Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5692
Peer-review started: May 30, 2023
First decision: July 4, 2023
Revised: July 11, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: August 26, 2023
Processing time: 87 Days and 4.4 Hours
Biliary adenomas that occur in the extrahepatic biliary tree are rare. It is difficult to distinguish it from cholangiocarcinoma or cholangiolithiasis by various imaging examinations, and it is very easy to be misdiagnosed.
To evaluate the cumulative experiences including clinical characteristics and treatments of nine patients diagnosed with extrahepatic biliary adenoma admitted to the First Affiliated Hospital of Xi’an Jiaotong University from 2016 to 2022.
A total of nine patients were included in our study. The laboratory examinations, disease diagnosis, therapy and pathological characteristics, and follow-up of every patient were evaluated.
Our cohort consisted of six females and three males with an average diagnosis age of 65.1 years (range 46-87). Six extrahepatic biliary adenomas were located in the common bile ducts and three in the hepatic duct. On initial presentation, all of the patients have symptom of biliary origin, including obstructive jaundice (4/9, 44.4%), abdominal pain (6/9, 66.7%), and fever (3/9, 33.3%). Preoperative imaging examination considered bile duct carcinoma in 6 cases and bile duct calculi in 3 cases. All the patients received surgical treatment and were confirmed by pathology as biliary adenoma. The symptoms improved significantly in all 9 patients after surgery. Seven of nine patients recovered well at follow-up without tumor recurrence. One patient died 2 mo after the surgery due to heart failure. One patient developed jaundice again 8 mo after surgery, underwent endoscopic retrograde cholangiopancreatography and biliary stent placement.
Benign extrahepatic biliary tumors are rare and difficult to diagnosis preoperatively. Intraoperative choledocho
Core Tip: Biliary adenomas that occur in the extrahepatic biliary tree are rare. It is difficult to distinguish it from cholangiocarcinoma or cholangiolithiasis by various imaging examinations, and it is very easy to be misdiagnosed. In this study, we present the cumulative experiences including clinical characteristics and treatments of nine patients diagnosed with extrahepatic biliary adenoma admitted to the First Affiliated Hospital of Xi’an Jiaotong University from 2016 to 2022. Benign extrahepatic biliary tumors are rare and difficult to diagnosis preoperatively. Intraoperative choledochoscopy and timely biopsy may offer great advantages.
- Citation: Li W, Tao J, Song XG, Hou MR, Qu K, Gu JT, Yan XP, Yao BW, Qin YF, Dong FF, Sha HC. Clinical study of extrahepatic biliary adenoma. World J Clin Cases 2023; 11(24): 5692-5699
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5692.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5692
Extrahepatic biliary adenoma is a rare entity arising from extrahepatic duct epithelium. They account for only 6% of all extrahepatic bile duct masses[1]. The main clinical symptoms are jaundice, pruritus, fever, abdominal pain and acute cholangitis, due to tumor enlargement or blockage of the biliary tract fragment[2]. The imaging examination findings may include extrahepatic or/and intrahepatic duct dilation, soft tissue density in the bile duct, or thickening and enhancement of the bile duct wall. However, these features are not typical and of limited use in differential diagnosis[2,3]. Clinically, preoperative diagnosis of extrahepatic biliary adenoma is rare and easily misdiagnosed as cholangiocarcinoma or cholangiolithiasis[4,5].
This paper summarizes the clinical characteristics of nine patients with extrahepatic biliary adenoma followed at our hospital during the past 6 years. Our cumulative experiences may help clinicians to better recognize, diagnose and treat this disease.
This study has been approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University and conducted in accordance with the approved guidelines. The informed consent was obtained from all subjects and/or their legal guardian(s). We reviewed the database and collected nine cases of extrahepatic biliary adenoma from 2016 to 2022. Patients with clinical presentations of obstructive jaundice, abdominal pain, chill and fever, cholangitis, and a pathological diagnosis of biliary adenoma were included in this cohort. The medical records of the included patients were reviewed. Tumor information were obtained from computed tomography (CT) scan or magnetic resonance cholangiopancreatography (MRCP) measurements. Follow-up data, including patients’ follow-up status and symptoms recovery, were acquired from hospital medical records or telephone interviews with patients, family members or general practitioners.
