Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5721
Peer-review started: April 5, 2023
First decision: May 15, 2023
Revised: May 24, 2023
Accepted: August 3, 2023
Article in press: August 3, 2023
Published online: August 26, 2023
Processing time: 141 Days and 19.4 Hours
Malignant melanoma of the prostate is rare. Twenty-five studies describing 45 cases have been reported. Prostate melanoma is characterized by an insidious onset and poor prognosis. The prognosis and treatment vary according to primary or secondary melanoma.
A 75-year-old man attended the hospital due to low back pain of 2 mo duration. He denied a history of trauma or abnormal urinary symptoms. Digital rectal examination showed indentation in the left lobe of the prostate, 1 cm in diameter. His prostate-specific antigen was 5.6 ng/mL and 18F-fluorodeoxyglucose positron emission tomography computed tomography (18F-FDG-PET/CT) showed focal glucose metabolism in the left lobe. Imaging showed bone metastases to T12 and bilateral ribs. Transperineal prostate biopsy was done and three tissue specimens on the left side showed prostate adenocarcinoma (Gleason score 3 + 3 = 6), but the specimen on the right side showed malignant melanoma. The patient underwent T12 tumor resection and pathology findings indicated metastatic malignant melanoma. The patient underwent gastroscopy and colonoscopy, and gastroscopy revealed multiple mucosal black spots in the gastric body and fundus. The patient was diagnosed with secondary malignant prostate melanoma and primary gastric disease.
Diagnosis of primary prostate melanoma requires caution and 18F-FDG-PET/CT may result in false-negative detection of melanoma.
Core Tip: Malignant melanoma of the prostate is rare. To date, 25 studies describing 45 cases have been reported. There is a significant difference in prognosis between primary and secondary cases. We report a case of secondary malignant prostate melanoma with primary gastric disease. We also review the literature on 10 cases of primary prostate melanoma, and found that most cases did not receive sufficient tests and 18F-fluorodeoxyglucose positron emission tomography computed tomography (18F-FDG-PET/CT) was false-negative. We conclude that caution should be used in the diagnosis of primary prostate melanoma, and 18F-FDG-PET/CT may result in false-negative detection of melanoma.
- Citation: Zhao H, Liu C, Li B, Guo JM. Malignant melanoma of the prostate: Primary or metastasis? A case report. World J Clin Cases 2023; 11(24): 5721-5728
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5721.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5721
Malignant melanoma, derived from melanocytes, mainly occurs in the skin, but can also be seen in the mucosa and internal organs, accounting for approximately 3% of all tumors[1]. Melanoma of the genitourinary tract is a rare disease, representing < 1% of all melanomas in men[2]. The majority develop from the penis and distal urethra, and melanoma of the prostate is even rarer; most are of prostatic urothelial origin or secondary to metastatic disease[3], and only 10 cases of primary melanoma of the prostate[4-13] and several cases of metastasis[14-17] have been reported in the English-language literature.
A 75-year-old man attended the hospital due to low back pain of 2 mo duration.
The patient had no history of trauma, tumor, and skin melanoma, and no recent weight loss. He also had no urinary symptoms.
The patient had a free previous medical history.
The patient denied family history of melanoma.
Digital rectal examination showed an indentation in the left lobe of the prostate, which was 1 cm in diameter.
Laboratory tests showed an abnormal level of prostate-specific antigen (PSA), which was 5.6 ng/mL, and free PSA/total PSA was 13.19%. His hemoglobin was 114 g/L and lactate dehydrogenase was 376 U/L. Other biochemical tests were within the reference range.
