Published online Aug 26, 2023. doi: 10.12998/wjcc.v11.i24.5678
Peer-review started: May 19, 2023
First decision: June 1, 2023
Revised: June 2, 2023
Accepted: August 3, 2023
Article in press: August 3, 2023
Published online: August 26, 2023
Processing time: 97 Days and 23 Hours
Hypertension is a common chronic disease that affects many people worldwide. Only a few reports related to the exploration of relevant indicators of the pre
To detect prethrombotic state-related indicators in patients with PH and analyze their differences in different patient populations to provide a laboratory basis for the clinical prevention and control of hypertensive thrombotic diseases.
The general data of patients with PH who attended the Department of Cardio
No significant differences were observed in age, sex, diabetes mellitus, smoking history, drinking history, body mass index, New York Heart Association functional classification, or the course of hypertension among the four groups (P > 0.05). The expressions of high-sensitivity C-reactive protein (hs-CRP), thrombomodulin (TM), hematocrit (Hct), erythrocyte sedimentation rate (ESR), P-selectin on platelet surface (CD62P), and fibrinogen (FIB) in the control group were < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group, and the expressions of platelet (PLT), activated partial thromboplastin time (APTT), prothrombin (PT), and plasma thrombin time (TT) in the control group was > Grade 1 hypertension group > Grade 2 hypertension group > Grade 3 hypertension group, and the difference was statistically significant (P < 0.05). The results of the multivariate logistic regression model showed that the expression of hs-CRP, TM, Hct, ESR, CD62P, PLT, APTT, PT, TT, and FIB in the included participants was related to the progression of PH. Among these, high expression of hs-CRP, TM, Hct, ESR, CD62P, APTT, PT, and TT, and low expression of PLT and FIB were risk factors for PH (OR > 1, P < 0.05). The results of the receiver operating characteristic curve analysis showed that the area under the curve of hs-CRP, TM, ESR, CD62P, APTT, PT, TT, and FIB for the prediction of PH were > 0.80, and the prediction value was ideal. Linear correlation analysis with bivariate Spearman showed that hs-CRP, TM, Hct, ESR, CD62P, APTT, PT, and TT were positively correlated with each other (r > 0, P < 0.05); PLT and FIB were negatively correlated with hs-CRP, TM, Hct, ESR, CD62P, APTT, PT, and TT (r < 0, P < 0.05); and PLT and FIB were positively correlated (r > 0, P < 0.05). Linear correlation analysis using bivariate Spearman showed that hs-CRP, TM, Hct, ESR, CD62P, and FIB were positively correlated with each other (r > 0, P < 0.05), whereas PLT, APTT, PT, and TT were negatively correlated with hs-CRP, TM, Hct, ESR, CD62P, and FIB (r < 0, P < 0.05). There was a positive correlation between PLT, APTT, PT, and TT (r > 0, P < 0.05).
The relevant indicators of the prethrombotic state in patients with PH, such as hs-CRP, TM, Hct, ESR, CD62P, PLT, APTT, PT, TT, and FIB, showed differences. High expression of hs-CRP, TM, Hct, ESR, CD62P, and FIB, and low expression of PLT, APTT, PT, and TT are the keys to the occurrence, progression, and thrombotic state of PH. Based on the above serum indicators’ expression in patients, targeted interventions can be administered to patients with abnormal expression levels to control the progression of their disease and reduce the risk of developing a prethrombotic state.
Core Tip: This study aimed to identify prethrombotic state-related indicators in primary hypertension (PH) patients and analyze their differences in different PH patient populations. The study found that high expression of inflammation-related indicators, vascular endothelial injury-related indicator, hemorheology-related indicators, platelet-related indicator, and coagulation function related indicators were risk factors for PH. Meanwhile, low expression of platelet and fibrinogen was also a risk factor. The study suggested that targeted interventions could be administered based on abnormal expression levels to control disease progression and reduce the risk of developing a prethrombotic state.
- Citation: Luo J, Yang T, Ding L, Xiong JH, Ying T, Xu F. Relevant detection indicator of prethrombotic state in patients with primary hypertension. World J Clin Cases 2023; 11(24): 5678-5691
- URL: https://www.wjgnet.com/2307-8960/full/v11/i24/5678.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i24.5678
Primary hypertension (PH) is a chronic disease characterized by increased systemic arterial blood pressure and is considered a risk factor for cardiovascular disease in older adults. If blood pressure is maintained at a high level for a long time without timely and effective treatment at an early stage of the disease, it will directly lead to heart failure, coronary heart disease, kidney disease, and other adverse complications, leading to an increased risk of death[1,2]. According to a global survey of chronic diseases, new cases of PH have been increasing annually in recent years, and a younger onset has become obvious. Currently, this disease is a major public health problem[3]. The World Health Organization’s reports emphasize that PH can significantly increase the risk of heart disease, stroke, kidney disease, and other health issues. In view of the high incidence of PH and the great difficulty in its control, some reports point out that early detection, diagnosis, and intervention are particularly important for reasonably controlling blood pressure levels and reducing morbidity risks such as cardiovascular complications and stroke[4,5].
The pathophysiology of PH and its complications have been the focus of many studies. Hypertension is the main cause of cardiovascular disease and death, and the prevention and treatment of hypertension have become the top priority in the prevention and treatment of cardiovascular disease. Impaired vascular endothelium and the release of inflammatory factors in patients with hypertension are the main factors leading to a prethrombotic state, which is an important intermediate link in the processes of myocardial infarction, stroke, and other complications in patients with hypertension[6,7]. Relevant studies have shown that hypertension leads to vascular inflammation and endothelial dysfunction. Inflammation can promote endothelial dysfunction, resulting in changes in the platelet, endothelial, coagulation, and fibrinolytic pathways, finally inducing the occurrence of a prethrombotic state or maintenance of a hypercoagulable state[8,9]. Therefore, observation of prethrombotic state-related indicators in patients with PH is particularly crucial for reducing the risk of thrombotic related diseases in these patients. Only a few reports related to the exploration of relevant indicators of the prethrombotic state in patients with PH in clinical settings were available. Therefore, we analyzed the differences in these indicators of prethrombotic state in patients with PH from different patient populations to provide a laboratory basis for the clinical prevention and control of the occurrence and development of hypertensive thrombotic diseases.