The nine patients consisted of six females (66.7%) and three males (33.3%) with an average diagnosis age of 65.1 years (ranged from 46 to 87) (Table 1). As for the location of extrahepatic biliary adenomas, six of them were located in the common bile duct (66.7%) and three in the hepatic duct (33.3%). The main symptoms of all the nine patients with extrahepatic biliary adenoma in the present study are presented in Table 1. All of the patients have symptoms of biliary origin, including obstructive jaundice (4/9, 44.4%), abdominal pain (6/9, 66.7%), chill and fever (3/9, 33.3%). However, these clinical features are of limited use in differential diagnosis.
| Patient | Male/female | Age, yr | Location | Presentation | Preoperative diagnosis | Treatment | Size (cm)/number of tumors | Histology | Outcome |
| 1 | Male | 87 | Distal CBD | Chills, fever | Choledocholithiasis | Local excision and T-tube drainage | 0.6 cm × 0.5 cm/1 | Papillary adenoma | Well at 2 yr after surgery |
| 2 | Male | 78 | Distal CBD | Painless jaundice | Distal cholangiocarcinoma | Pancreaticoduodenectomy | 1.2 cm × 1 cm/1 | Papillary adenoma | Well at 7 mo after surgery |
| 3 | Female | 58 | Common hepatic duct, right and left intrahepatic ducts | Abdominal pain, jaundice, dark urine | Multiple hepatic exterior and interior calculus of bile duct | Local lesion biopsy and T-tube drainage | 2 cm × 0.8 cm (biggest)/several | Papillary adenoma (biliary papillomatosis) | 8 mo after surgery, jaundice, ERCP + plastic biliary stent |
| 4 | Female | 46 | Common hepatic duct | Abdominal pain, jaundice | Hilar cholangiocarcinoma | Local excision, Roux-en-Y hepatojejunostomy | 1 cm × 0.8 cm/1 | Tubulovillous adenoma | Well at 3 yr after surgery |
| 5 | Male | 60 | Distal CBD | Abdominal pain and distention | Distal cholangiocarcinoma | Pancreaticoduodenectomy | 1 cm × 1 cm/1 | Tubular adenoma | Well at 6 yr after surgery |
| 6 | Female | 64 | Distal CBD | Abdominal pain, fever, nausea and vomiting | Common bile duct neoplasms | Local excision, Roux-en-Y hepatojejunostomy | 1 cm × 1.2 cm/1 | Tubular adenoma | Well at 6 yr after surgery |
| 7 | Female | 82 | Distal CBD | Abdominal pain, fever, nausea and vomiting | Choledocholithiasis | Local excision and T-tube drainage | 0.9 cm × 0.7 cm/1 | Tubular adenoma | Died after 2 mo due to heart failure |
| 8 | Female | 49 | Distal CBD | Painless jaundice | Distal cholangiocarcinoma | Pancreaticoduodenectomy | 2 cm × 2 cm/1 | Tubulovillous adenoma | Well at 4 yr after surgery |
| 9 | Female | 62 | Left hepatic and common hepatic ducts | Abdominal pain, and fever | Hilar cholangiocarcinoma | Local excision, Roux-en-Y hepatojejunostomy | 3 cm × 2 cm/1 | Tubular adenoma | Well at 5 yr after surgery |
The patients’ laboratory test results were as follows: As for the liver function tests, six of nine patients showed an elevated aspartate transaminase level ranging from 58 to 470 U/L (normal ≤ 40), five of nine patients showed an increased alanine transaminase level ranging from 63 to 253 U/L (normal ≤ 40), eight of nine patients showed an elevated γ-glutamyl transpeptidase level ranging from 85 to 1143 U/L (normal 10-60), six of nine patients showed an elevated alkaline phosphatase level ranging from 144 to 804 U/L (normal 45-125), four of nine patients showed an elevated total bilirubin (TBIL) level ranging from 32.8 to 195 /μmol/L (normal 3.4-17.1), four of nine patients showed an elevated direct (conjugated) bilirubin level ranging from 32.8-195 /μmol/L (normal ≤ 3.4). Tumor marker tests showed that carbohydrate antigen 19-9 was elevated in case 2 (45.3 U/mL) and case 3 (3808 U/mL) with normal level ≤ 39 U/mL. Other tumor markers, including cancer antigen 125 and carcinoembryonic antigen, were normal. Tests for hepatitis B and C virus were negative in all patients.
