Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5742
Peer-review started: April 16, 2023
First decision: May 19, 2023
Revised: June 25, 2023
Accepted: July 31, 2023
Article in press: July 31, 2023
Published online: August 26, 2023
Processing time: 130 Days and 22.7 Hours
Rationale: No other treatment besides lung transplant is effective for idiopathic pulmonary fibrosis (IPF). Patients with IPF have poor prognosis, which may eventually lead to death. Patient concerns: Two female patients were diagnosed with IPF. In our recent follow-up, both these patients maintained a good quality of life.
Diagnosis: Both patients had dry cough and progressive dyspnea. Interventions: The first patient was treated with prednisone, and the second patient was treated with prednisone and tripterygium glycosides. However, the symptoms did not improve and fibrosis was not controlled. Thus, the Feibi recipe was used. Outcomes: No deterioration was observed after the treatment, and the dry cough and its effect were ameliorated. Furthermore, they are still alive and the quality of their lives has improved.
These two cases suggest that the Feibi recipe and other traditional Chinese medicine therapies could be beneficial for IPF treatment.
Core Tip: In this report, we present the cases of two patients diagnosed with idiopathic pulmonary fibrosis with progressive fibrosing who received traditional Chinese medicine (TCM). The disease progression slowed down, and the symptoms were relieved. After several years of follow-up, we collected their medical history, computed tomography scans, and found that the patients had a moderate quality of life after the TCM treatment.
- Citation: Liu ZH, Li GD, Hao QX, Cao F, Cheng Y, Kou MJ, Jiao Y. Acute exacerbation of idiopathic pulmonary fibrosis treated using the Feibi recipe: Two case reports. World J Clin Cases 2023; 11(24): 5742-5748
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5742.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5742
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrosing interstitial pneumonia of unknown cause and a fatal lung disease[1]. The incidence of IPF has been increasing annually, and the median survival of patients diagnosed with IPF is only 2-3 years, with the 5-year survival rate being < 40%[2]. The World Health Organization has listed IPF as one of the refractory diseases and a danger to human health in the 21st century.
The hypothesis proposed was that the pathogenesis of IPF is closely associated with the role of alveolar-capillary barrier basement membrane in retaining the architecture of the injured lung and the contribution of transforming growth factor-β-persistent antigens, bone marrow-derived progenitor cells, and other factors[3].