In this study, general data on patients with PH who attended the Department of Cardiovascular Medicine, The First Affiliated Hospital of Jiangxi Medical College, from January 2022 to December 2022 was collected retrospectively while strictly adhering to the relevant provisions of the Medical Ethics Committee. The enrolled patients were divided into three groups based on their PH Grade: 40 patients in the Grade 1 hypertension experimental group (28 males and 12 females); 40 patients in the Grade 2 hypertension experimental group (25 males and 15 females); and 40 patients in the Grade 3 hypertension experimental group (23 males and 17 females). The baseline data of 40 volunteers who underwent physical examinations in our hospital during the same period but were not diagnosed with PH were collected and included in the control group (25 males and 15 females). The baseline data of the above four groups of participants were compared. The balance was ideal (P > 0.05), and the study was conducted (details of baseline data comparison are shown in Table 1).
| Baseline data | Grade 1 hypertension experimental group (n = 40) | Grade 2 hypertension experimental group (n = 40) | Grade 3 hypertension experimental group (n = 40) | Control group (n = 40) | Statistical values | P value | |
| Age (yr) | < 60 | 31 | 29 | 30 | 28 | χ2 = 0.646 | 0.886 |
| ≥ 60 | 9 | 11 | 10 | 12 | |||
| Sex | Male | 28 | 25 | 23 | 25 | χ2 = 1.369 | 0.713 |
| Female | 12 | 15 | 17 | 15 | |||
| Combined diabetes | Yes | 10 | 8 | 9 | 10 | χ2 = 0.387 | 0.946 |
| No | 30 | 32 | 31 | 30 | |||
| BMI (kg/m2) | ≤ 24 | 15 | 14 | 15 | 13 | χ2 = 0.300 | 0.960 |
| > 24 | 25 | 26 | 25 | 27 | |||
| Smoking history | Yes | 12 | 10 | 12 | 11 | χ2 = 0.340 | 0.952 |
| No | 28 | 30 | 28 | 29 | |||
| Drinking history | Yes | 10 | 11 | 13 | 11 | χ2 = 0.587 | 0.899 |
| No | 30 | 29 | 27 | 29 | |||
| NYHA cardiac functional classification | Grade I | 33 | 31 | 32 | 33 | Z = 0.588 | 0.557 |
| Grade II | 6 | 7 | 8 | 7 | |||
| Grade III | 1 | 2 | 0 | 0 | |||
| Grade IV | 0 | 0 | 0 | 0 | |||
| Course of hypertension (mean ± SD, yr) | 5.25 ± 2.15 | 5.35 ± 2.25 | 5.47 ± 2.17 | - | F = 0.110 | 0.896 | |
(1) All patients with PH referred to the relevant contents in the China Guidelines for the Prevention and Treatment of Hypertension (Revised Version in 2018)[10]: In the resting state without using any antihypertensive drugs, the systolic blood pressure (SBP) of the patients was ≥ 140 mmHg and/or the diastolic blood pressure (DBP) was ≥ 90 mmHg. The PH was determined by taking measurements three times on a different day or above for all patients; (2) All enrolled patients were aware of the purpose and method of this study, and signed the consent form voluntarily; and (3) Baseline data of the included patients, including laboratory results, were well-maintained for the purposes of this study.
(1) Patients with organic diseases such as hypertensive encephalopathy and hypertensive nephropathy; (2) complications of thrombotic hemorrhagic diseases; (3) previous medication history of combined administration of anti-anxiety and anti-depression drugs, which led to decreased compliance of patients and prevented them from cooperating with the research smoothly; (4) combined use of drugs and other bad habits; (5) complications of cachexia such as tumor; (6) patients who took antiplatelet and anticoagulant drugs such as warfarin, aspirin enteric-coated tablet, and heparin within 3 mo of enrollment; (7) pregnant women and those who menstruating; (8) co-infection or immune system disease, and for those for whom the clinical treatment effect is not ideal; and (9) combination of decreased coagulation system function.
Based on the purpose and study method, the statistical table of baseline data was designed to include sex (male/female), age (< 60 years/≥ 60 years), accompanied with diabetes (Yes/No, diabetes was diagnosed according to the diagnosis and treatment standards in China Guidelines for the Prevention and Treatment of Type 2 Diabetes, 2017 Edition)[11], body mass index (BMI) (≤ 24 kg/m2, > 24 kg/m2, which was divided into: low weight: < 18.5 kg/m2, normal: 18.5-23.9 kg/m2, and overweight/obesity: ≥ 24 kg/m2) according to the relevant contents of China Guidelines for Prevention and Control of Adult Overweight and Obesity[11,12], smoking history (Yes/No, Continuous/cumulative smoking for more than half a year), drinking history (Yes/No, and drinking more than once a month in the past 12 mo), New York Heart Association (NYHA)[13] cardiac function classification (Grade I: Daily activities were not restricted, and common physical activities did not cause patients to feel shortness of breath, palpitation, and fatigue; Grade II: Mild limitation, and easy daily physical activity, may cause patients to have shortness of breath, palpitations, fatigue, and other uncomfortable feelings; Grade III: Limitation is obvious, and lower than normal daily activity level cause patients to experience shortness of breath, palpitation, fatigue, and other uncomfortable feelings; Grade IV: Limited activities and unable to carry out any activity, patients to experience shortness of breath, palpitation, fatigue, and other uncomfortable feelings even in the rest state), course of PH, and blood pressure status.
According to the DBP and SBP of patients, they were divided into: Grade 1 hypertension: SBP 140-159 mmHg and/or DBP 90-99 mmHg; Grade 2 hypertension: SBP 160-179 mmHg and/or DBP 100-109 mmHg; Grade 3 hypertension: SBP ≥ 180 mmHg and/or DBP ≥ 110 mmHg. When DBP and SBP belonged to different grades, whichever came first was determined to be the higher grade.
High-sensitivity C-reactive protein (hs-CRP): 5 mL of peripheral venous blood from all participants in the fasting state was collected shortly after waking up in the morning, and the supernatant was collected after centrifugation at 3000 r/min (centrifugal radius: 15 cm) for 15 min. The hs-CRP levels in serum samples were detected by immunoturbidimetry.