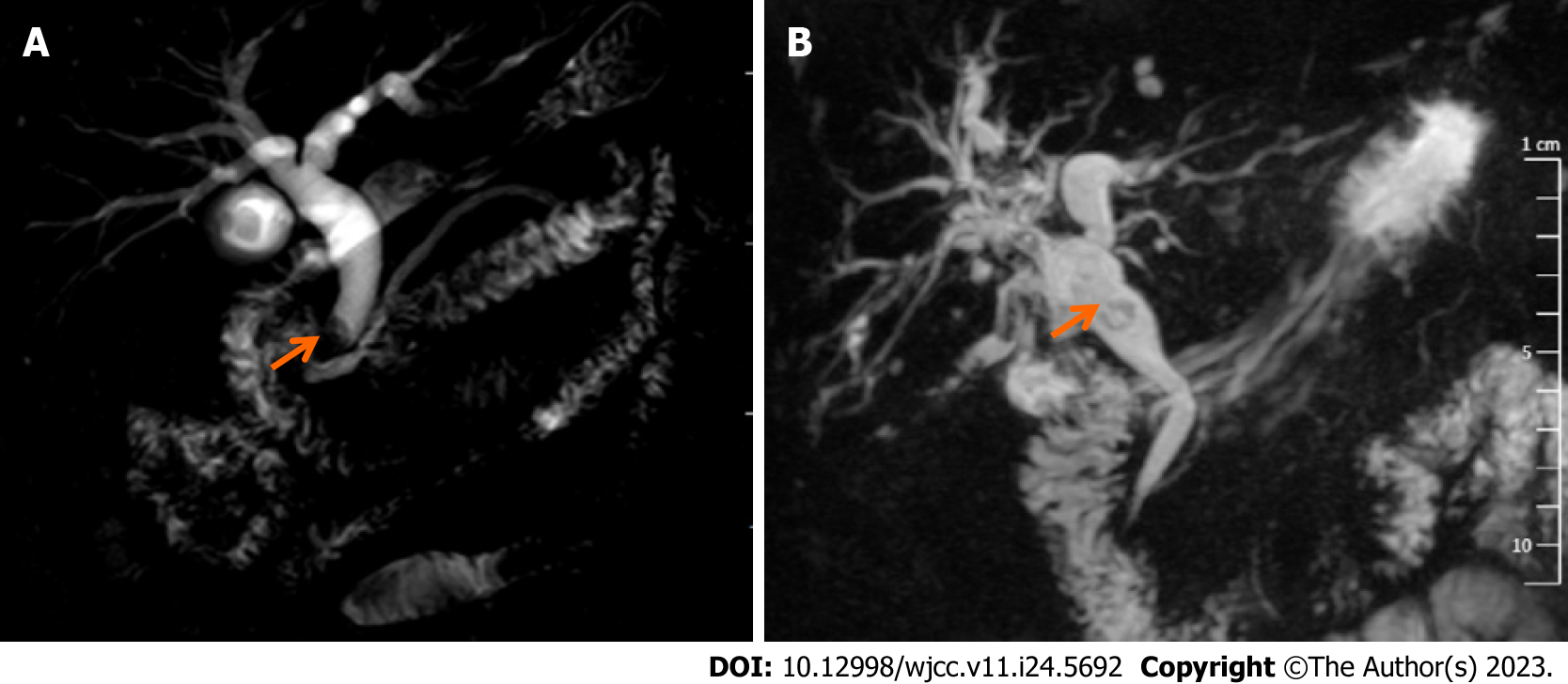
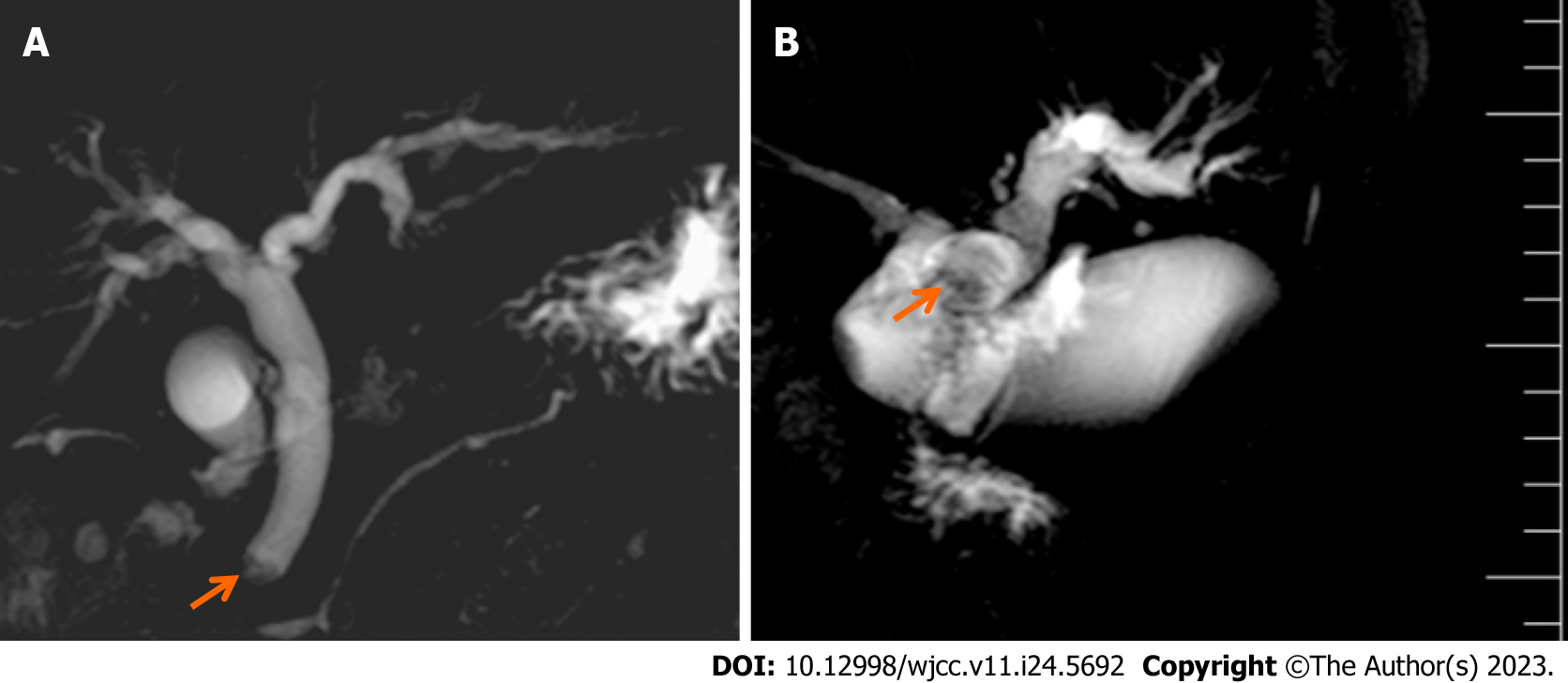
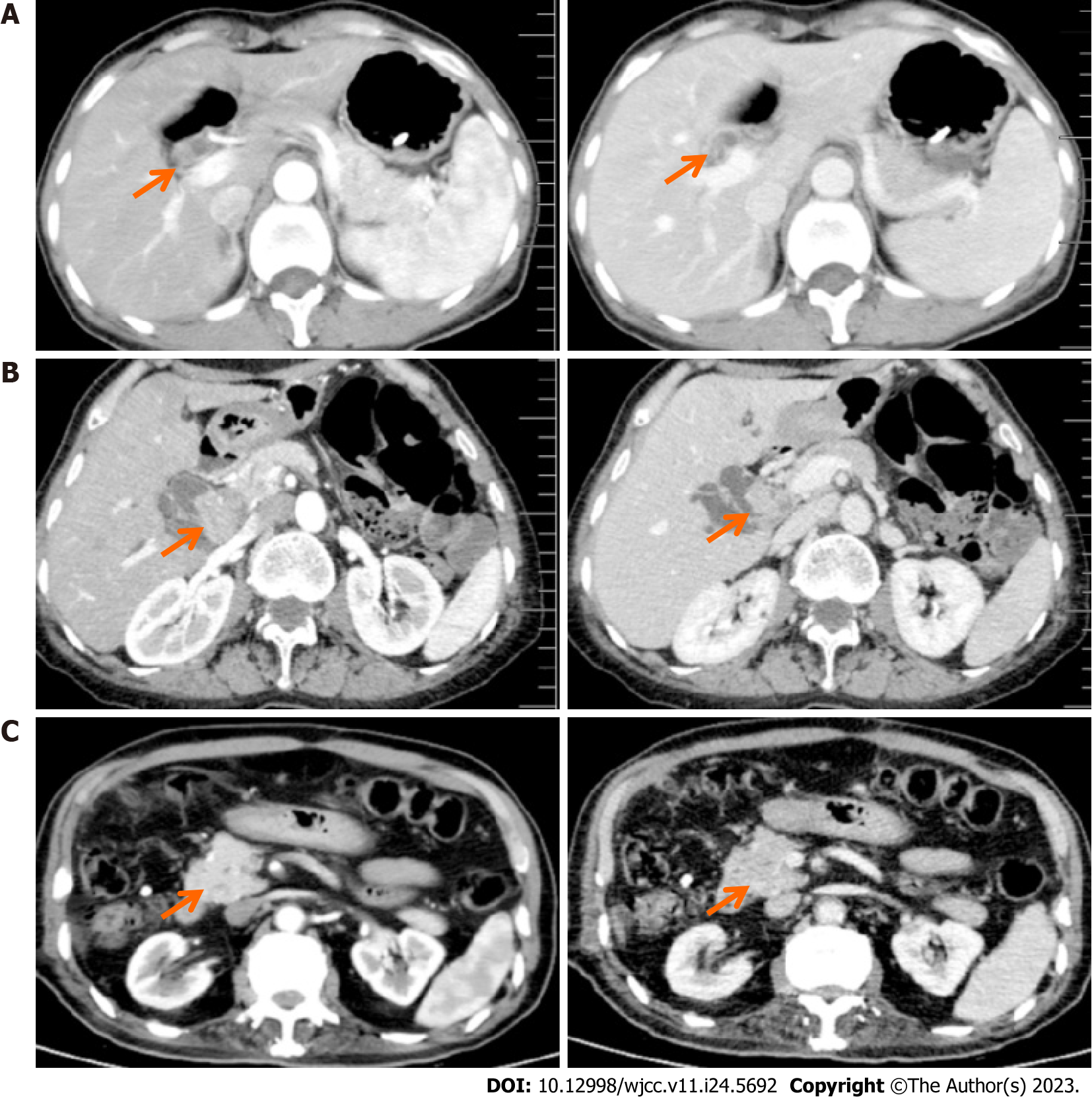
Upon diagnosis, the biliary lesions were identified by abdominal enhanced CT in 8 of 9 cases, MRCP in 7 of 9 cases and ultrasonography in all cases in our patient cohort. Preoperative imaging examinations considered that 6 cases were cholangiocarcinoma and 3 cases were cholangiolithiasis. On the basis of MRCP finding, gallbladder and common bile duct stones were suspected in case 1, while multiple hepatic exterior and interior calculus of bile duct were suspected