, may also be helpful for differential diagnosis. Glomerular vessels and surface scales exhibit high sensitivity and specificity in BD[14,15]. Milia-like cysts and cerebriform appearance are considered highly sensitive to SK[16,17]. Shiiya et al[18] proposed that fine scales arranged orbicularly, scattered fine black dots or globules and the absence of glomerular vessels could aid in the precise diagnosis of HAS. However, no dermoscopic features of malignant HAS have yet been documented. In this study, besides fine scales and hyperkeratosis, our dermoscopic images showed that linear telangiectasis were also exhibited in the papilla, which has not been reported before. Therefore, we speculate that the appearance of telangiectasis may contribute to the differential diagnosis of malignant HAS and HAS.
Although malignant HAS is a malignant adnexal adenoma, prompt surgical resection can achieve good therapeutic results. As a clinically uncommon tumour, malignant HAS is often misdiagnosed as BD or SK. Precise diagnosis depends on histopathological examination, and immunohistochemical analysis is also valuable. Furthermore, we are the first to display dermoscopic features of malignant HAS and found linear telangiectasis that had not been reported in studies of HAS. Therefore, we speculate that telangiectasis appearance may contribute to the differential diagnosis of malignant and benign forms of HAS and that dermoscopy may be a valuable tool for the early diagnosis of malignant HAS.
| 1. | Coburn JG, Smith JL. Hidroacanthoma simplex; an assessment of a selected group of intraepidermal basal cell epitheliomata and of their malignant homologues. Br J Dermatol. 1956;68:400-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 79] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Sun Kim M, Rae Lee H, Lee JH, Son SJ, Song KY. Malignant hidroacanthoma simplex: arising in hidroacanthoma simplex mimicking clonal seborrheic keratosis. Int J Dermatol. 2013;52:258-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Lee JY, Lin MH. Pigmented malignant hidroacanthoma simplex mimicking irritated seborrheic keratosis. J Cutan Pathol. 2006;33:705-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Bardach H. Hidroacanthoma simplex with in situ porocarcinoma. A case suggesting malignant transformation. J Cutan Pathol. 1978;5:236-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kohli N, Kim SS, Jiang SI. Malignant hidroacanthoma simplex treated with Mohs surgery. Dermatol Surg. 2015;41:518-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Piqué E, Olivares M, Espinel ML, Fariña M, Martín L, Barat A, Requena L, Castro A. Malignant hidroacanthoma simplex. A case report and literature review. Dermatology. 1995;190:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Ansai S, Koseki S, Hozumi Y, Tsunoda T, Yuda F. Malignant transformation of benign hidroacanthoma simplex. Dermatology. 1994;188:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Lee WJ, Seo YJ, Yoon JS, Suhr KB, Lee JH, Park JK, Suh KS. Malignant hidroacanthoma simplex: a case report. J Dermatol. 2000;27:52-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Takano Y, Nishimura M, Urabe A, Hayashi N, Toshitani S. Malignant hidroacanthoma simplex. J Dermatol. 1989;16:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Ishida M, Hotta M, Kushima R, Okabe H. A case of porocarcinoma arising in pigmented hidroacanthoma simplex with multiple lymph node, liver and bone metastases. J Cutan Pathol. 2011;38:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Yang L, Zhao Y, Zhang W, Lu Q. Malignant hidroacanthoma simplex on the foot. Asian J Surg. 2022;45:1976-1977. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Battistella M, Langbein L, Peltre B, Cribier B. From hidroacanthoma simplex to poroid hidradenoma: clinicopathologic and immunohistochemic study of poroid neoplasms and reappraisal of their histogenesis. Am J Dermatopathol. 2010;32:459-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Takanashi M, Urabe A, Nakayama J, Hori Y. Distribution of epithelial membrane antigen in eccrine poroma. Dermatologica. 1991;183:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Zalaudek I, Kreusch J, Giacomel J, Ferrara G, Catricalà C, Argenziano G. How to diagnose nonpigmented skin tumors: a review of vascular structures seen with dermoscopy: part I. Melanocytic skin tumors. J Am Acad Dermatol. 2010;63:361-74; quiz 375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Zalaudek I, Argenziano G, Leinweber B, Citarella L, Hofmann-Wellenhof R, Malvehy J, Puig S, Pizzichetta MA, Thomas L, Soyer HP, Kerl H. Dermoscopy of Bowen's disease. Br J Dermatol. 2004;150:1112-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Sahin MT, Oztürkcan S, Ermertcan AT, Güneş AT. A comparison of dermoscopic features among lentigo senilis/initial seborrheic keratosis, seborrheic keratosis, lentigo maligna and lentigo maligna melanoma on the face. J Dermatol. 2004;31:884-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Sato Y, Fujimura T, Tamabuchi E, Haga T, Aiba S. Dermoscopy findings of hidroacanthoma simplex. Case Rep Dermatol. 2014;6:154-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Shiiya C, Hata H, Inamura Y, Imafuku K, Kitamura S, Fujita H, Shimizu H. Dermoscopic features of hidroacanthoma simplex: Usefulness in distinguishing it from Bowen's disease and seborrheic keratosis. J Dermatol. 2015;42:1002-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dermatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, France; Lourencao P, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Liu JH