Except for lung transplantation, no other treatments are effective for IPF[1]. Moreover, lung transplantation has certain limitations, such as high cost and lack of lung sources.
The Feibi recipe, a formula developed by Professor Ping’an Zhou with more than 50 years of clinical experience, was used to treat pulmonary fibrosis and evaluate the symptoms of the patients in this study.
Case 1: A 78-year-old woman was admitted to our hospital in November 2012.
Case 2: A 64-year-old woman with an 18-month history of persistent dry cough and progressive dyspnea on exertion was referred to our hospital in October 2002.
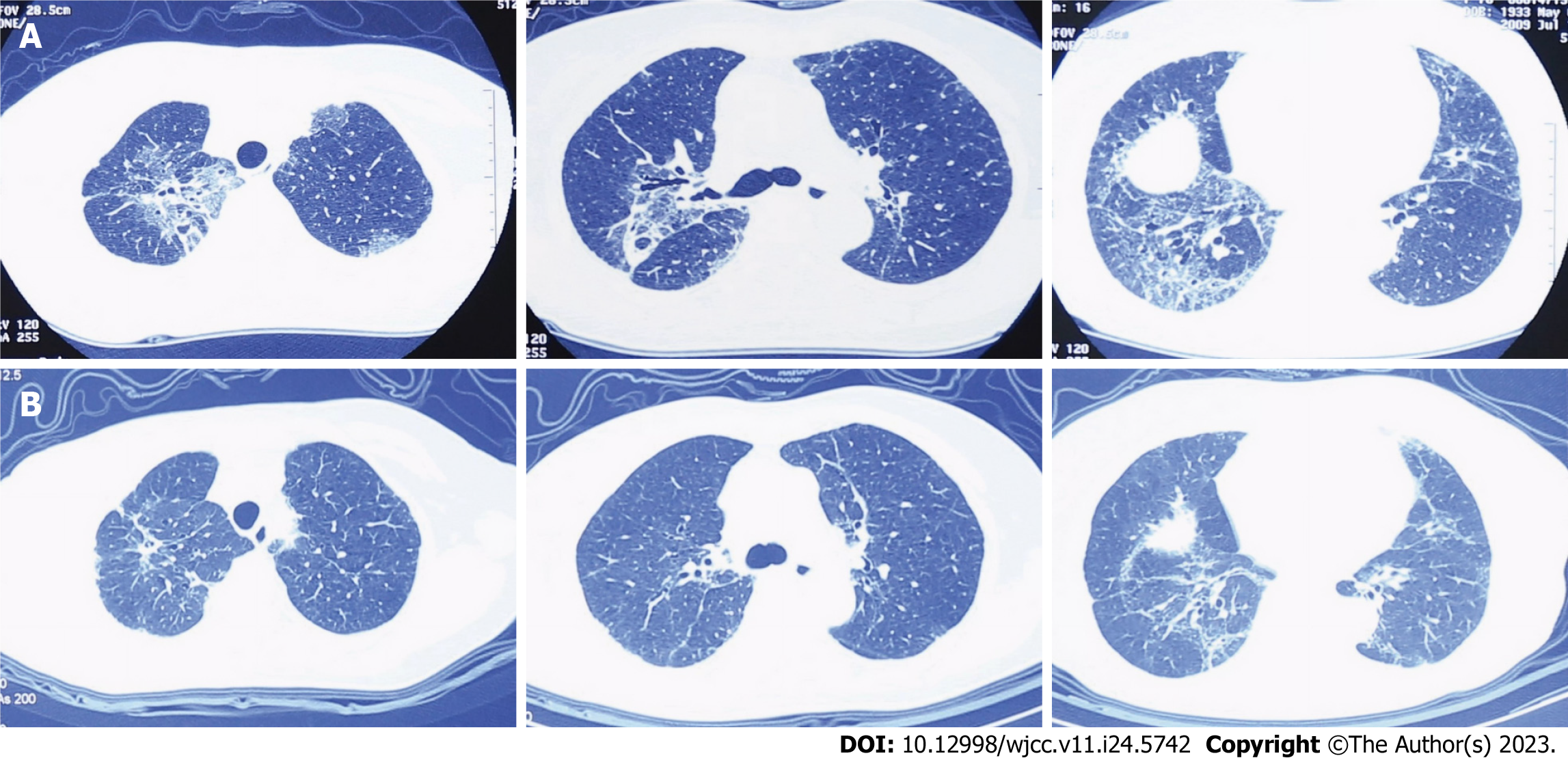
Case 1: The reason of admission was dry cough and worsening dyspnea within the last 3 years. Three years ago, she was diagnosed with IPF in Beijing Chaoyang Hospital, and the diagnosis was based on the clinical symptoms and medical examination findings. High-resolution computed tomography (HRCT) revealed subpleural reticular opacities predominantly in the bilateral lobes (Figure 1A). The bronchoalveolar lavage fluid contained 72.5% neutrophils, which was apparently higher than the normal range (< 3%). The patient was treated with two antibiotics because of lung inflammation and an increase in the leukocyte count (8.27 × 109/L). Prednisone was used to treat IPF. Although the infection was contained, the dry cough became more frequent and the dyspnea worsened.
Case 2: The patient was referred to our hospital in October 2002 for an 18-month history of persistent dry cough and progressive dyspnea on exertion. She was diagnosed with IPF at Peking Union Medical College Hospital based on her symptoms and physical and chemical examination findings. HRCT of the chest revealed basal and peripheral predominant reticular abnormality with honeycombing (Figure 2A). Velcro rales could be heard over both lung fields. The pulmonary function test revealed a total lung capacity of 1.41 L (64.3% of the predicted) and a forced vital capacity of 2.10 L (90.4% of the predicted). Meanwhile, the diffusion for carbon monoxide was 55.2% of the predicted. The results of fiberoptic bronchoscopy were normal. Meanwhile, the rheumatoid factors and other tests excluded secondary pulmonary fibrosis. She was receiving prednisone and tripterygium glycosides from the hospital for nearly a year and a half, but there was no improvement.
The patient had no chronic disease and no history of surgery.
The patient had no personal and family history.
Case 1: The vital signs were as follows: Temperature: 36.4 °C, blood pressure: 132/77 mmHg, heart rate: 91 beats per minute, and respiratory rate: 23 breaths per minute.
Case 2: Her vital signs were as follows: Temperature: 36.3 °C, blood pressure: 138/82 mmHg, heart rate: 71 beats per minute, and respiratory rate: 20 breaths per minute.
Case 1: The results of the blood routine test were normal.