in case 3 (Figure 1). Distal cholangiocarcinoma and hilar cholangiocarcinoma were suspected in case 5 and case 9, respectively, because of the intra- and extra-hepatic bile duct dilatation and mass in the extrahepatic bile duct (Figure 2). Contrast-enhanced CT revealed a mild enhancement of the mass in arterial phase, along with an enhanced drop or maintenance of enhancement in venous phase (Figure 3). Case 4 and 9 were suspicious for cholangiocarcinoma of the hilar bile duct, while case 2, 5, 6 and 8 were suspicious for cholangiocarcinoma of the distal bile duct.
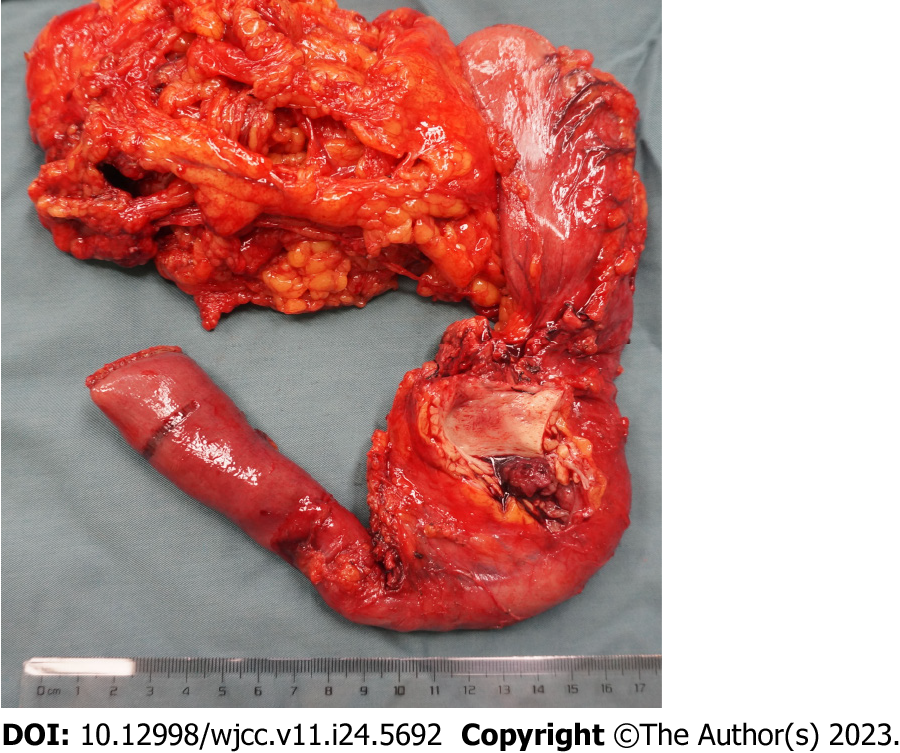
The available treatment modalities used in our patients are presented in Table 1. All the cases received surgical resection [8 in our hospital, 1 in the other hospital (case 3)], involving pancreaticoduodenectomy (PD) (3/9) (Figure 4), partial resection of bile duct and Roux-en-Y hepatojejunostomy (3/9), local lesion resection and T-tube drainage (2/9), local lesion biopsy (direct cholangioscopy with multiple biopsies revealed biliary papillomatosis) and T-tube drainage (1/9). For all the 9 patients, biliary symptoms were improved after the surgery.