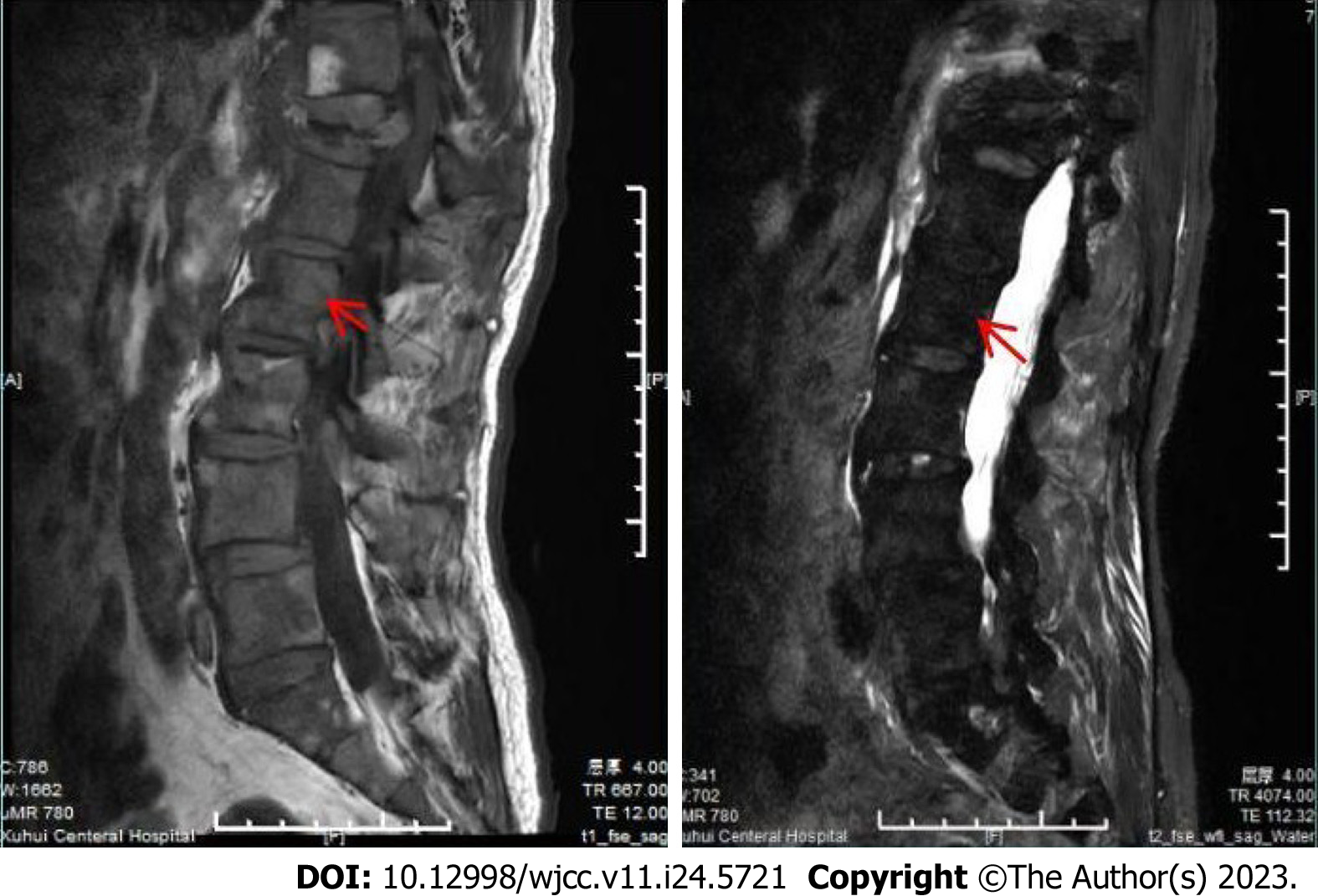
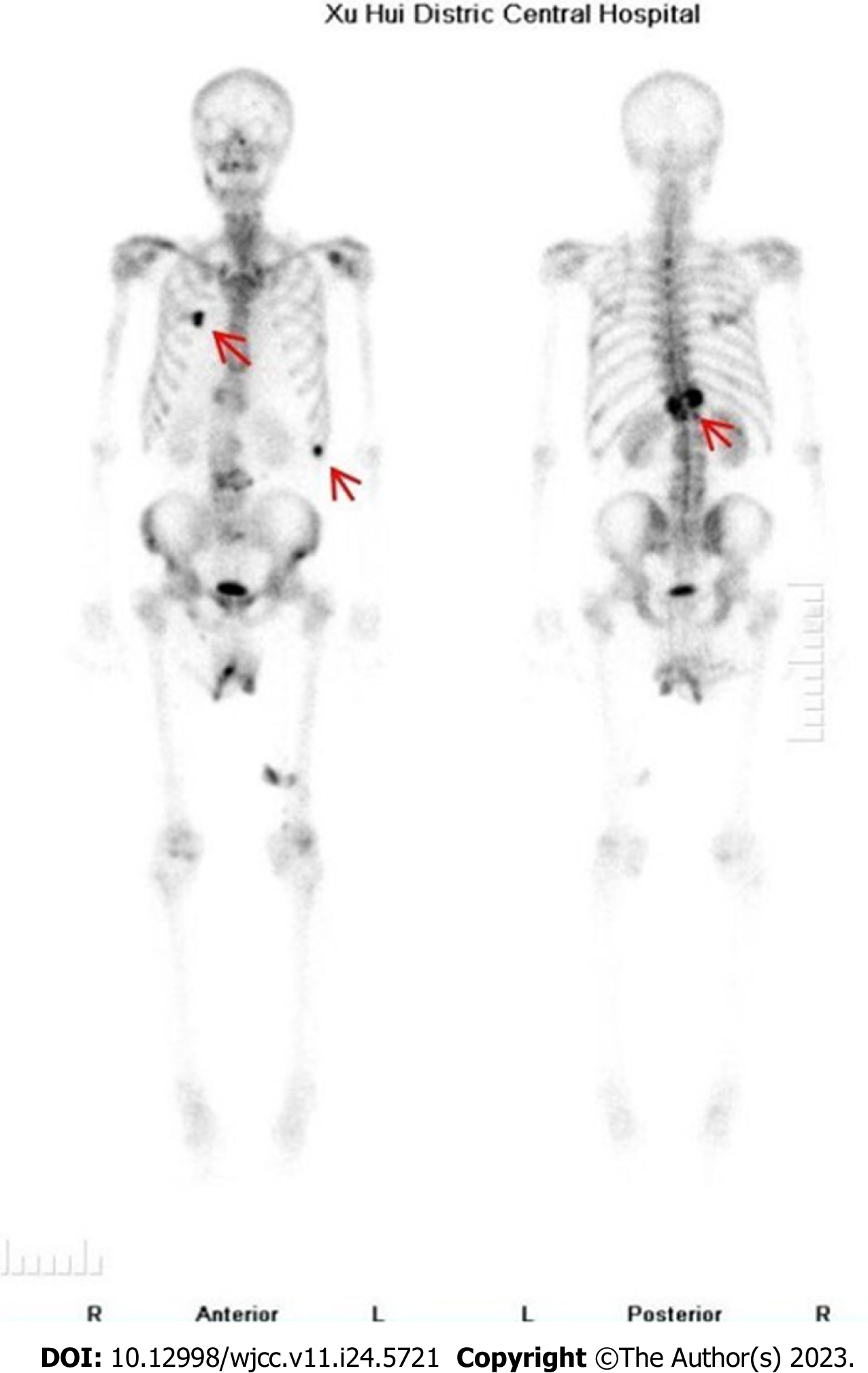
Lumbar magnetic resonance imaging (MRI) showed multiple abnormal signals in the bone, indicating possible metastatic tumors, and compressed bone in thoracic (T12) vertebrae (Figure 1), with a high T1 signal and a low T2 signal. Bone single photon emission computed tomography showed T12 compression of whole body bone, with a concentration of radioactivity in bilateral ribs, and bone metastases were considered (Figure 2).
