Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5710
Peer-review started: July 10, 2023
First decision: July 18, 2023
Revised: July 22, 2023
Accepted: July 31, 2023
Article in press: July 31, 2023
Published online: August 26, 2023
Processing time: 46 Days and 0.4 Hours
Chronic atrophic gastritis is a persistent disorder of the digestive system where the gastric mucosa epithelium and glands undergo atrophy, leading to a decrease in their number and thinning of the gastric mucosa. It is worth noting that the prevalence of chronic atrophic gastritis is higher in China compared to the global average, and it is also considered a precancerous condition for gastric cancer.
To evaluate the efficacy of Huangqi Jianzhong decoction in treating chronic atrophic gastritis. Chronic atrophic gastritis is a persistent illness characterized by the progressive disappearance of healthy gastric glands due to repeated injury. Huangqi Jianzhong decoctions are widely used in China to treat chronic atrophic gastritis. However, there is limited scientific evidence regarding their efficacy in treating this illness.
The present meta-analysis adhered to the PRISMA guidelines and used the Cochrane Collaboration methodology. We performed a comprehensive search for clinical trials investigating the use of Huangqi Jianzhong decoction in treating chronic atrophic gastritis published until January 2023. The risk of bias and the quality of the included studies were evaluated using the Cochrane Handbook guidelines. Finally, a meta-analysis was conducted using the RevMan 5.4 soft
This study included a total of 13 articles, comprising 1269 samples. The meta-analysis was conducted on these 13 articles, yielding the following results: I2 = 0%, P = 0.60, [RR = 1.24, 95%CI: 1.18 to 1.30, P < 0.00001]. The forest plot analysis of the Helicobacter pylori clearance rate revealed I2 = 0%, P = 0.36, [RR = 1.20, 95%CI: 1.05 to 1.38, P = 0.009]. The forest plot of PG-I level showed I2 = 99%, P < 0.00001, [MD = 4.99, 95%CI: -1.59 to 11.58, P = 0.14]. The forest plot of stomach pain demonstrated I2 = 54%, P = 0.04, [MD = -0.63, 95%CI: -0.68 to -0.58, P < 0.00001]. The forest plot of reflux indicated I2 = 82%, P = 0.0009, [MD = -0.48, 95%CI: -0.63 to -0.33, P < 0.00001]. The forest plot of recurrence rate exhibited I2 = 0%, P = 0.92, [RR = 0.15, 95%CI: 0.04 to 0.66, P = 0.01]. The forest plot of adverse reactions showed no heterogeneity in outcome data, [RR = 1.07, 95%CI: 0.53 to 2.17, P = 0.86].
This study demonstrated that Huangqi Jianzhong decoction improved various factors in adults with chronic atrophic gastritis. These factors included the total effective rate, Helicobacter pylori clearance rate, symptoms such as stomachache and acid reflux alleviation, and recurrence rates.
Core Tip: Chronic atrophic gastritis is a chronic disease featured by the gradual disappearance of normal glands after being damaged repeatedly. We conducted a meta-analysis using Revman 5.4, and the study showed that Huangqi Jianzhong Tang (HQT) can effectively improve the symptoms of chronic atrophic gastritis and enhance patients’ quality of life. We advocate the integration of traditional Chinese medicine in clinical practice for treating diseases, and our research provides theoretical support for the clinical use of HQT in the treatment of chronic atrophic gastritis.
- Citation: Yan XP, Si W, Ding MS, Tian YF, Guo Z. Efficacy and safety of Huangqi Jianzhong decoction in the treatment of chronic atrophic gastritis: A meta-analysis. World J Clin Cases 2023; 11(24): 5710-5720
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5710.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5710
Chronic atrophic gastritis is characterized by the loss of gastric glandular structures caused by inflammation[1]. The prevalence rate of chronic atrophic gastritis in China is higher than the global incidence of 0%–10.9%[2]. This illness is currently classified as a precancerous stomach lesion[3]. Gastric cancer is the fifth most common cancer worldwide and the third leading cause of cancer-related deaths[4]. Given these facts, appropriate management of chronic atrophic gastritis is critical[5]. The majority of chronic atrophic gastritis cases are associated with Helicobacter pylori (H. pylori) infection, which should be treated in most cases[6]. However, recent studies have indicated that the eradication rate of H. pylori has decreased below 80% owing to increased antibiotic resistance, lack of compliance, and adverse effects[7]. Therefore, alternative treatments that can effectively and safely reduce the symptoms of chronic atrophic gastritis and improve patients’ quality of life must be investigated.
Traditional Chinese medicine (TCM) is currently a viable treatment option[8]. TCM doctors assess a patient’s condition by examining tongue manifestations, pulse palpation, and specific symptoms before prescribing a medicine[9]. In TCM, a syndrome refers to a group of symptoms experienced by a patient[10]. Patients with chronic atrophic gastritis may present with the cold–heat complex syndrome, which is characterized by symptoms including distending stomach pain, belching, and a yellow tongue coating.
Huangqi Jianzhong decoction is a traditional Chinese herbal formula comprising seven herbs, including Fructus Jujube (Dazao), Radix Astragali (Huangqi), Rhizoma Zingiberis Recens (Shengjiang), Ramulus Cinnamomi (Guizhi), Radix Glycyrrhizae (Gancao), Paeoniae Radix Alba (Baishao), and Saccharum Granorum (Yitang)[11]. This decoction is used to treat gastrointestinal diseases, including chronic gastritis, atrophic gastritis, peptic ulcers, and H. pylori-related gastritis, and was first mentioned in Zhongjing Zhang’s Synopsis of Prescriptions of the Golden Chamber[11]. A previous study found that Huangqi Jianzhong decoction can reduce tumor necrosis factor, Interleukin 1, and interferon levels[12]. However, the safety and efficacy of this herbal formula remain debatable.
Herein, a systematic review of relevant literature was conducted to assess the efficacy and safety of Huangqi Jianzhong decoction in treating chronic atrophic gastritis. The study analyzed the total effective rate, H. pylori clearance rate, pepsinogen I (PG-I) levels, TCM syndrome scores (including stomachache and acid reflux), recurrence rates, and any adverse effects associated with the use of Huangqi Jianzhong decoction.