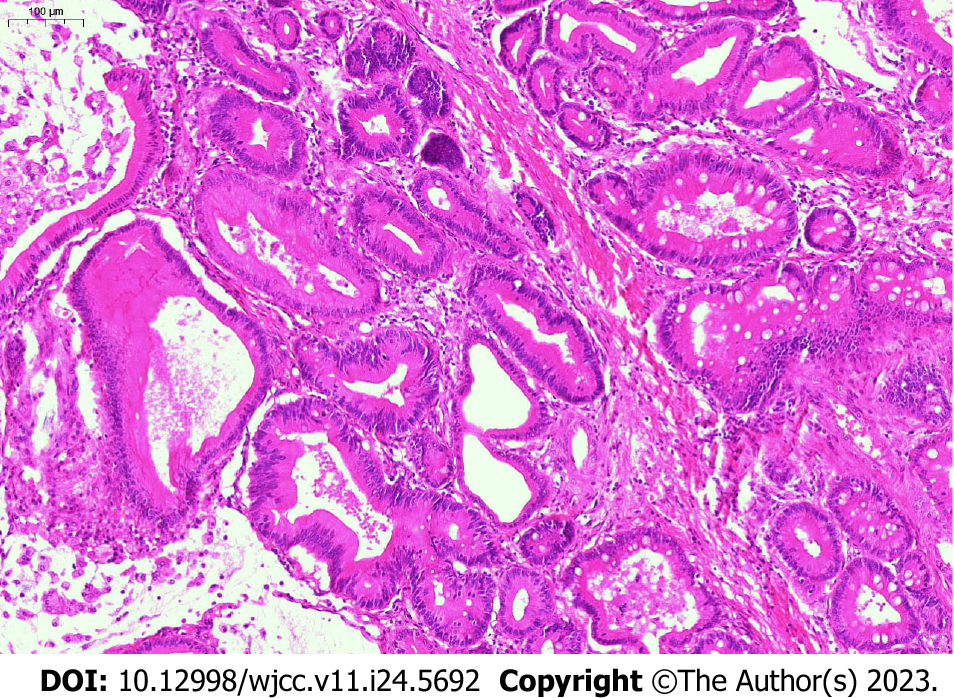
Histological examination (hematoxylin-eosin staining) of surgical specimens revealed adenomas (Figure 5). Histopathologic subtypes from pathological examination results showed that there were 9 cases of simple adenoma tissues (3 papillary adenoma, 4 tubular adenoma and 2 tubulovillous adenoma), 4 cases of mild dysplasia, and 2 cases of moderate to severe dysplasia. The location of adenomas was in the common bile duct (6/9, 66.7%) and common hepatic duct (2/9, 22.2%). One case (1/9, 11.1%) involved multiple ducts in continuity.
Most of the patients had good outcomes and recovered well at follow-up without tumor recurrence (7/9, 77.8%). Long-term follow-up (> 1 year) and short-term follow-up (< 1 year) were in 6 and 3 cases, respectively. By the time of follow-up in April 2023, one patient had died 2 mo after the surgery due to heart failure (case 7). Another patient developed jaundice again after pulling out the T-tube. The obstruction was caused by hilar bile duct obstruction. Subsequently she underwent endoscopic retrograde cholangiopancreatography (ERCP) with plastic stent insertion for the management biliary obstruction (case 3). The longest follow-up were 6 years and the patients (cases 5 and 6) still alive without tumor recurrence.