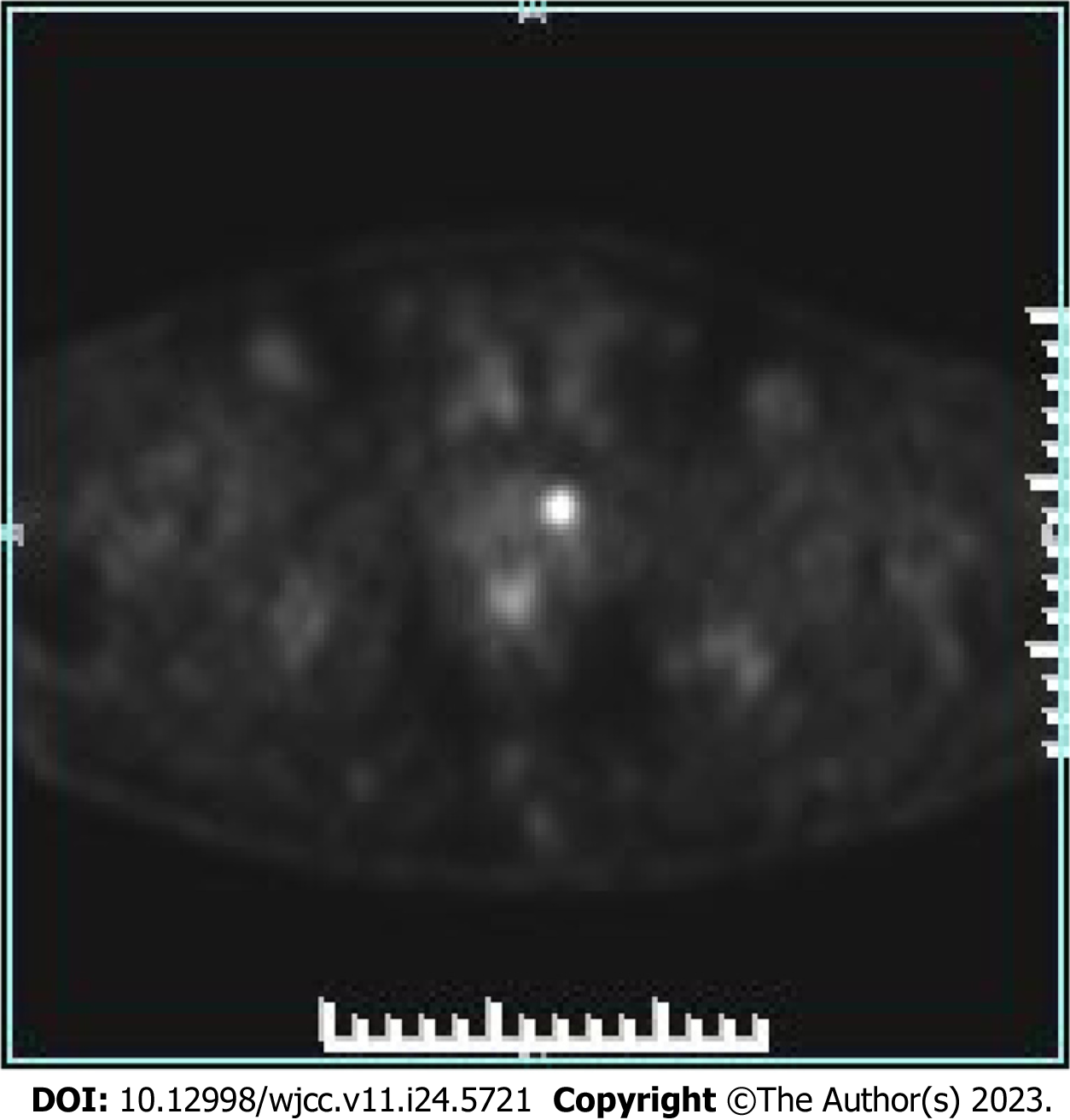
Positron emission tomography (PET)/computed tomography (CT) showed benign prostate hyperplasia, focal glucose metabolism in the left lobe, a malignant tumor waiting to be excreted, and puncture biopsy was recommended. Extensive bone lesions in the whole body were considered large metastases, and multiple small lymph nodes in the retroperitoneal and left pelvic wall required close follow-up (Figure 3).