Hematocrit (Hct) and erythrocyte sedimentation rate (ESR): 5 mL of venous blood was collected from patients in the fasting state. Hct was detected using a BC-5000 automatic blood cell analyzer (Shenzhen Meirui Biomedical Electronics Co., Ltd.), and ESR was detected using a SECCO SD-100 dynamic ESR instrument.
Thrombomodulin (TM): 5 mL of fasting venous blood collected from the morning onwards was centrifuged at 3000 r/min (centrifugal radius: 15 cm) for 15 min, and the supernatant was collected. Serum TM expression was measured using ELISA.
Platelet count (PLT) and P-selectin on the platelet surface (CD62P): 5 mL fasting elbow venous blood was collected, PLT was detected using a BC-5000 automatic blood cell analyzer (Shenzhen Meirui Biomedical Electronics Co., Ltd.), and CD62P expression was detected using flow cytometry (Beckman Coulter EPICSXL, US).
Activated partial thromboplastin time (APTT), prothrombin time (PT), plasma thrombin time (TT), and fibrinogen (FIB): 5 mL of fasting venous blood from morning onwards was collected and centrifuged at high speed (3000 r/min) for 10 min at 4℃ for the effective separation of upper serum. The expression levels of APTT, PT, TT, and FIB were determined using a Hitachi 7600 automatic biochemical analyzer (Hitachi, Japan). All indicators were tested in strict accordance with the manufacturer’s instructions.
SPSS24.0 software was used for the data processing. All measurement data were subjected to the Shapiro-Wilk normality test. Data that conformed to the normal distribution were expressed as mean ± SD. An independent sample t-test was used for comparisons between groups, and repeated-measures analysis of variance was used for comparisons of single indicators among multiple groups. The count data were expressed as percentages using χ2 test, and the correlation between variables was analyzed using bivariate Spearman linear correlation analysis. The relationship between each indicator and patients with PH was tested using Logistic regression analysis. The receiver operating characteristic (ROC) curves of the patients were drawn to test the predictive value of the main indicators in PH. The following definitions were used for evaluation by the area under the curve (AUC), AUC ≤ 0.50: no predictive value, 0.50 < AUC ≤ 0.70: low predictive value, 0.70 < AUC ≤ 0.90: medium predictive value, and AUC > 0.90: high predictive value. P < 0.05 indicated that the difference was statistically significant.
There was no significant difference in age, sex, concomitant diabetes, smoking history, drinking history, BMI, and NYHA functional classification data of subjects among the four groups (P > 0.05). No significant difference was observed in the course of disease of patients with hypertension among the three groups (P > 0.05), as shown in Table 1.
The expression levels of hs-CRP and TM in the control group were < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group, and the differences were statistically significant (P < 0.05). The results are shown in Table 2.
| Group | Number of patients | hs-CRP (mmol/L) | TM (μg/L) |
| Grade 1 hypertension experimental group | 40 | 2.05 ± 0.85 | 20.15 ± 1.05 |
| Grade 2 hypertension experimental group | 40 | 4.15 ± 1.05 | 22.85 ± 1.35 |
| Grade 3 hypertension experimental group | 40 | 5.45 ± 1.20 | 26.12 ± 2.25 |
| Control group | 40 | 1.72 ± 0.32 | 5.41 ± 1.20 |
| F value | - | 149.720 | 1420.213 |
| P value | - | < 0.001 | < 0.001 |
The expressions of Hct and ESR in the control group were < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group. The differences were statistically significant (P < 0.05) as shown in Table 3.
| Group | Number of cases | Hct (%) | ESR (mm/h) |
| Grade 1 hypertension experimental group | 40 | 40.85 ± 3.15 | 30.25 ± 5.85 |
| Grade 2 hypertension experimental group | 40 | 41.25 ± 3.45 | 42.15 ± 6.25 |
| Grade 3 hypertension experimental group | 40 | 43.25 ± 4.25 | 60.15 ± 8.15 |
| Control group | 40 | 39.56 ± 2.85 | 25.12 ± 5.05 |
| F value | - | 7.810 | 234.107 |
| P value | - | < 0.001 | < 0.001 |
CD62P expression in the control group was < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group. The PLT expression in the control group was > Grade 1 hypertension group > Grade 2 hypertension group > Grade 3 hypertension group and showed statistically significant differences (P < 0.05), as listed in Table 4.
| Group | Number of patients | CD62P (%) | PLT (× 109) |
| Grade 1 hypertension experimental group | 40 | 8.85 ± 1.18 | 115.68 ± 40.15 |
| Grade 2 hypertension experimental group | 40 | 11.12 ± 1.35 | 108.56 ± 35.85 |
| Grade 3 hypertension experimental group | 40 | 13.10 ± 1.75 | 95.25 ± 30.12 |
| Control group | 40 | 6.25 ± 1.05 | 135.25 ± 50.12 |
| F value | - | 190.280 | 7.052 |
| P value | - | < 0.001 | < 0.001 |
The expressions of APTT, PT, and TT in the control group were > Grade 1 hypertension group > Grade 2 hypertension group > Grade 3 hypertension group, and the expression of FIB in the control group was < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group. The differences were statistically significant (P < 0.05), as shown in Table 5.
| Group | Number of patients | APTT (s) | PT (s) | TT (s) | FIB (g/L) |
| Grade 1 hypertension experimental group | 40 | 29.25 ± 2.71b | 11.98 ± 0.50b | 15.95 ± 1.08b | 3.20 ± 0.51b |
| Grade 2 hypertension experimental group | 40 | 27.25 ± 2.24a | 10.82 ± 0.92a | 15.01 ± 0.89a | 5.12 ± 0.68a |
| Grade 3 hypertension experimental group | 40 | 25.42 ± 1.52a | 10.02 ± 0.34a | 14.02 ± 0.65a | 6.19 ± 1.15a |
| Control group | 40 | 30.12 ± 2.65 | 12.31 ± 0.52 | 16.08 ± 1.12 | 3.01 ± 0.42 |
| F value | - | 315.25 | 105.522 | 174.218 | 82.151 |
| P value | - | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The expression of the aforementioned indicators in the serum of the enrolled patients was taken as a covariate and PH as a dependent variable (1 = Yes, 0 = No) to establish a multivariate Logistic regression model. The results showed that the expressions of hs-CRP, TM, Hct, ESR, CD62P, PLT, APTT, PT, TT, and FIB in the enrolled patients were related to the progression of PH. High expressions of hs-CRP, TM, Hct, ESR, CD62P, APTT, PT, and TT and low expressions of PLT and FIB were risk factors for PH (OR > 1, P < 0.05), as shown in Table 6.