This meta-analysis adhered to the PRISMA guidelines and the Cochrane Collaboration methodology[13]. A comprehensive search was conducted across nine electronic databases, including PubMed, Web of Science, Cochrane Library, Embase, Medline, CNKI, Wanfang, Cqvip, and SinoMed. The search was performed from the inception of the databases until January 2023. The following keywords were used in the search strategy: (atrophic gastritis OR gastritis, atrophic OR CAG OR chronic gastritis OR gastric atrophy OR gastric mucosal atrophy) AND (“huangqi jianzhong decoction” OR “huangqi jianzhong tablets” OR “huangqi jianzhong formula” OR “huangqi jianzhong pills” OR “huangqi jianzhong capsules” OR “huangqi jianzhong tang”). This strategy aimed to identify relevant studies on the use of Huangqi Jianzhong decoction for treating chronic atrophic gastritis.
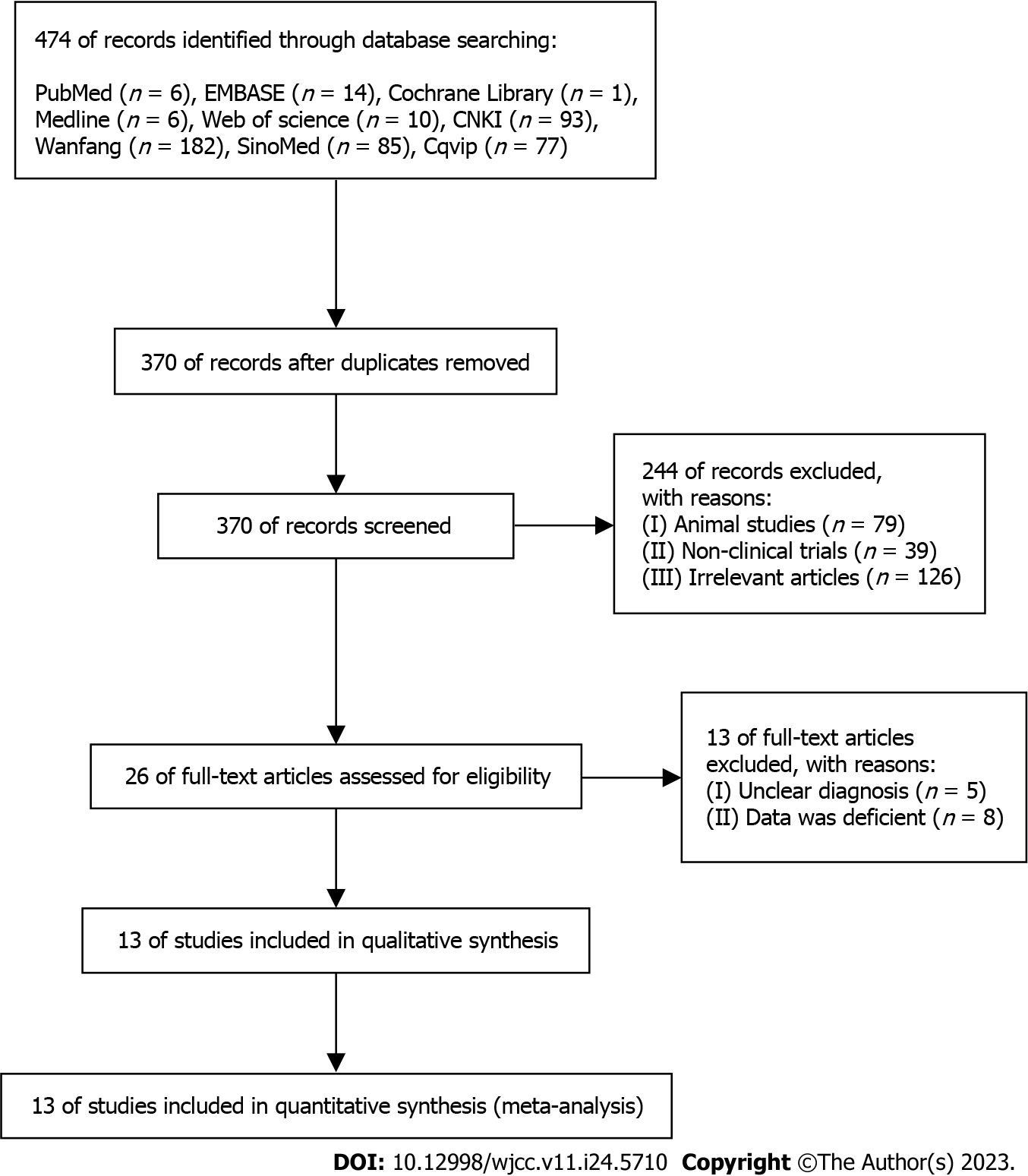
The meta-analysis included studies that met the following inclusion criteria: (1) Patients diagnosed with chronic atrophic gastritis based on endoscopy and pathology; (2) participants aged ≥ 18 years; and (3) the primary outcomes of interest were the total effective rate, H. pylori clearance rate, PG-I levels, TCM syndrome scores, recurrence rates, and adverse effects. Studies were excluded based on the following criteria: (1) Non-clinical trials; (2) studies without full-text availability; and (3) studies lacking comprehensive data. A total of 13 studies that met the inclusion criteria were included in the meta-analysis.