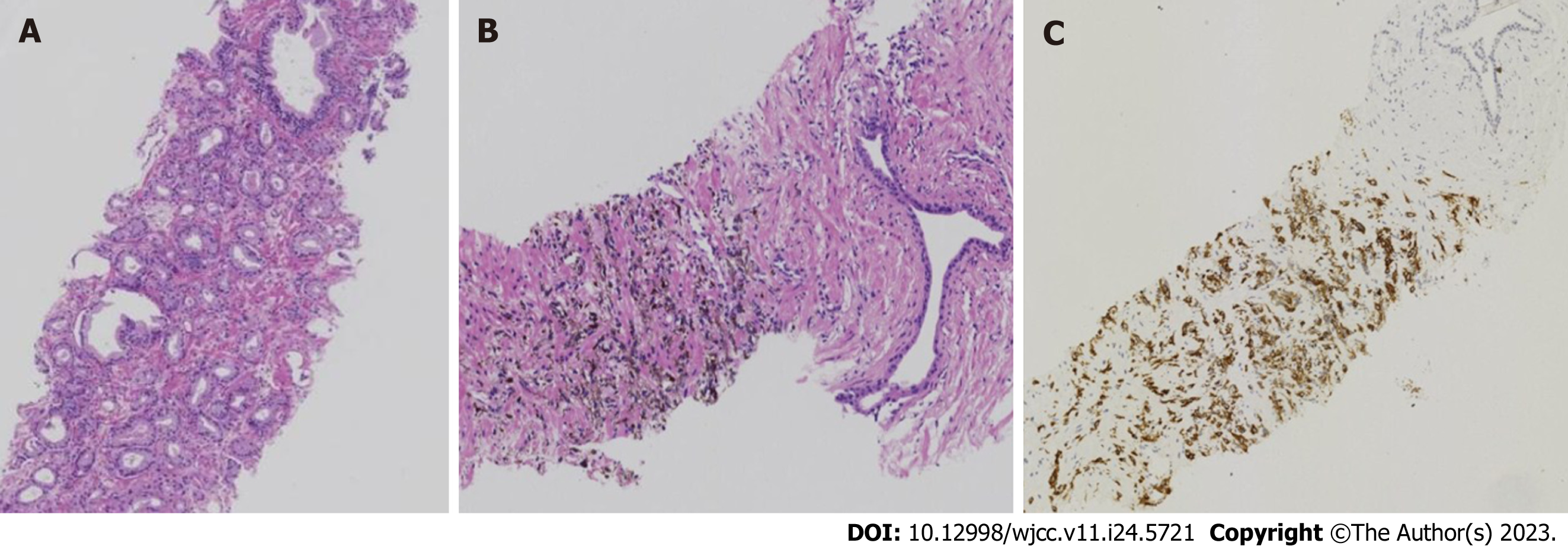
The patient underwent a transperineal prostate biopsy. Six tissue specimens were obtained. Three on the left side showed prostate adenocarcinoma (Gleason score 3+3=6), grade 1 (Figure 4A), and one on the right side showed small foci of melanocytes in the proliferative prostate tissue (Figure 4B). Immunohistochemical results were as follows: S100+, Ki-67+ 10%, CD68, SOX10+, Melan A+, P40, and HMB45+ (Figure 4C).