| Variable | B | SE | Wals | P value | OR value | 95%CI | |
| Upper limit | Lower limit | ||||||
| Constant | -2.717 | 0.665 | 16.720 | < 0.001 | 0.066 | - | - |
| hs-CRP | 1.535 | 0.307 | 25.086 | < 0.001 | 4.643 | 2.546 | 8.468 |
| TM | 0.684 | 0.248 | 7.584 | 0.006 | 1.982 | 1.218 | 3.226 |
| Hct | 0.184 | 0.058 | 10.219 | 0.001 | 1.202 | 1.074 | 1.346 |
| ESR | 0.226 | 0.042 | 29.282 | < 0.001 | 1.254 | 1.155 | 1.361 |
| CD62P | 2.261 | 0.486 | 21.684 | < 0.001 | 9.592 | 3.704 | 24.842 |
| PLT | 0.017 | 0.005 | 12.958 | < 0.001 | 1.017 | 1.008 | 1.027 |
| APTT | 0.716 | 0.128 | 11.664 | < 0.001 | 1.926 | 1.421 | 2.158 |
| PT | 0.805 | 0.148 | 15.660 | < 0.001 | 2.192 | 1.605 | 2.840 |
| TT | 0.924 | 0.108 | 13.348 | < 0.001 | 2.490 | 1.808 | 3.418 |
| FIB | 5.212 | 0.863 | 19.566 | < 0.001 | 88.423 | 18.137 | 427.013 |
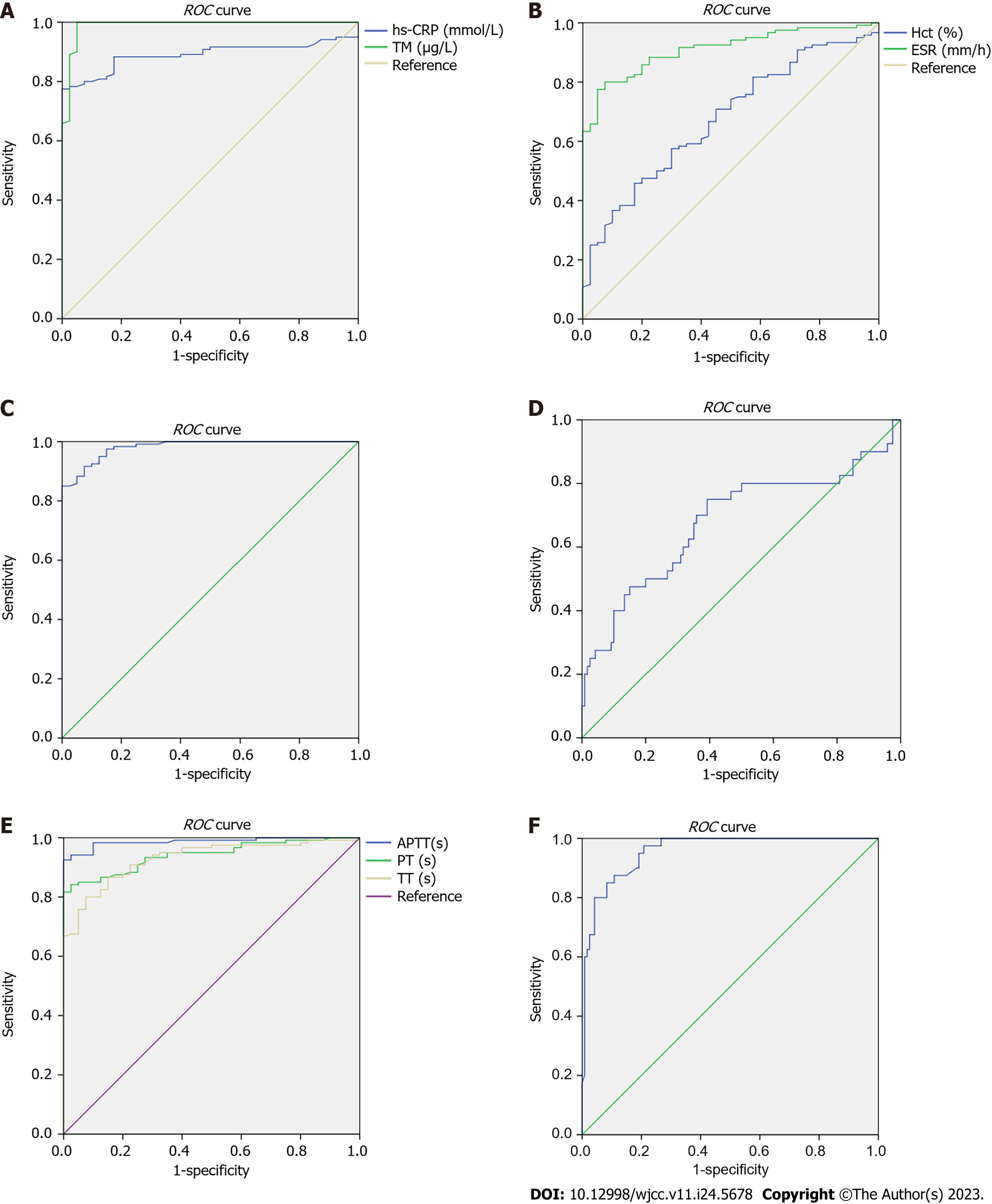
The expression of the aforementioned serum indicators in the enrolled patients was taken as a test variable, and the condition of PH was taken as state variable (1 = Yes, 0 = No) to draw the ROC curve (Figure 1). The results of ROC curve analysis showed that the AUC of hs-CRP, TM, ESR, CD62P, APTT, PT, TT, and FIB for the prediction of PH was > 0.80, and the prediction value was ideal, as shown in Table 7.