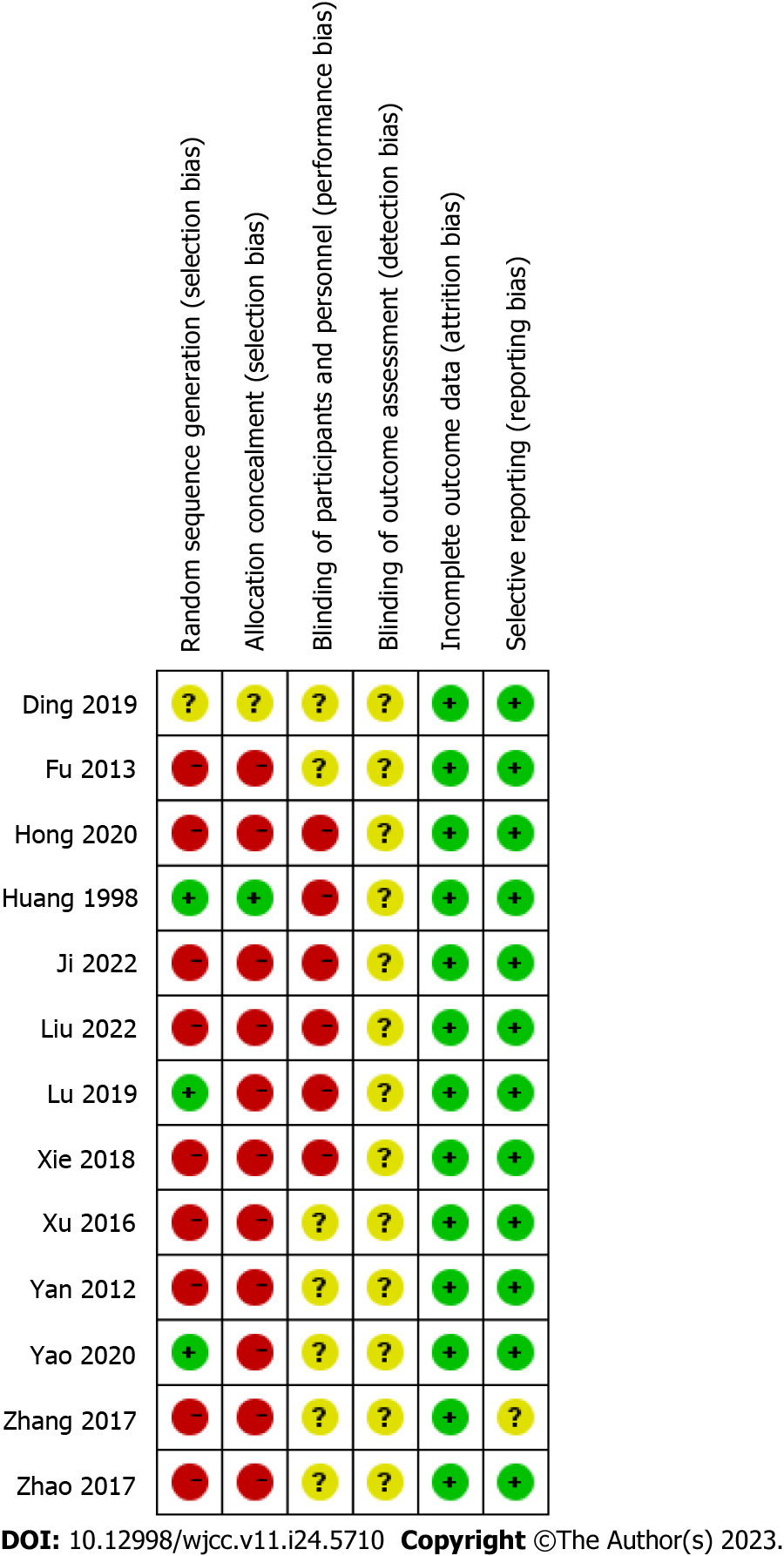
The literature reviews were conducted independently by two researchers. They reviewed the titles and abstracts of the identified studies based on the established exclusion and inclusion criteria. Studies that did not meet the criteria were excluded. The researchers then reviewed the full text of the selected studies for final inclusion. Disagreements between the two researchers were assessed by a third-party researcher during the screening process and resolved through negotiation. Data including authors, publication details, participant age and sex, intervention, study duration, and results were extracted from the selected studies. The risk of bias of all included studies was independently assessed by the two researchers, and any disagreements were resolved by a third-party researcher. The included studies’ risk of bias was assessed following the Cochrane Handbook guidelines, evaluating seven key domains: Random sequence generation, allocation concealment, caregiver blinding, patients, outcome assessors, incomplete outcome data collection, and selective reporting. Each domain was assigned a score, with 2, 1, and 0 indicating a high risk, unclear risk, and low risk of bias, respectively. The overall risk of bias was classified as follows: a score of 0–2 indicated a low risk of bias (high quality), that of 3–5 indicated a moderate risk of bias (moderate quality), and that of 6–8 indicated a high risk of bias (low quality).
In this study, several outcome indicators were used, including the total effective rate, H. pylori clearance rate, PG-I levels, TCM syndrome scores, recurrence rates, and adverse effects. The total effective rate was calculated using the following formula: (number of clinical recoveries + number of effective cases + number of markedly effective cases) divided by the total number of cases and the resulting value multiplied by 100%. Additionally, statistical analysis was conducted using RevMan 5.4. Categorical variables were expressed as relative risks (RRs) with 95% confidence intervals (CIs), whereas continuous variables were expressed as mean differences (MDs) with 95%CIs. A P value of < 0.05 was considered statistically significant. The I² statistic was used to examine heterogeneity among the included studies. A fixed-effects model was used if I2 was < 50% (low heterogeneity), whereas a random-effects model was used if I2 was > 50% (significant heterogeneity). A sensitivity analysis was performed to evaluate the reliability and stability of the results by removing the study with the greatest weight from the analysis. Funnel plots were used to assess publication bias in randomized controlled trials (RCTs) involving ≥ 10 participants. Funnel plots can help identify potential bias or asymmetry in data. These statistical analyses were performed to assess the robustness and validity of the findings of our meta-analysis.
The sequential steps in the literature search process are shown in Figure 1. A total of 13 studies involving 1269 participants were included in the meta-analysis. Initially, the database searches yielded 474 studies. Of these, 104 duplicates were removed, and the remaining 370 studies were further evaluated. From these, 13 studies were selected for the meta-analysis (Figure 1). Table 1 provides a comprehensive overview of the study characteristics, including age, sex, number of cases, interventions, and outcomes assessed.