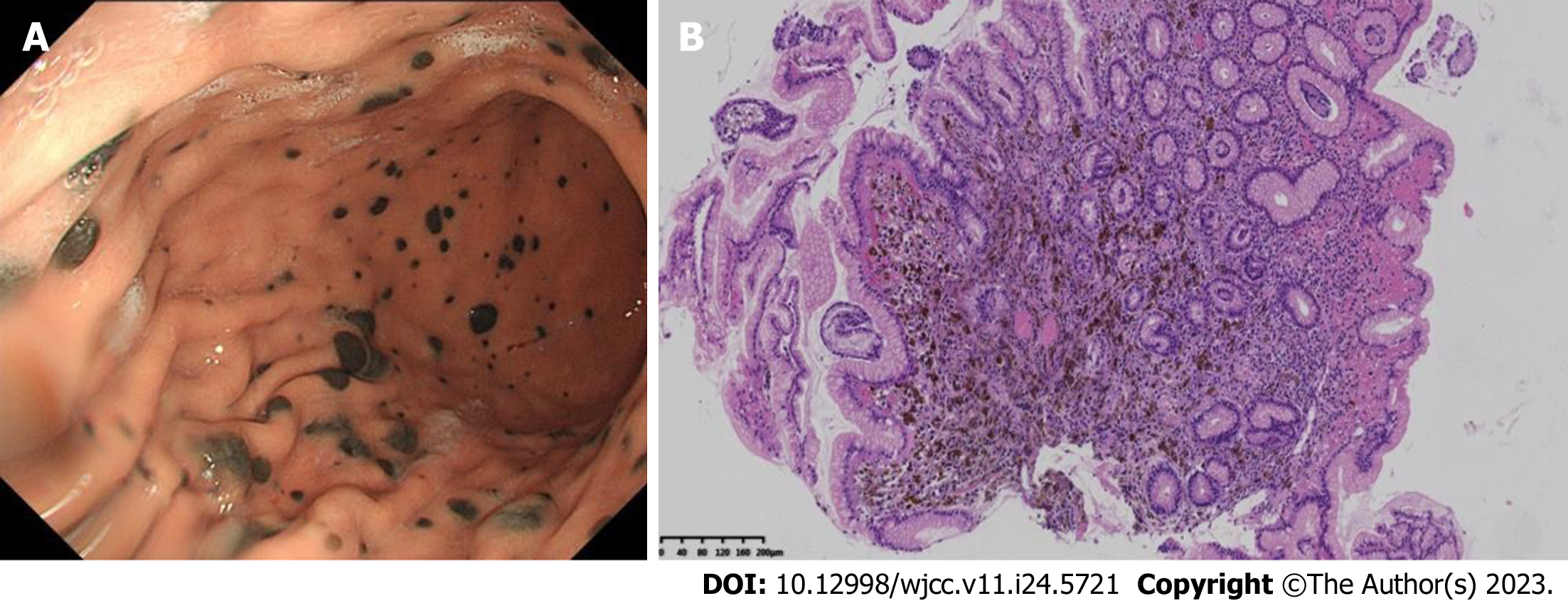
The patient underwent gastroscopy and colonoscopy, and gastroscopy revealed multiple mucosal black spots in the body and fundus of the stomach (Figure 5A). A mucosal biopsy showed acute chronic nonatrophic gastritis in the antrum. Alcian Blue-Periodic Acid-Schiff in the gastric body suggested malignant melanoma. Immunohistochemical results were as follows: tumor cells S100+, HMB45+, AE1/AE3, p53+, Ki-67+ 3%, CD68, SOX10+, and Melan A+ (Figure 5B).
The patient underwent T12 tumor resection, spinal canal decompression and vertebroplasty. Black lesions were found on the thoracic vertebrae and lumbar appendages. Pathology findings indicated metastatic malignant melanocytoma.
This patient was diagnosed with primary prostate adenocarcinoma, gastric melanoma with bone metastases, and prostate metastases.
Following bone surgery (T12 tumor resection, spinal canal decompression and vertebroplasty), the patient received dacarbazine + cisplatin chemotherapy and (Rh-endostatin) targeted therapy for four courses.
The patient died within 11 mo.
Melanoma is a malignant tumor arising from pigment-containing cells, known as melanocytes, which are mainly located in cutaneous tissue. Prostate malignant melanoma may be primary or secondary[2], and a total of 46 cases have been reported to date including our patient. The median age of the patients was 61 years ranging from 29 to 84 years[1].
The most common presentation is obstructive lower urinary tract symptoms. Diagnosis needs histological analysis during transurethral resection of the prostate or core-biopsy material. When a diagnosis of prostate melanoma is made, it is important to distinguish between primary and secondary lesions, so it is important to look for melanoma lesions in other sites.
Our patient had primary gastric malignant melanoma with multiple metastases to the prostate and bone. In this case,
MRI manifestations of bone metastases from prostate carcinoma are generally T1 signal hypointensity and T2 hyperintensity. MR images in our patient were characterized by T1 enhancement and T2 attenuation, which can be used to differentiate between melanoma and prostate carcinoma of bone metastasis.