Case 2: The results of blood routine tests and rheumatoid factor were normal.
Case 1: HRCT revealed fibrosis in both lungs, with ground-glass density and grid-like changes.
Case 2: HRCT of the chest revealed basal and peripheral predominant reticular abnormality with honeycombing and bronchiectasis.
Case 1: Radix astragali 20 g, Flos lonicerae japonicae 20 g, Fritillaria 10 g, Radix et Rhizoma Glycyrrhizae 10 g, Radix Angelicae sinensis 6 g, Rhizoma dioscoreae nipponicae 15 g, Folium pyrrosiae 15 g, Radix trichosanthis 15 g, Ganoderma 15 g, Radix et rhizoma rhodiolae crenulatae 15 g, Radix adenophorae 15 g, and Bulbus lilii 15 g.
Case 2: Radix astragali 20 g, Flos lonicerae japonicae 20 g, Radix et rhizoma glycyrrhizae 10 g, Radix angelicae sinensis 10 g, Ganoderma 15 g, Radix et rhizoma rhodiolae crenulatae 15 g, Semen armeniacae amarum 9 g, Radix puerariae lobatae 15 g, Radix paeoniae alba 15 g, Folium mori 30 g, and Caulis perillae 10 g.
The final diagnosis was IPF. Both patients had dry cough and progressive dyspnea.
The patients received the Feibi recipe continuously. In the first patient, the symptoms were controlled. On HRCT, there was no superimposition on the background of lung fibrosis, and improvements were observed after the treatment (Figure 1B). In the second patient, most of the symptoms had improved, and there was no new progression based on HRCT (Figure 2B).
Visual analog scale, Leicester cough questionnaire, and chronic cough impact questionnaire were used to evaluate the effect of the disease before and after the treatment, such as on physiological and social function, mental health, and vitality. To an extent, that could reflect the efficacy of the Feibi recipe. The results revealed that the treatment relieved the cough and its effects as well as the quality of life of the patients (Tables 1-4).
| Score | |||
| VAS (day/night) | LCQ | CCIQ | |
| Before the treatment | 7.8/4.8 | 7.36 | 86 |
| After the treatment | 2.4/1.5 | 16.51 | 41 |
| Score | ||
| Before the treatment | After the treatment | |
| Physical function | 2 | 4.38 |
| Psychological function | 2.86 | 6.14 |
| Social function | 2.5 | 6 |
| Total | 7.36 | 16.51 |
| Score | |||
| VAS (day/night) | LCQ | CCIQ | |
| Before the treatment | 8.3/5.1 | 9.54 | 77 |
| After the treatment | 2.0/2.0 | 17.92 | 37 |
| Score | ||
| Before the treatment | After the treatment | |
| Physical function | 3 | 5.5 |
| Psychological function | 3.29 | 5.42 |
| Social function | 3.25 | 7 |
| Total | 9.54 | 17.92 |
Herein, we report the cases of two patients who were diagnosed with IPF based on HRCT and fiberoptic bronchoscopy. Despite treatment with prednisone, the course of pulmonary fibrosis was not controlled. Therefore, traditional Chinese medicine (TCM) was used as an alternative for treating IPF. After treatment with the Feibi recipe, HRCT revealed no deterioration, and the dry cough and its effects were ameliorated. Furthermore, the patients are still alive, and their quality of their lives has improved.
In TCM thesis, IPF belongs to the category of “lung paralysis” and “lung asthenia.” Its basic pathogenesis is mainly related to lung Qi deficiency and exogenous pathogenic factors that attack and penetrate the cells, causing heat and toxin accumulation.
The Feibi recipe is a TCM formula that is composed of Radix astragali, Flos lonicerae japonicae, and other components. Radix astragali nourishes Qi and resolves the toxins. Flos lonicerae japonicae clears the heat and dissolves the toxins.
Pharmacological studies have demonstrated that Radix astragali contains astragalus saponins and polysaccharides, which play a vital role in immunoregulation[4]. Astragalus polysaccharides have been found to protect rat lung tissue from pulmonary fibrosis, showcasing their protective effects. Additionally, astragaloside, amino acids, and selenium present in Radix astragali exhibit anti-aging properties, counteract free radical damage, prevent lipid peroxidation, and possess anti-inflammatory effects.