Benign tumors of extrahepatic bile duct are less common than malignant tumors. They used to be divided into five different types: Tubular, papillary, tubulopapillary, biliary cystadenoma and papillomatosis[6]. In 2019, the World Health Organization classification divides benign epithelial gallbladder and extrahepatic bile duct tumors into adenoma, biliary intraepithelial neoplasia, intracystic papillary neoplasm and intraductal papillary neoplasm of the bile ducts[7]. Extrahepatic biliary adenoma appears to be a disease of older patients, with greater frequency in the sixth decade of human life[2]. The pathogenesis of this disease is not completely understood. It is uncertain whether it is a reactive, hamartomatous, or neoplastic lesion[8].
Early diagnosis is a great challenge because extrahepatic bile duct adenoma does not appear obvious symptoms before the bile duct is blocked by the tumor completely[3]. The most common presenting signs were obstructive jaundice, abdominal pain, fever, cholangitis and abnormal liver function[2,3,6]. However, these atypical symptoms cannot be distinguished from other diseases that cause biliary obstruction, such as cholangiocarcinoma and cholangiolithiasis. Abdominal ultrasound, contrast-enhanced CT scan or MRCP may reveal the dilatation of the intra- or/and extrahepatic bile ducts, a mild enhancement of the soft tissue densities in the bile ducts, or thickened bile duct walls. But these lesions radiologically could mimic carcinoma and choledocholithiasis[4,9]. ERCP combined with choledochoscopy may be an effective diagnostic method, with the unique advantage of obtaining tissue for histopathological analysis[3]. However, sometimes preoperative diagnosis might still not be achieved in spite of repeated ERCP with brush cytology or choledochoscopy with biopsy[10]. In such cases, surgical treatment is necessary.
Surgical resection might be appropriate in patients with apparently localized disease or biliary obstruction. Local endoscopic resection (papillectomy) with sphincterotomy can be successfully carried out in distal intraductal adenomas[11]. Radical resection should be recommended if a malignant tumor is suspected or the tumor exceeds 20 mm[12]. PD should be selected for patients involving cancer of the distal common bile ducts. While local bile duct resection with Roux-en-Y reconstruction might also be curative for high-risk patients who are considered to have benign tumors[6,12]. Liver transplantation is the only curative treatment for patients with diffused biliary papillomatosis. A combination of liver transplantation and PD might be an effective treatment strategy for diffused lesions[3,13].
Histopathology is a gold standard in diagnosis of adenoma. Multiple biopsies are required because of the variation of atypical/dysplasia in the lesion or the development of adenocarcinoma[2]. Differentiating biliary adenoma from cholangiocarcinoma remains a potential challenge, and immunostaining for high mobility group protein A2, thymus cell antigen 1, forkhead box protein P1, forkhead box class O 3a and desmogleins may be helpful[14-16].
Although adenoma is a rare extrahepatic biliary benign tumor, it should be considered in the differential diagnosis of patients with obstructive jaundice or cholangitis. Since there are no special clinical or radiologic features have been described to distinguish it from cholangiocarcinoma or cholangiolithiasis, surgical intervention including cholangioscopy with biopsy is very effective for diagnosis and treatment.
Biliary adenomas occurring in extrahepatic biliary trees are rare. Various imaging tests are difficult to distinguish from cholangiocarcinoma or cholangiolithiasis.
As a rare benign tumor, extrahepatic biliary adenoma is very easy to be misdiagnosed.
To summarize the clinical characteristics and treatment experience of nine patients with extrahepatic biliary adenoma in the First Affiliated Hospital of Xi’an Jiaotong University from 2016 to 2022.
Our study included a total of 9 patients. Laboratory examination, disease diagnosis, treatment, pathological features and follow-up of each patient were evaluated.
Our cohort included 6 women and 3 men with a mean age of diagnosis of 65.1 years (range 46-87). Six cases of extrahepatic biliary adenomas were located in the common bile duct and 3 in the hepatic duct. All patients had biliary symptoms at first, including obstructive jaundice (4/9, 44.4%), abdominal pain (6/9, 66.7%), and fever (3/9, 33.3%). Preoperative imaging showed cholangiocarcinoma in 6 cases and cholangiolithiasis in 3 cases. All patients underwent surgical treatment and were pathologically confirmed as biliary adenomas. The postoperative symptoms of all the 9 patients were significantly improved. Seven of the 9 patients recovered well after surgery without tumor recurrence. One patient died of heart failure 2 mo after surgery. Jaundice recurred in 1 patient 8 mo after surgery, and endoscopic retrograde cholangiopancreatography and biliary stent placement were performed.