| Ref. | Age | Male/Female | Number of cases | Intervention | Duration of intervention | Outcome |
| Ji et al[14], 2022 | C: 52.55 ± 9.57; T: 51.34 ± 10.52 | C = 34/26; T = 35/25 | C: n = 60; T: n = 60 | C: Amoxicillin + Clarithromycin + Rabeprazole; T: Amoxicillin + Clarithromycin + HQJZD + Rabeprazole | 8 wk | Total effective rate; PG-I |
| Liu et al[15], 2022 | C: 52.27 ± 13.63; T: 51.94 ± 12.86 | C=20/18; T=22/16 | C: n = 38; T: n = 38 | C: Omeprazole; T: Omeprazole + HQJZD | 8 wk | Total effective rate; Stomachache |
| Zhao et al[16], 2017 | C: 45.32 ± 5.61; T: 46.78 ± 5.89 | C: 19/17; T: 20/16 | C: n = 36; T: n = 36 | C: Rabeprazole + Hydrotalcite; T: Rabeprazole + Hydrotalcite + HQJZD | 8 wk | Total effective rate; Stomachache; Acid reflux; Adverse reaction; Recurrence rate |
| Ding et al[17], 2019 | C: 50.27 ± 10.12; T: 51.03 ± 9.89 | C: 34/21; T: 32/23 | C: n = 55; T: n = 55 | C: Amoxicillin + Clarithromycin + Omeprazole; T: Amoxicillin + Clarithromycin + HQJZD + Omeprazole | 12 wk | Total effective rate; Stomachache; Acid reflux; PG-I |
| Huang et al[18], 1998 | C: 54.3 ± 4.5; T: 53.5 ± 4.7 | C: 25/20; T: 26/19 | C: n = 45; T: n = 45 | C: Amoxicillin + Clarithromycin + Omeprazole + Folate; T: Amoxicillin + Clarithromycin + Omeprazole+ Folate + HQJZD | 4 wk | Total effective rate |
| Yao et al[19], 2020 | C: 54.3 ± 4.5; T: 53.5 ± 4.7 | C: 25/20; T: 26/19 | C: n = 45; T: n = 45 | C: Amoxicillin + Clarithromycin + Rabeprazole+ Colloidal bismuth pectin citrate; T: Amoxicillin + Clarithromycin + Rabeprazole+ Colloidal bismuth pectin citrate +HQJZD | 2 wk | Total effective rate; Stomachache; Acid reflux; PG-I; Adverse reaction; H. pylori eradication rate |
| Lu et al[20], 2019 | C: 46.8 ± 16.5; T: 47.4 ± 16.7 | C: 56/37; T: 58/35 | C: n = 93; T: n = 93 | C: Weifuchun; T: HQJZD | 12 wk | Total effective rate; PG-I |
| Hong et al[21], 2020 | C: 63.53 ± 6.81; T: 65.18 ± 5.37 | C: 36/23; T: 33/26 | C: n = 59; T: n = 59 | C: Weifuchun; T: HQJZD | 12 wk | Total effective rate; Stomachache |
| Xu et al[22], 2016 | C: 68.17 ± 10.58; T: 68.26 ± 10.63 | C: 22/17; T: 21/18 | C: n = 39; T: n = 39 | C: Vitacoenzyme; T: HQJZD | 8 wk | Total effective rate; Stomachache; Acid reflux; Adverse reaction; Recurrence rate |
| Yan et al[23], 2012 | C: 40 ± 9.6; T: 41 ± 8.3 | C: 38/22; T: 40/25 | C: n = 60; T: n = 65 | C: Weifuchun; T: HQJZD | 12 wk | Total effective rate; H. pylori eradication rate |
| Zhang et al[24], 2017 | NS | NS | C: n = 40; T: n = 40 | C: Weifuchun; T: HQJZD | 8 wk | Total effective rate; Adverse reaction |
| Fu et al[25], 2013 | C: 54.60 ± 5.64; T: 49.60 ± 6.11 | C: 38/22; T: 16/14 | C: n = 30; T: n = 30 | C: Vitacoenzyme; T: HQJZD | 8 wk | Total effective rate; Adverse reaction |
| Xie et al[26], 2018 | C: 46.0 ± 2.7; T: 44.5 ± 2.6 | C: 19/15; T: 17/17 | C: n = 34; T: n = 34 | C: Rabeprazole + Hydrotalcite; T: HQJZD | 8 wk | Total effective rate; Stomachache; Acid reflux |
Among the included studies, eight had a high risk of bias, while five had a moderate risk of bias. The most prevalent biases among the studies were related to random sequence generation and allocation concealment. Figure 2 depicts a graphical representation of the risk-of-bias assessment.
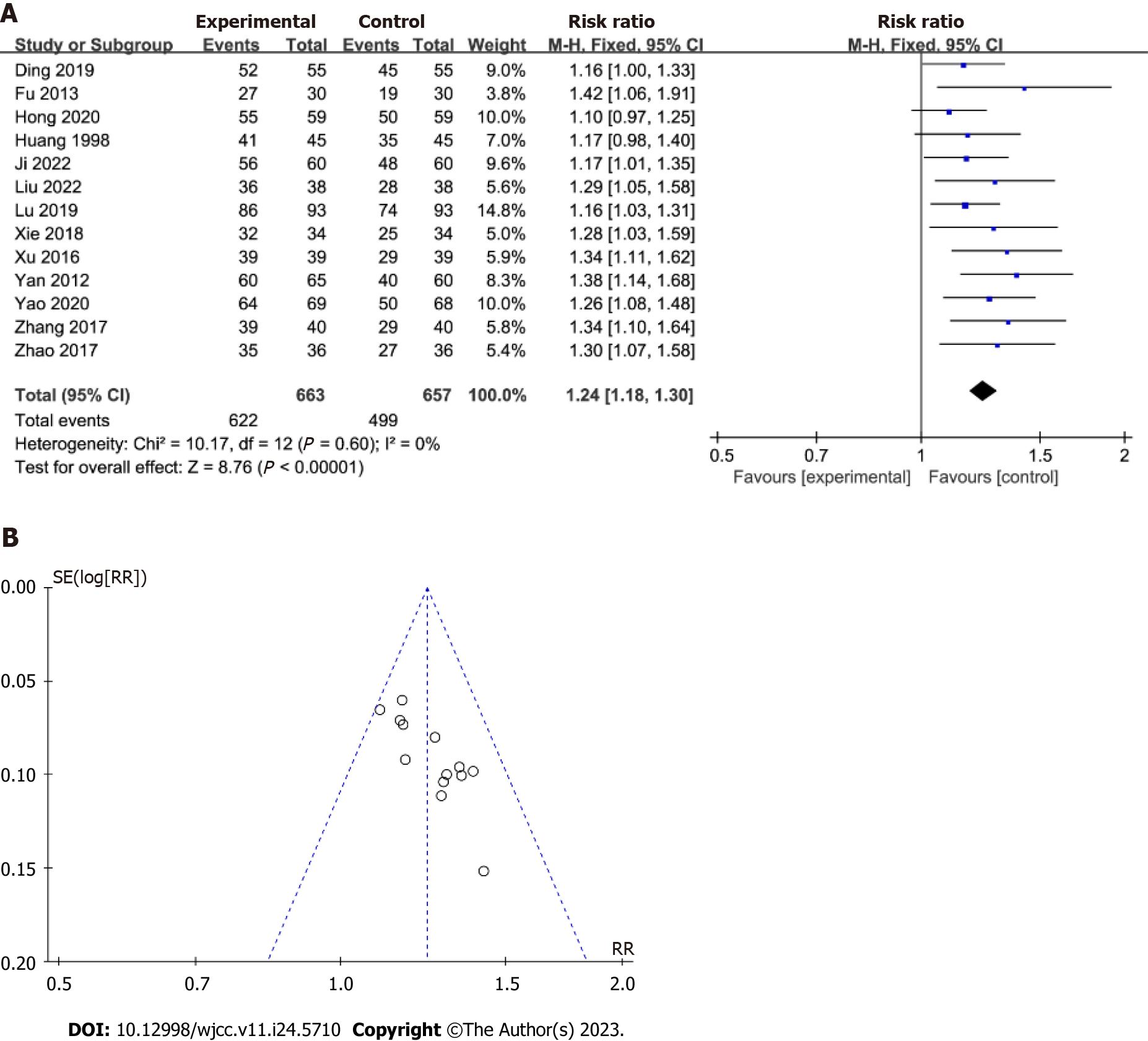
Total effective rate: Thirteen studies were included in the total effective rate analysis. As shown in Figure 3, the experimental group showed a significant decrease in the total effective rate [RR = 1.24; 95%CI: 1.18 to 1.30, P < 0.001], with a low heterogeneity (I2 = 0%; P = 0.6), compared with the control group. Furthermore, evaluation of the funnel plot asymmetry revealed little evidence of publication bias. Moreover, the data did not change significantly after excluding the study with the highest weight from the analysis.