| Indicator | AUC | 95%CI for AUC | Standard error | P value | Cut-off value | Sensitivity | Specificity |
| hs-CRP | 0.890 | 0.840-0.941 | 0.026 | < 0.001 | 1.135 | 0.950 | 0.975 |
| TM | 0.989 | 0.972-1.000 | 0.009 | < 0.001 | 18.345 | 0.992 | 0.05 |
| Hct | 0.676 | 0.586-0.766 | 0.046 | 0.001 | 34.990 | 0.967 | 0.975 |
| ESR | 0.911 | 0.867-0.955 | 0.022 | < 0.001 | 17.365 | 0.992 | 0.975 |
| CD62P | 0.982 | 0.966-0.997 | 0.008 | < 0.001 | 6.635 | 0.992 | 0.325 |
| PLT | 0.680 | 0.572-0.788 | 0.055 | 0.001 | 47.290 | 0.975 | 0.975 |
| APTT | 0.987 | 0.973-1.000 | 0.007 | < 0.001 | 38.650 | 0.992 | 0.650 |
| PT | 0.942 | 0.908-0.975 | 0.017 | < 0.001 | 12.810 | 0.992 | 0.875 |
| TT | 0.929 | 0.890-0.968 | 0.020 | < 0.001 | 12.680 | 0.992 | 0.975 |
| FIB | 0.957 | 0.929-0.986 | 0.015 | < 0.001 | 2.110 | 0.975 | 0.267 |
The linear correlation analysis using bivariate Spearman showed that hs-CRP, TM, Hct, ESR, CD62P, and FIB were positively correlated with each other (r > 0, P < 0.05), whereas PLT, APTT, PT, and TT were negatively correlated with hs-CRP, TM, Hct, ESR, CD62P, and FIB (r < 0, P < 0.05). There was a positive correlation between PLT, APTT, PT, and TT levels (r > 0, P < 0.05), as shown in Table 8.
| Indicator | hs-CRP | TM | Hct | ESR | CD62P | PLT | APTT | PT | TT | FIB |
| hs-CRP | - | 0.792a | 0.237 (0.003) | 0.782a | 0.753a | -0.296a | -0.794a | -0.652a | -0.631a | 0.802a |
| TM | 0.792a | - | 0.300a | 0.818a | 0.813a | -0.329a | -0.856a | -0.734a | -0.682a | 0.846a |
| Hct | 0.237 (0.003) | 0.300a | - | 0.317a | 0.279a | -0.113 (0.153) | -0.295a | -0.201 (0.011) | -0.279a | 0.318a |
| ESR | 0.782a | 0.818a | 0.317a | - | 0.788a | -0.267 (0.001) | -0.821a | -0.665a | -0.626a | 0.830a |
| CD62P | 0.753a | 0.813a | 0.279a | 0.788a | - | -0.246 (0.002) | -0.850a | -0.707a | -0.668a | 0.814a |
| PLT | -0.296a | -0.329a | -0.113 (0.153) | -0.267 (0.001) | -0.246 (0.002) | - | 0.331a | 0.214 (0.007) | 0.223 (0.005) | -0.272 (0.001) |
| APTT | 0.794a | 0.856a | 0.295a | 0.821a | 0.850a | -0.331 | - | 0.677a | 0.681a | -0.843a |
| PT | 0.652a | 0.734a | 0.201 (0.011) | 0.665a | 0.707a | -0.214 (0.007) | 0.677a | - | 0.534a | -0.739a |
| TT | 0.631a | 0.682a | 0.279a | 0.626a | 0.668a | -0.223 (0.005) | 0.681a | 0.534a | - | -0.674a |
| FIB | 0.802a | 0.846a | 0.318a | 0.830a | 0.814a | -0.272 (0.001) | -0.843a | -0.739a | -0.674a | - |
At present, the pathogenesis of PH has not been fully elucidated in clinical practice. It is widely accepted that increased smooth muscle tension in peripheral arterioles and their increased responsiveness to vasoactive substances, such as angiotensin, 5-hydroxytryptamine, and catecholamine, induce corresponding functional changes. These changes can, in turn, cause structural changes in blood vessels, and ultimately result in hypertension-related symptoms due to increased peripheral vascular resistance[15,16]. The prethrombotic state, also known as hypercoagulable state, is a pathological process in which many factors cause dysfunction in coagulation, hemostasis, fibrinolysis, anticoagulation, and other systems. The pre-thrombotic state in PH is closely associated with target organ damage and long-term prognosis. Therefore, correcting coagulation and fibrinolysis dysfunction while reasonably controlling blood pressure is very important for reducing the risk of its complications[17,18].