Benign extrahepatic biliary tumors are rare and difficult to diagnosis preoperatively. Intraoperative choledochoscopy and timely biopsy may offer great advantages.
The differential diagnosis of obstructive jaundice or cholangitis should be taken into account with extrahepatic biliary adenoma. Since cholangiocarcinoma and cholangiolithiasis do not have clear clinical and imaging features to distinguish them from extrahepatic biliary adenoma, surgical interventions including cholangiographic biopsy are very effective in diagnosis and treatment.
| 1. | Munshi AG, Hassan MA. Common bile duct adenoma: case report and brief review of literature. Surg Laparosc Endosc Percutan Tech. 2010;20:e193-e194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Harvey SC. DNA structural dynamics: longitudinal breathing as a possible mechanism for the B in equilibrium Z transition. Nucleic Acids Res. 1983;11:4867-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Xiao Y, Zhao J, Wu H, Xie KL, Wan Y, Xu XW, Zhang YG. Surgical treatment of malignant biliary papillomatosis invading adjacent organs: A case report. World J Clin Cases. 2019;7:253-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Morris-Stiff GJ, Senda Y, Verbeke CS, Lodge PA. Papillary adenoma arising in the left hepatic duct: an unusual tumour in an uncommon location. Eur J Gastroenterol Hepatol. 2010;22:886-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Kim WJ, Hwang S, Lee YJ, Kim KH, Park KM, Ahn CS, Moon DB, Ha TY, Song GW, Jung DH, Park GC, Kim MH, Lee SK, Seo DW, Park do H, Lee SS, Lee SG. Clinicopathological Features and Long-Term Outcomes of Intraductal Papillary Neoplasms of the Intrahepatic Bile Duct. J Gastrointest Surg. 2016;20:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Čekas K, Rudaitis V, Beiša V, Jotautas V, Rutkauskaitė D, Meškauskas R, Stratilatovas E. Common bile duct villous adenoma: a case report and review of the literature. J Med Case Rep. 2016;10:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2758] [Article Influence: 459.7] [Reference Citation Analysis (3)] |
| 8. | Gonzalez RS, Raza A, Propst R, Adeyi O, Bateman J, Sopha SC, Shaw J, Auerbach A. Recent Advances in Digestive Tract Tumors: Updates From the 5th Edition of the World Health Organization "Blue Book". Arch Pathol Lab Med. 2021;145:607-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Yusif-Zade K, Musayev J, Yeler M. Tubulopapillary adenoma of the common bile duct presenting with jaundice. Ulus Cerrahi Derg. 2016;32:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Sotona O, Cecka F, Neoral C, Ferko A, Rejchrt S, Podhola M, Subrt Z, Jon B. Papillary adenoma of the extrahepatic biliary tract--a rare cause of obstructive jaundice. Acta Gastroenterol Belg. 2010;73:270-273. [PubMed] |
| 11. | Bohnacker S, Soehendra N, Maguchi H, Chung JB, Howell DA. Endoscopic resection of benign tumors of the papilla of vater. Endoscopy. 2006;38:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kim BS, Joo SH, Joo KR. Carcinoma in situ arising in a tubulovillous adenoma of the distal common bile duct: a case report. World J Gastroenterol. 2008;14:4705-4708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Imvrios G, Papanikolaou V, Lalountas M, Patsiaoura K, Giakoustidis D, Fouzas I, Anagnostara E, Antoniadis N, Takoudas D. Papillomatosis of intra- and extrahepatic biliary tree: Successful treatment with liver transplantation. Liver Transpl. 2007;13:1045-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | He J, Yang Z, Wu Z, Wang L, Xu S, Zou Q, Yuan Y, Li D. Expression of FOXP1 and FOXO3a in extrahepatic cholangiocarcinoma and the implications in clinicopathological significance and prognosis. Onco Targets Ther. 2019;12:2955-2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Liu R, Yang Z, Huang S, Li D, Zou Q, Yuan Y. The expressions of HMGA2 and Thy1 in extrahepatic cholangiocarcinoma and their clinicopathological significances. Surg Oncol. 2019;29:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Xu S, Huang S, Li D, Zou Q, Yuan Y, Yang Z. Negative Expression of DSG1 and DSG2, as Prognostic Biomarkers, Impacts on the Overall Survival in Patients with Extrahepatic Cholangiocarcinoma. Anal Cell Pathol (Amst). 2020;2020:9831646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Aoki H, Japan; Ker CG, Taiwan; Yildiz K, Turkey S-Editor: Wang JJ L-Editor: A P-Editor: Yuan YY