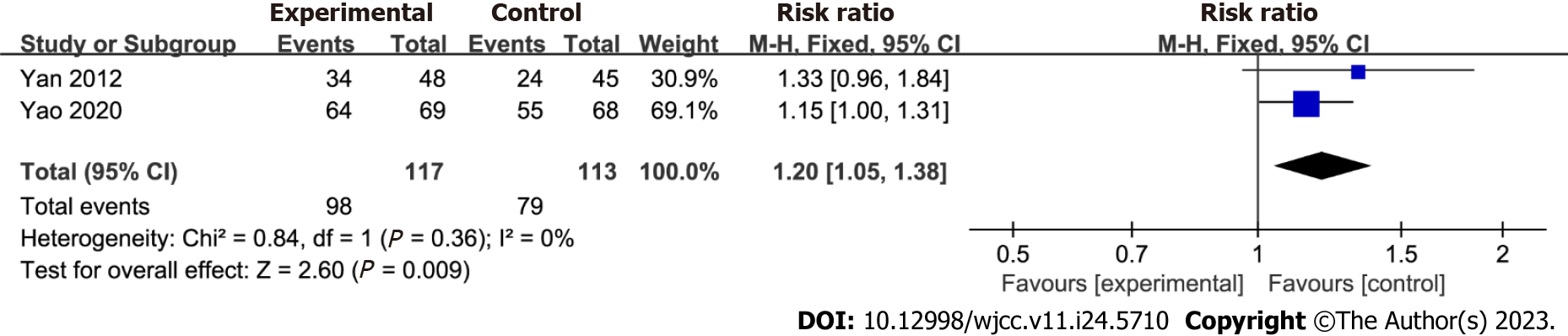
H. pylori clearance rate: This meta-analysis included two studies that reported improvements in H. pylori clearance rates. Figure 4 shows the effect of chronic atrophic gastritis on the total H. pylori clearance rate, demonstrating a significant effect [RR = 1.20; 95%CI: 1.05 to 1.38, P = 0.009]. Low heterogeneity was observed (I2 = 0%; P = 0.36), indicating a consistent outcome across the studies. Furthermore, the data remained unchanged after excluding the study with the highest weight from the analysis.
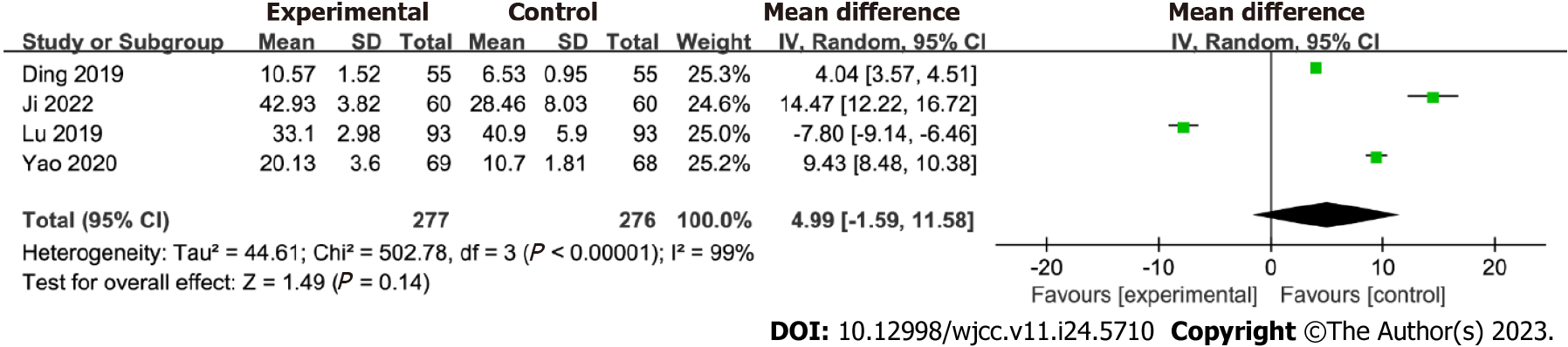
PG-I levels: Four studies that analyzed PG-I levels were included in this meta-analysis, and the effect of Huangqi Jianzhong decoction on these levels is shown in Figure 5. No significant difference in PG-I levels was observed between the two groups [MD = 4.99; 95%CI: -1.59 to 11.58, P = 0.14]. However, there was no significant heterogeneity (I2 = 99%; P < 0.001), indicating variability among the studies. Furthermore, the data did not exhibit significant changes after excluding the study with the highest weight from the analysis.