Following MRI, PET/CT and prostate biopsy, our patient underwent gastroenteroscopy, which revealed the primary lesion in the stomach. In the 10 cases of primary prostate melanoma reviewed in the present study (Table 1), it was found that there was a lack of thorough examination in most cases. Only three cases underwent endoscopy of the gastrointestinal tract and two received 18F-FDG-PET/CT. The prognosis in these patients also varied, ranging from 1 to 84 mo[1-14]. We suggest that some patients diagnosed with primary prostate melanoma may have metastatic lesions at the time of diagnosis.
| Ref. | Age | History disease | Symptoms | Other test | Treatment | Stage | Time to recurrence | Metastases | Secondly treatment | OS |
| Berry and Reese[4], 1953 | 38 | No | Luts | n/m | Cystoprostatectomy | n/m | 7 mo | n/m | n/m | 36 mo |
| Hübler et al[5], 1980 | n/f | n/f | n/f | n/f | n/f | n/f | n/f | n/f | n/f | n/f |
| Wang[6], 2001 | 61 | n/m | BPH | (1) Physical examination of the whole body skin surface, oral cavity, and mucosa and optic fundus; (2) CT and MRI of the brain, abdo_x005f men and pelvic cavity; and (3) endoscopy of gastrointestinal tract | TURP | T1N0Mo | n/m | n/m | No | 84 mo |
| Wong and Bell[8], 2006 | 71 | n/f | Urinary retention | n/f | TURP | n/f | n/f | n/f | n/f | 5 mo |
| Wong et al[7], 2008 | n/f | n/f | n/f | n/f | n/f | n/f | n/f | n/f | n/f | n/f |
| Doublali et al[9], 2010 | 75 | n/m | Urinary tract obstruction. Urethroscopy had revealed black discoloration of the prostate | (1) Physical examination of body skin surface, mucosa; (2) CT of brain, abdomen and pelvis; and (3) colonoscopy and gastroscopy | TURP | n/m | n/m | n/m | No | 1 mo |
| Ma et al[10], 2010 | 29 | n/m | Disuria Digital rectal examination and Transrectal Ultrasound of a mass | Pelvic CT scan with contrast | Radical prostatectomy | T2N0M0 | n/m | n/m | n/m | 3 mo |
| Tosev et al[11], 2015 | 37 | Hodgkin’s disease | Hematuria and urinary retention. In-house cystoscopy showed an asymmetric prostate enlargement with purple discoloration Prostate biopsy | (1) Skin of the body; (2) colonoscopy and gastroscopy; (3) CT of chest and abdomen; and (4) pelvic MRI | Open retropubic radical prostatectomy with extended lymph-node dissection | n/m | 4 mo | Lung | Dacarbazine, ipilimumab, nivolumab | 16 mo |
| Li et al[12], 2019 | 42 | No | Hematuria Accepy PVP. Intraoperatively, a dark lesion was noted in the patient’s prostatic urethra | (1) CT of the chest, abdomen, and pelvis; (2) brain MRI; (3) bone scan; and (4) 18F-FDG PET/CT scan | RRP and PLND | T2N1M0 | 3 mo | Lesion along the right iliac artery, pulmonary nodule | Biochemotherapy for 6 cycles dacarbazine, vinblastine, cisplatin, IL2 | 84 mo |
| Parmar et al[13], 2019 | 65 | No | Acute urinary retention | (1) Physical examination of whole body skin surface, oral cavity and anal mucosa; and (2) PET-CT | TURP, Dacarbazine, Chemotherapy | n/m | 3 mo | n/m | n/m | n/m |
In the only systematic review presented to date on all cases of prostate melanoma, Caputo et al[14] summarized 45 cases both in English and non-English literature. The median age of patients was 61 years and only 10 primary prostatic cases have been reported so far. Caputo’s team found that patients with prostatic metastases from melanoma had a dismal prognosis with a median survival of 3 mo (range 7 d to 6 mo). The prognosis of primary prostatic melanoma was not as bad as expected. Of the seven available cases with at least 1 year of follow-up, two survived for > 5 years, while the remaining five died after an average of 1 year.