The synthesis rate and amount of hs-CRP, a marker of the inflammatory response, are both low in a healthy individual. When the body suffers from inflammation and injury, the expression of hs-CRP can increase rapidly within a short time, playing an important role in regulating the function of phagocytes, activating complement, and eliminating apoptosis, necrosis, and damaged cell tissues in the body[19,20]. The results of this study showed that the expression of hs-CRP in the control group was < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group, indicating that high expression of hs-CRP might be an important factor leading to the progression of PH and induction of a prethrombotic state. The reasons for this analysis may be as follows: First, the chronic inflammatory state induced by hs-CRP leads to the transformation of vascular endothelium to pro-inflammatory and pro-coagulation surfaces, leading to damage to the vascular system and causing endothelial dysfunction. Local hypercoagulable states lead to the formation of fibrin, platelet aggregation, and other coagulation-fibrinolysis system disorders, finally inducing PH; if not properly controlled, it will further induce a prethrombotic state[21,22]. Second, the inflammatory state of the body causes urate crystals to deposit in the blood vessels, which over time causes the blood to hypercoagulate and induce thrombosis[23]. Hct indirectly reflects the volume and size of red blood cells and determines the effect of red blood cells on blood viscosity[24]. The high expression of Hct leads to a reduction of erythrocyte deformability and volume, increase in blood viscosity, significant increase in blood pressure on one hand, and a retardation of blood flow due to the increase in blood viscosity and blood flow resistance on the other hand. This leads to the accumulation of metabolic indicators in the blood, such as uric acid and glucose, in large quantities on the vascular wall, affecting the body's blood pressure level[25,26]. ESR refers to the rate of erythrocyte sedimentation at rest. Owing to the interference of the negative charge on the cell membrane surface, the ESR is dispersed and decreases. The higher the ESR is, the higher the ESR becomes. Therefore, ESR is often used as an evaluation index for tumors and inflammation in clinic[27,28]. The results of this study showed that the expressions of Hct and ESR in the control group were < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group, suggesting that the high expressions of Hct and ESR were related to the induction of PH. The reasons for analysis results may be as follows: (1) High expression of Hct can lead to increased red blood cell count and blood viscosity, increased vascular resistance, and significantly increased cardiovascular load, which in turn can lead to increased body blood pressure and the occurrence of PH; (2) The increase of blood viscosity leads to slow blood flow, hypoxia of organs and tissues, and intracellular release of cytokines such as angiotensin, resulting in thickening and decreased elasticity of the vascular wall, and inducing PH; and (3) The red blood cell surface is negatively charged. When Hct is highly expressed, the number of blood cells decreases and the electrical load is alleviated. The agglutination reaction occurs at an increased speed, and precipitation is accelerated. As a result, the blood becomes hypercoagulable, with the flow rate reducing and blood pressure increasing, which is more likely to induce PH[29-31]. Platelet activation, endothelial damage, and the subsequent release of bioactive substances are important processes during the prethrombotic state[32]. TM, a specific endothelial cell receptor, mainly distributed as a single-chain glycoprotein on the surface of vascular endothelial cells, which mediates the rapid activation of the anticoagulant factor protein C and causes specific changes in the thrombin substrate; thus, playing an important role in the body's anticoagulant reaction. When its expression in plasma is increased, it accelerates platelet accumulation, which is closely related to cardiovascular and cerebrovascular diseases[33-35]. Endothelial cells are the only interface where tissue contacts the blood. Local endothelial cells exhibit antithrombotic activity and are vulnerable to functional interface[36]. The results of this study showed that the expression level of TM in the control group was as follows: Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group, suggesting that TM was related to the formation of PH. The possible reasons for this observation were as follows: When the blood pressure increased, the vascular endothelium was damaged and thrombogenic endothelial components such as microfibrils and collagen were exposed; the damaged endothelial cells released substances such as von Willebrand factor and P-selectin, causing local leukocyte aggregation, platelet adhesion, and smooth muscle contraction. Persistent abnormal blood circulation occurred, which in turn accelerated blood viscosity and caused PH[37,38]. The biological effects of PLT include the release of substances rich in hemostatic components, acceleration of blood coagulation, and prevention of vascular endothelial damage. High PLT expression can induce thrombosis[39]. CD62P, an adhesion molecule belonging to the selectin family, is distributed on the surface of the platelet membrane. When its high expression in the blood promotes thrombosis, it is followed by CD62P, which belongs to the lysosomal membrane-intact membrane glycoprotein. When platelets are activated, they are released into the blood along with their shedding. Their high expression in the peripheral blood is a key indicator for the clinical evaluation of platelet activation and release function[40,41]. P-selectin is synthesized in platelets and endothelial cells, and is primarily located in the α particles of endothelial cell coryneform bodies and platelets. P-selectin plays a key role in platelet adhesion to endothelial cells, and also mediates interaction between platelets, platelet monocytes, and neutrophils. This interaction activates neutrophils, releases many vasoactive substances, such as active enzymes and cytokines, and damages endothelial cells. This causes several biological effects, such as increased permeability of the basement membrane, decreased fibrinolytic activity, proliferation of extracellular matrix, and deposition of fibrinogen, and ultimately initiates thrombosis[42,43]. The results of this study showed that the expression of CD62P in the control group was < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group, and the expression of PLT in the control group was > Grade 1 hypertension group > Grade 2 hypertension group > Grade 3 hypertension group, indicating that the high expression of CD62P and low expression of PLT might be related to PH and the prethrombotic state. The causes were analyzed as follows: (1) For patients with continuously elevated blood pressure, spastic contraction of blood vessels results in impaired vascular endothelium, activated platelet activity, activated large numbers of platelets, simultaneously activated thromboxane A2, increased release of serotonin, enhanced platelet adhesion and agglutination, and slowed blood flow rate, causing hypertension and prethrombotic state; and (2) When blood pressure increased, the α particles of endothelial coryneform bodies and platelets fused with the cell membrane in a short time, and P- selectin showed persistently high expression on the cell membrane surface, which initiated and expanded thrombosis[44,45]. Coagulation is a process involving many coagulation factors and enzymes that require positive and negative feedback regulatory pathways to jointly maintain demand. In a healthy state, the coagulation and anticoagulant systems contain each other and are in a dynamically balanced state to ensure the health of the blood system. When any link is abnormal, the dynamic balance is disrupted, resulting in an abnormal blood system that mainly manifests as thrombosis or hemorrhage. APTT, PT, TT, and FIB are collectively referred to as the coagulation four items, including different coagulation pathways, which are effective indicators of the body's anticoagulant activity, coagulation factor synthesis ability, and coagulation conditions. Prothrombin is synthesized in the liver and converted into thrombin after activation, which promotes the conversion of fibrinogen into fibrin and plasma coagulation and causes a prethrombotic state[46,47]. FIB is an indispensable glycoprotein bridging molecule with a relatively high molecular weight during platelet aggregation. It not only directly participates in blood coagulation, but also increases blood viscosity and changes hemodynamics by promoting platelet adhesion and aggregation[48]. The results of this study showed that the expressions of APTT, PT, and TT in the control group were > Grade 1 hypertension group > Grade 2 hypertension group > Grade 3 hypertension group, and the expression of FIB in the control group was < Grade 1 hypertension group < Grade 2 hypertension group < Grade 3 hypertension group, suggesting that APTT, PT, TT, and FIB were also related to PH and its prethrombotic state. Based on the biological effects of the above indices, it is speculated that these serum indices are closely related to the occurrence of PH and its prethrombotic state.