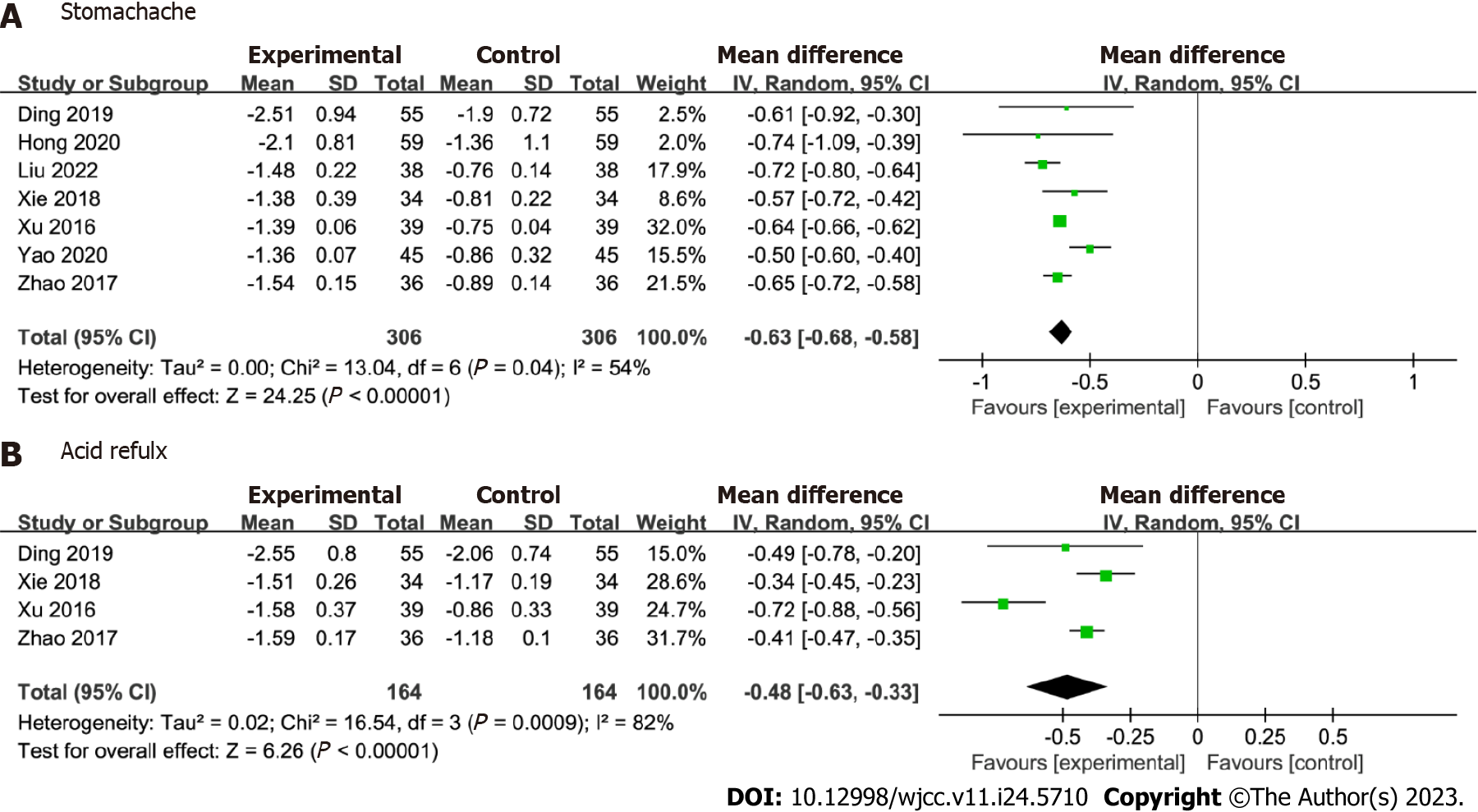
TCM syndrome scores: This meta-analysis included seven studies focusing on stomachache improvement. Figure 6 shows the role of Huangqi Jianzhong decoction in alleviating stomachache. The Huangqi Jianzhong decoction group exhibited a significant improvement in stomachache compared with the control group [MD = -0.63; 95%CI: -0.68 to -0.58, P < 0.001; Figure 6A]. However, substantial heterogeneity (I2 = 54%; P = 0.04) was observed, indicating variability among the studies. The analysis also revealed a significant difference in acid reflux between the two groups [MD = -0.48; 95%CI: -0.63 to -0.33, P < 0.001], despite marked heterogeneity (I2 = 82%; P < 0.001; Figure 6B). The results showed no significant changes after excluding the study with the highest weights from the analysis.
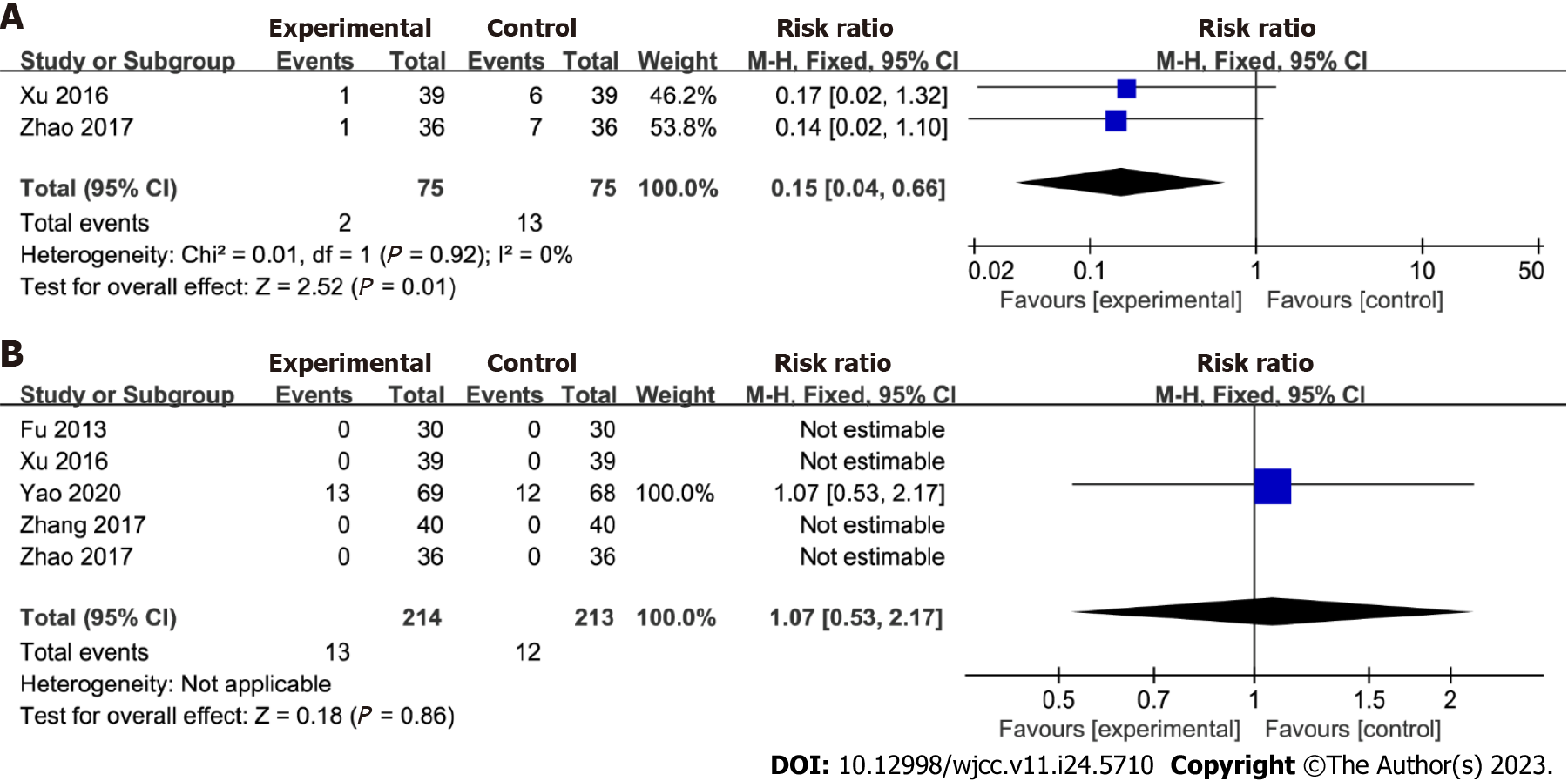
Recurrence rates and adverse effects: Two studies comparing Huangqi Jianzhong decoction with a control group were included in the pooling data for the recurrence rates of patients with chronic atrophic gastritis. A significant difference in recurrence rates was observed between the Huangqi Jianzhong decoction and control groups [RR = 0.15; 95%CI: 0.04 to 0.66, P = 0.01]. Low heterogeneity was observed (I2 = 0%; P = 0.92), indicating a consistent outcome across the studies. Furthermore, five studies were pooled to compare the adverse effects in the Huangqi Jianzhong decoction and control groups. However, no significant difference in adverse effects was observed between the two groups [RR = 1.07; 95%CI: 0.53 to 2.17, P = 0.86; Figure 7]. Moreover, the data did not exhibit significant changes after excluding the study with the highest weight from the analysis.