It is important to distinguish between primary and secondary melanoma. Radical surgery followed by adjuvant chemo-/immunotherapy represents the most reasonable therapeutic strategy. For patients with primary disease, a more aggressive approach may provide better benefits.
Malignant melanoma of the prostate is rare. There have been 46 cases reported including our patient. There is a significant difference in the prognosis between primary and secondary cases[14] (3 mo vs 12 mo). The diagnosis of primary prostate melanoma has important implications for treatment. For accurate diagnosis, physical examination of the body skin surface, CT of the whole body, and endoscopy including the urinary tract and gastrointestinal tract should be conducted. 18F-FDG-PET may result in false-negative findings in the detection of melanoma both in the prostate and gastric mucosa.
| 1. | Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol. 2016;70:106-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 1271] [Article Influence: 127.1] [Reference Citation Analysis (2)] |
| 2. | Sánchez-Ortiz R, Huang SF, Tamboli P, Prieto VG, Hester G, Pettaway CA. Melanoma of the penis, scrotum and male urethra: a 40-year single institution experience. J Urol. 2005;173:1958-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Paner GP, Aron M, Hansel DE, Amin MB. Non-epithelial neoplasms of the prostate. Histopathology. 2012;60:166-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Berry NE, Reese L. Malignant melanoma which had its first clinical manifestations in the prostate gland. J Urol. 1953;69:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Hübler J, Pajor L, Kincses I. [Primary malignant melanoma of the prostate]. Acta Chir Acad Sci Hung. 1980;21:239-243. [PubMed] |
| 7. | Wong J, Wise GJ, Clark B. Malignant melanoma of the prostate: a case report. Can J Urol. 2008;15:4027-4029. [PubMed] |
| 8. | Wong JA, Bell DG. Primary malignant melanoma of the prostate: case report and review of the literature. Can J Urol. 2006;13:3053-3056. [PubMed] |
| 9. | Doublali M, Chouaib A, Khallouk A, Tazi MF, El Fassi MJ, Farih MH, Elfatmi H, Bendahou M, Benlemlih A, Lamarti O. Primary malignant melanoma of prostate. Urol Ann. 2010;2:76-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ma L, Liu W, Sun F. Primary malignant melanoma of the prostate. Int J Urol. 2010;17:94-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Tosev G, Kuru TH, Huber J, Freier G, Bergmann F, Hassel JC, Pahernik SA, Hohenfellner M, Hadaschik BA. Primary melanoma of the prostate: case report and review of the literature. BMC Urol. 2015;15:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Li R, Zhang M, Duplisea JJ, Troncoso P, Ward JF. Detection and Treatment of Primary Prostatic Melanoma. Urology. 2019;123:16-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Parmar K, Khanna A, Singh SK, Sharma M. A rare cause of acute urinary retention- Primary malignant melanoma of prostate. Asian J Urol. 2019;6:380-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Caputo A, Addesso M, Zeppa P, D'Antonio A. Malignant melanoma of the prostate gland: A systematic review. Pathol Res Pract. 2021;226:153594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Sella A, Ro JY. Renal cell cancer: best recipient of tumor-to-tumor metastasis. Urology. 1987;30:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Grignon DJ, Ro JY, Ayala AG. Malignant melanoma with metastasis to adenocarcinoma of the prostate. Cancer. 1989;63:196-198. [PubMed] [DOI] [Full Text] |
| 17. | Snow H, Hazell S, Francis N, Mohammed K, O'Neill S, Davies E, Mansfield D, Messiou C, Hujairi N, Nicol D, Harrington K, Smith M. Prostate-specific membrane antigen expression in melanoma metastases. J Cutan Pathol. 2020;47:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Teoh EJ, Tsakok MT, Bradley KM, Hyde K, Subesinghe M, Gleeson FV. Recurrent Malignant Melanoma Detected on 18F-Fluciclovine PET/CT Imaging for Prostate Cancer. Clin Nucl Med. 2017;42:803-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bagheri-Mohammadi S, Iran; Sahin TT, Turkey; Sarier M, Turkey S-Editor: Fan JR L-Editor: Kerr C P-Editor: Zhang YL