Flos lonicerae japonicae, on the other hand, contains flavonoids and chlorogenic acid, which contribute to its broad-spectrum antibacterial and antiviral effects. Furthermore, the components found in Flos lonicerae japonicae exhibit anti-inflammatory, antipyretic, and antiendotoxin properties. These components have the ability to regulate immunity, enhance the phagocytic function of leukocytes, reduce inflammatory cells in lung tissues, and decrease hydroxyproline content, indicating their potential therapeutic benefits[5].
A report has suggested upregulation of transforming growth factor beta (TGF-β) ligands is observed in major pulmonary diseases, including pulmonary fibrosis[6]. The Feibi recipe has been reported to exhibit inhibitory effects on the phosphorylation of P38 mitogen-activated protein kinase, resulting in a decrease in the expression of TGF-β1. Moreover, it has been found to downregulate the expression of interleukin-6 (IL-6) in lung tissue and reduce the content of type III collagen and hyaluronic acid in the serum. These findings suggest that the Feibi recipe possesses the potential to inhibit fibrosis and attenuate immunoinflammatory injury[7]. Moreover, it can reduce pathological response and inflammatory mediators, including IL-6, IL-13, IL-17, monocyte chemoattractant protein-1, tumor necrosis factor-α, and plasma glutathione peroxidase, in rats with PM2.5-induced lung injury. No. 2 Feibi recipe appears to attenuate lung injury in rats induced by PM2.5[8]. Moreover, clinical studies have shown that the Feibi recipe can improve the quality of life of patients with IPF[9,10]. Other molecules such as obeticholic acid and components from plants like Curdione and Paeoniflorin also have the potential to treat pulmonary fibrosis[11-13].
The two cases presented here suggest that the Feibi recipe is effective and beneficial for the treatment of IPF. However, further clinical experience and studies are needed to verify this finding.
| 1. | Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5557] [Cited by in RCA: 5442] [Article Influence: 362.8] [Reference Citation Analysis (0)] |
| 2. | Fernández Pérez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, Yi ES, Ryu JH. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Zhang Y, Li JT, Liu YQ, Li J, SU Y, Yan CL, Nie L. [Effect of Astragalus polysaccharides on Th1 / Th2 cytokine balance and NO level in serum of pulmonary fibrosis rats]. Zhongguo Laonianxue Zazhi. 2009;29:35. [DOI] [Full Text] |
| 5. | Zhang QX. [Research progress on pharmacological function and application of honeysuckle]. Shandong Chemical Industry. 2023;52:121-122+126. [DOI] [Full Text] |
| 6. | Saito A, Horie M, Nagase T. TGF-β Signaling in Lung Health and Disease. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 389] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 7. | Jiao Y, Guan TY, Zhou PA. [Effect of FBR on p38MAPK and TGF - ß1 in lung tissue of pulmonary fibrosis rats]. JTCM. 2007;48:259-261. [DOI] [Full Text] |
| 8. | Liu Z, Wang W, Cao F, Liu S, Zou X, Li G, Yang H, Jiao Y. Number 2 Feibi Recipe Reduces PM2.5-Induced Lung Injury in Rats. Evid Based Complement Alternat Med. 2018;2018:3674145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Fu XF, Wu ZS, Cao F. [Clinical observation on 30 cases of IPF treated by FBR]. Beijing Zhongyiyao Daxue Xuebao. 2015;22:26-28. [DOI] [Full Text] |
| 10. | Cao F, Wu ZS, Fu XF, Li H, Jiao Y. [Treatment of 22 cases of idiopathic pulmonary interstitial fibrosis with FBR]. Huanqiu Zhongyiyao. 2015;8:87-89. [DOI] [Full Text] |
| 11. | Comeglio P, Filippi S, Sarchielli E, Morelli A, Cellai I, Corno C, Pini A, Adorini L, Vannelli GB, Maggi M, Vignozzi L. Therapeutic effects of obeticholic acid (OCA) treatment in a bleomycin-induced pulmonary fibrosis rat model. J Endocrinol Invest. 2019;42:283-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Liu P, Miao K, Zhang L, Mou Y, Xu Y, Xiong W, Yu J, Wang Y. Curdione ameliorates bleomycin-induced pulmonary fibrosis by repressing TGF-β-induced fibroblast to myofibroblast differentiation. Respir Res. 2020;21:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Ji Y, Dou YN, Zhao QW, Zhang JZ, Yang Y, Wang T, Xia YF, Dai Y, Wei ZF. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol Sin. 2016;37:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cheng TH, Taiwan; Vignozzi L, Italy S-Editor: Fan JR L-Editor: A P-Editor: Zhao S