This study aimed to conduct a comprehensive review of the scientific literature on the efficacy and safety of Huangqi Jianzhong decoction in patients with chronic atrophic gastritis. The results suggest that Huangqi Jianzhong decoction is an effective treatment for this illness. The study demonstrated that the Huangqi Jianzhong decoction group exhibited significant improvements in the total effective rate, H. pylori clearance rates, and TCM syndrome scores (associated with stomach pain and acid reflux), as well as decreased recurrence rates compared with the control group.
Huangqi Jianzhong decoction has been used to treat various symptoms, including abdominal upset or pain, belching, abdominal bloating, nausea, vomiting, loose stools, sensations of fullness, upper abdominal burning, and lower extremity coldness or weakness. Existing data support its use in the treatment of chronic gastritis[8], suggesting that Huangqi Jianzhong decoction has many therapeutic targets in treating chronic gastritis[8]. The results of our meta-analysis support previously reported findings[12-15]. Furthermore, using Huangqi Jianzhong decoction, the total effective rate and H. pylori clearance rate significantly improved, whereas the scores for stomachache and acid reflux and the recurrence rates of patients significantly decreased. These findings are statistically significant and consistent with earlier findings[12-15].
We also assessed the safety of Huangqi Jianzhong decoction by examining its adverse effects. There were no significant differences in adverse effects between the Huangqi Jianzhong decoction and control groups. However, it is essential to note that only one study reported adverse effects in both groups. In the control group, adverse effects including nausea, mild gastrointestinal reactions, and bloating were reported, while in the Huangqi Jianzhong decoction group, adverse effects included headache, rash, nausea, and mild gastrointestinal reactions[16].
This study had several limitations. First, among the included trials, only three provided information on how participants were randomly assigned to experimental groups, and none provided follow-up data. This could introduce potential biases in the results. Second, while H. pylori infection is the most prevalent cause of chronic atrophic gastritis, other factors can also contribute to this illness. The specific causes in the included studies may not have been fully investigated. Third, all the clinical trials included in this meta-analysis were conducted and published in China, which could limit the generalizability of the findings to other populations. Notably, the overall effect rate was used as the primary outcome measure in all trials to assess the efficacy of Huangqi Jianzhong decoction in treating chronic atrophic gastritis.
This review emphasizes the efficacy of Huangqi Jianzhong decoction in improving the total effective rate, H. pylori clearance rate, TCM syndrome scores, and recurrence rates in adults with chronic atrophic gastritis. Furthermore, we recommend conducting additional high-quality RCTs to further evaluate this treatment strategy.
Chronic atrophic gastritis is a persistent disorder of the digestive system where the gastric mucosa epithelium and glands undergo atrophy, leading to a decrease in their number and thinning of the gastric mucosa. It is worth noting that the prevalence of chronic atrophic gastritis is higher in China compared to the global average, and it is also considered a precancerous condition for gastric cancer.
Currently, Western medicine treatments for chronic atrophic gastritis can only slow down the progression of the disease and are not capable of providing an effective cure. Therefore, we aim to investigate the therapeutic effects of Huangqi Jianzhong Decoction on chronic atrophic gastritis from the perspective of traditional Chinese medicine, aiming to enhance patients’ quality of life.
To assess the therapeutic efficacy of Huangqi Jianzhong Decoction for chronic atrophic gastritis.
We conducted a literature review on the treatment of chronic atrophic gastritis with Huangqi Jianzhong Decoction, focusing on publications up until January 2023. The collected literature was analyzed for heterogeneity, and a meta-analysis was performed using Revman 5.4 and the Cochrane Handbook.
We included a total of 13 articles, comprising 1269 patients. The findings of our analysis revealed that the use of Huangqi Jianzhong Decoction in the treatment of chronic atrophic gastritis led to significant improvements in various aspects. These improvements included an increased clearance rate of Helicobacter pylori, alleviation of symptoms such as stomach pain, and a reduction in the recurrence rate of patients. However, there was no significant change observed in the levels of Pepsin I.
Huangqi Jianzhong Decoction has a significant therapeutic effect on patients with chronic atrophic gastritis, including improvements in the clearance rate of Helicobacter pylori, reduction of stomachache, alleviation of gastric acid reflux, and decreased disease recurrence rate.