To verify the above hypothesis, the multivariate Logistic regression model established in this study revealed that the expressions of hs-CRP, TM, Hct, ESR, CD62P, PLT, APTT, PT, TT, and FIB in participants were related to the progression of PH. Among these, high expression of hs-CRP, TM, Hct, ESR, CD62P, and FIB and low expression of APTT, PT, TT, and PLT were risk factors for PH. The results of the ROC curve showed that the AUC of hs-CRP, TM, ESR, CD62P, APTT, PT, TT, and FIB in the prediction of PH was > 0.80, and the prediction values were all ideal. This suggests that the risk of PH and its prethrombotic state in patients can be determined clinically by observing the above serum indicators in research participants, thus guiding rational clinical intervention measures. The results of the linear correlation analysis with bivariate Spearman showed that hs-CRP, TM, Hct, ESR, CD62P, and FIB had a positive correlation with each other (r > 0, P < 0.05), whereas PLT, APTT, PT, and TT had a negative correlation with hs-CRP, TM, Hct, ESR, CD62P, and FIB (r < 0, P < 0.05). There was a positive correlation between PLT, APTT, PT, and TT, and the above indicators interacted with each other, which may be because the indicators are related to the body's coagulation mechanism and platelet function, and a change in one indicator will definitely cause a change in the other related indicators. However, the specific mechanism of action of these indicators has not yet been identified and needs to be defined in future targeted research. In addition, it should be noted that the number of enrolled samples in this study was single, and the baseline data of enrolled patients was all covered by our hospital. The inclusion of a retrospective analysis was limited, and all covered indicators were selected at a certain time point without performing dynamic real-time detection of their indicators. There was still a bias in the conclusion of the study, and the credibility of the conclusion should be verified by expanding the sample size and conducting multicenter prospective research in the future to provide a scientific and effective reference basis for the clinical evaluation of the disease occurrence and progression in relevant patients.
The relevant indicators of the prethrombotic state in patients with PH, such as hs-CRP, TM, Hct, ESR, CD62P, PLT, APTT, PT, TT, and FIB, showed differences; high expression of hs-CRP, TM, Hct, ESR, CD62P, and FIB and low expression of PLT, APTT, PT, and TT are the keys to the occurrence, progression, and thrombotic state of PH. In clinical settings, according to the expression of the above serum indicators, targeted interventions can be administered to patients with abnormal expression to control the progression of the patient's disease and reduce the risk of a prethrombotic state.
Hypertension is a common chronic disease that affects many people worldwide. Hypertension can lead to various complications, including prethrombotic state and thrombotic diseases, which pose a significant risk to patients' health.
The study collected data from patients with different grades of hypertension and compared them with a control group. The results showed that certain indicators, such as high-sensitivity C-reactive protein (hs-CRP), thrombomodulin (TM), hematocrit (Hct), erythrocyte sedimentation rate (ESR), P-selectin on platelet surface (CD62P), platelet (PLT), activated partial thromboplastin time (APTT), prothrombin (PT), plasma thrombin time (TT), and fibrinogen (FIB), were associated with PH progression, and their expression levels differed among the groups. High expression of some indicators, such as hs-CRP, TM, Hct, ESR, CD62P, and fibrinogen, and low expression of others, such as PLT, APTT, PT, and TT, were identified as risk factors for PH. The findings suggest that targeted interventions based on these indicators' expression levels could help control the progression of PH and reduce the risk of developing a prethrombotic state.
The research objectives were to investigate the relationship between these indicators and the progression of PH and identify risk factors for the development of prethrombotic state in hypertensive patients.
This study retrospectively collected general data from patients with primary hypertension. The patients were divided into three groups based on their hypertension grade, and a control group was included. Relevant indicators of prethrombotic state were compared among the groups, including inflammation-related indicators, hemorheology-related indicators, vascular endothelial injury-related indicators, platelet-related indicators, and coagulation function-related indicators. Multivariate logistic regression models and receiver operating characteristic curve analyses were used to analyze the relationship between these indicators and PH progression. Linear correlation analysis was also performed.
The study results showed that certain indicators, including hs-CRP, TM, Hct, ESR, CD62P, PLT, APTT, PT, TT, and FIB, were associated with PH progression. The expressions of these indicators varied among hypertensive patients with different grades of hypertension and the control group. High expression of hs-CRP, TM, Hct, ESR, CD62P, and FIB, and low expression of PLT, APTT, PT, and TT, were identified as risk factors for PH.
This study identified the relevant indicators associated with prethrombotic state in patients with primary hypertension. The expressions of these indicators varied among hypertensive patients with different grades of hypertension and the control group. High expression of certain indicators and low expression of others were identified as risk factors for PH progression and the development of a prethrombotic state. The study findings suggest that monitoring and controlling the expression levels of these indicators could help prevent and treat hypertensive thrombotic diseases. The results provide a laboratory basis for clinical prevention and control of hypertensive thrombotic diseases, offering valuable insights into the mechanisms underlying the development of thrombotic events in hypertensive patients.