Traditional Chinese medicine represents a longstanding treatment approach in China. When approaching the treatment of chronic atrophic gastritis from a traditional Chinese medicine perspective, our focus lies not only on symptom relief but also on studying treatment methods that effectively address the condition. By doing so, we aim to improve the overall quality of life for patients, going beyond mere alleviation of the disease process.
| 1. | Vannella L, Lahner E, Annibale B. Risk for gastric neoplasias in patients with chronic atrophic gastritis: a critical reappraisal. World J Gastroenterol. 2012;18:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (5)] |
| 2. | Du Y, Bai Y, Xie P, Fang J, Wang X, Hou X, Tian D, Wang C, Liu Y, Sha W, Wang B, Li Y, Zhang G, Shi R, Xu J, Huang M, Han S, Liu J, Ren X, Wang Z, Cui L, Sheng J, Luo H, Zhao X, Dai N, Nie Y, Zou Y, Xia B, Fan Z, Chen Z, Lin S, Li ZS; Chinese Chronic Gastritis Research group. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterol. 2014;14:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 3. | Li Y, Xia R, Zhang B, Li C. Chronic Atrophic Gastritis: A Review. J Environ Pathol Toxicol Oncol. 2018;37:241-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1383] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 5. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3309] [Article Influence: 551.5] [Reference Citation Analysis (6)] |
| 6. | Ishikura N, Usui Y, Ito H, Kasugai Y, Oze I, Kato S, Yatabe Y, Nakamura S, Matsuo K. Helicobacter pylori (HP) infection alone, but not HP-induced atrophic gastritis, increases the risk of gastric lymphoma: a case-control study in Japan. Ann Hematol. 2019;98:1981-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology. 2021;161:1325-1332.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 321] [Article Influence: 64.2] [Reference Citation Analysis (3)] |
| 8. | Tang XD, Lu B, Zhou LY, Zhan SY, Li ZH, Li BS, Gao R, Wang FY, Wang P, Yang JQ, Liu G, Zhang YQ, Che GX, Lin M, Bian LQ, Zhao YP; China Academy of Chinese Medical Sciences, Beijing. Clinical practice guideline of Chinese medicine for chronic gastritis. Chin J Integr Med. 2012;18:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Wang J, Wong YK, Liao F. What has traditional Chinese medicine delivered for modern medicine? Expert Rev Mol Med. 2018;20:e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 10. | Xie R, Xia Y, Chen Y, Li H, Shang H, Kuang X, Xia L, Guo Y. The RIGHT Extension Statement for Traditional Chinese Medicine: Development, Recommendations, and Explanation. Pharmacol Res. 2020;160:105178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Hu J, He T, Liu J, Jia S, Li B, Xu W, Liao M, Guo L. Pharmacological and molecular analysis of the effects of Huangqi Jianzhong decoction on proliferation and apoptosis in GES-1 cells infected with H. pylori. Front Pharmacol. 2022;13:1009705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Nöst X, Pferschy-Wenzig EM, Nikles S, He X, Fan D, Lu A, Yuk J, Yu K, Isaac G, Bauer R. Identification of Constituents Affecting the Secretion of Pro-Inflammatory Cytokines in LPS-Induced U937 Cells by UHPLC-HRMS-Based Metabolic Profiling of the Traditional Chinese Medicine Formulation Huangqi Jianzhong Tang. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51894] [Article Influence: 10378.8] [Reference Citation Analysis (2)] |
| 14. | Ji MY, Luo FL. Efficacy of Huangqi Jianzhong Tang combined with rabeprazole in the treatment of chronic atrophic gastritis of the weak spleen and stomach type. Guizhou Medicine. 2022;46:75-76. |
| 15. | Liu D. Clinical observation of Huangqi Jianzhong Tang in the treatment of chronic atrophic gastritis with evidence of spleen and stomach deficiency cold. Modern Distance Education in Chinese Traditional Medicine. 2022;20:71-73. |
| 16. | Zhao ZH, Dong J. Clinical observation of 36 cases of Hp-positive chronic atrophic gastritis patients with spleen and stomach deficiency cold type treated with Huangqi Jianzhong Tang as an adjunct. Sichuan Traditional Chinese Medicine. 2017;35:158-160. |
| 17. | Ding HR, Wang F, Li JN. Effectiveness and mechanism of Huangqi Jianzhong Tang with evidence-based addition and subtraction in the treatment of chronic atrophic gastritis. Linchuang Yixue Yanjiuyushijian. 2019;4:128-130. |
| 18. | Huang ZG, Huang L, Huang S, Chen SL, Zeng DB. Effects of Huangqi Jianzhong Tang combined with Liangfen Wan on gastric mucosal blood flow and serum oxidative stress index in patients with chronic atrophic gastritis. Xiandai zhongxiyi Zazhi. 2020;29:1998-2002. |
| 19. | Yao MW, Xu L, Huang GH. Efficacy of Huangqi Jianzhong Tang combined with Hedeng Matching Point Moxibustion in the treatment of Helicobacter pylori-positive chronic atrophic gastritis in the deficient-cold spleen and stomach type. Xiandai zhongxiyi Zazhi. 2020;29:124-128. |
| 20. | Lu WW. Treatment of chronic atrophic gastritis with intestinal epithelial hyperplasia with addition of Huangqi Jianzhong Tang. Acta Chinese Medicine. 2019;34:1531-1534. |
| 21. | Hong WH, Lu BQ, Li ZJ, Wu PC. Clinical efficacy of Huangqi Jianzhong Tang in the treatment of precancerous lesions in the stomach. Zhonghua Laonianxue Zazhi. 2020;40:2503-2505. |
| 22. | Xu JW. Efficacy of Huangqi Jianzhong Tang with evidence-based addition and subtraction in the treatment of chronic atrophic gastritis due to chronic superficial gastritis extension. Shaanxi Traditional Chinese Medicine. 2016;37:142-144. |
| 23. | Yan XJ, Luo T, Meng NN. Efficacy of Huangqi Jianzhong Tang in treating 65 cases of chronic atrophic gastritis. Liaoning Zhongyiyao Zazhi. 2012;39:688-689. |
| 24. | Zhang GP. Efficacy of Huangqi Jianzhong Tang combined with acupuncture point patching in the treatment of chronic atrophic gastritis. Shanxi Yixue Zazhi. 2017;46:1850-1851. |
| 25. | Fu Q, Wang ZL, Jiang SQ. Huangqi Jianzhong Tang for the treatment of 30 cases of chronic atrophic gastritis with deficiency cold in the spleen and stomach. Zhongyi Zazhi. 2013;54:1600-1601. |
| 26. | Xie ZM, Chen CH, Wu ZX. Effect of adjuvant treatment with Huangqi Jianzhong Tang on Hp-positive chronic atrophic gastritis with spleen and stomach deficiency and cold. Gannan Yixueyuan Xuebao. 2018;38:545-548. |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim KM, South Korea; Pierce T, Australia S-Editor: Liu JH L-Editor: A P-Editor: Yuan YY