The findings of this study could pave the way for future research to investigate the mechanisms underlying the relationship between prethrombotic state and hypertension progression. Further studies could explore the efficacy of targeted interventions based on these indicators' expression levels to prevent and treat hypertensive thrombotic diseases. Additionally, future research could investigate the potential of these indicators as biomarkers for monitoring the progression of PH and predicting the development of thrombotic events in hypertensive patients. The study's results provide valuable insights into the pathophysiology of hypertensive thrombotic diseases and offer a basis for the development of personalized and effective prevention and treatment strategies for patients with PH.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hackam DJ, United States; Pocate-Cheriet K, France S-Editor: Wang JL L-Editor: A P-Editor: Yu HG
| 1. | Vareta G, Georgianos PI, Vaios V, Sgouropoulou V, Roumeliotis S, Georgoulidou A, Dounousi E, Eleftheriadis T, Papagianni A, Balaskas EV, Zebekakis PE, Liakopoulos V. Epidemiology of Hypertension among Patients on Peritoneal Dialysis Using Standardized Office and Ambulatory Blood Pressure Recordings. Am J Nephrol. 2022;53:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Haarman MG, Kerstjens-Frederikse WS, Vissia-Kazemier TR, Breeman KTN, Timens W, Vos YJ, Roofthooft MTR, Hillege HL, Berger RMF. The Genetic Epidemiology of Pediatric Pulmonary Arterial Hypertension. J Pediatr. 2020;225:65-73.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Black E, Lee J, Flynn JT, McCulloch CE, Samuels JA, Seth D, Warady B, Furth S, Mitsnefes M, Ku E. Discordances between pediatric and adult thresholds in the diagnosis of hypertension in adolescents with CKD. Pediatr Nephrol. 2022;37:179-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 894] [Article Influence: 178.8] [Reference Citation Analysis (0)] |
| 5. | Shen G, Huang JY, Yu YL, Liu L, Chen CL, Zhang B, Huang YQ, Feng YQ. J-shaped association between serum uric acid and acute coronary syndrome in patients with essential hypertension. Postgrad Med J. 2020;96:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Cai W, Lang M, Jiang X, Yu Q, Zhou C, Zou W, Zhang X, Lei J. Correlation among high salt intake, blood pressure variability, and target organ damage in patients with essential hypertension: Study protocol clinical trial (SPIRIT compliant). Medicine (Baltimore). 2020;99:e19548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Liu H, Huang W, Liu J, Zhao N, Wang H. Association between Arteriosclerosis and Uncontrolled Blood Pressure in Patients with Essential Hypertension: Cross-Sectional Observational Study Results of the BEST Study. J Vasc Res. 2022;59:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Luft FC. Did you know? Acta Physiol (Oxf). 2020;229:e13469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Deliyanti D, Alrashdi SF, Touyz RM, Kennedy CR, Jha JC, Cooper ME, Jandeleit-Dahm KA, Wilkinson-Berka JL. Nox (NADPH Oxidase) 1, Nox4, and Nox5 Promote Vascular Permeability and Neovascularization in Retinopathy. Hypertension. 2020;75:1091-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | China Hypertension Prevention and Treatment Guidelines Revision Committee; Hypertension Alliance (China); Chinese Medical Association Cardiovascular Disease Credit Association Chinese Physicians Association Hypertension Professional Committee; Chinese Medical Care International Exchange Promotion Association Hypertension Branch; Chinese Geriatric Society Hypertension Branch. [2018 Chinese guidelines for the management of hypertension]. Zhongguo Xinxueguan Zazhi. 2019;24:24-56. [DOI] [Full Text] |
| 11. | Chinese Diabetes Association. [Guidelines for the prevention and control of type 2 diabetes in China, 2017 Edition)]. Zhonghua Tangniaobing Zazhi. 2018;10:4-67. [DOI] [Full Text] |
| 12. | Department of Disease Control, Ministry of Health of the People's Republic of China. [Guidelines for the prevention and control of overweight and obesity in adults in China]. Beijing: People's Health Publishing House, 2006. |
| 13. | Demers C, McKelvie RS, Yusuf S. Interobserver reliability and validity of the New York Heart Association Functional Classification (NYHA-FC) in heart failure patients. Eur J Heart Fail. 2000;2:73-74. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Saha I, Paul B, Mukherjee A, Biswas R, Dasgupta A. Validity of the WHO criteria for adolescent hypertension. East Afr J Public Health. 2011;8:135-137. [PubMed] |
| 15. | Le Ribeuz H, To L, Ghigna MR, Martin C, Nagaraj C, Dreano E, Rucker-Martin C, Girerd B, Bouligand J, Pechoux C, Lambert M, Boet A, Issard J, Mercier O, Hoetzenecker K, Manoury B, Becq F, Burgel PR, Cottart CH, Olschewski A, Sermet-Gaudelus I, Perros F, Humbert M, Montani D, Antigny F. Involvement of CFTR in the pathogenesis of pulmonary arterial hypertension. Eur Respir J. 2021;58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Bełtowski J. Salt Intake, Aldosterone Secretion, and Obesity: Role in the Pathogenesis of Resistant Hypertension. Am J Hypertens. 2021;34:588-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Choudhary MK, Värri E, Matikainen N, Koskela J, Tikkakoski AJ, Kähönen M, Niemelä O, Mustonen J, Nevalainen PI, Pörsti I. Primary aldosteronism: Higher volume load, cardiac output and arterial stiffness than in essential hypertension. J Intern Med. 2021;289:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Han J, Yuan X, Song W, Cheng Y, Lu Y, Zhang Y, Liu Y, Jiang Y. The Correlation of Fibroblast Growth Factor 23 with Cardiac Remodeling in Essential Hypertension with Normal Renal Function. Cardiology. 2022;147:271-280. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Qu CH, Liu H, Tang JY. [Correlation between high-sensitivity C-reactive protein and brachial-ankle pulse wave velocity among patients with essential hypertension]. Zhongguo Linchuang Baojian Zazhi. 2020;23:79-83. [DOI] [Full Text] |
| 20. | Shanmugarajan D, Girish C, Harivenkatesh N, Chanaveerappa B, Prasanna Lakshmi NC. Antihypertensive and pleiotropic effects of Phyllanthus emblica extract as an add-on therapy in patients with essential hypertension-A randomized double-blind placebo-controlled trial. Phytother Res. 2021;35:3275-3285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Kang XP, Guo XH, Su Yp, Ping Z, Wang RX. [Correlation between plasma HCY and hs-CRP levels and carotid atherosclerosis in patients with hypertension and its risk factors]. Neimenggu Yixueyuan Xuebao. 2020;42:345-349. |
| 22. | Zhang QK, Yang JL, Wang Z. [Value of serum lipoprotein-associated phospholipase A2 and high-sensitivity C-reactive protein in diagnosis of essential hypertension with carotid atherosclerotic plaque]. Linchuang Yu Bingli Zazhi. 2022;42:2613-2619. |
| 23. | Liu FF, Zhang Q, Yang LH, Sun CH, Xu XT, Fang SJ, Liu Y, Yao F. [Correlation between Serum hs-CRP, Homocysteine and Nocturnal Blood Pressure Variability in Patients with Essential Hypertension]. Zhongguo Quanke Yixue. 2020;23:1292-1297. [DOI] [Full Text] |
| 24. | Strauch B, Petrák O, Wichterle D, Zelinka T, Holaj R, Widimský J Jr. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am J Hypertens. 2006;19:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Gou TT, Zhou WD. [Effects of Hematocrit on the Parameters of Thromboelastography in Healthy Adults]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29:901-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Alende-Castro V, Alonso-Sampedro M, Vazquez-Temprano N, Tuñez C, Rey D, García-Iglesias C, Sopeña B, Gude F, Gonzalez-Quintela A. Factors influencing erythrocyte sedimentation rate in adults: New evidence for an old test. Medicine (Baltimore). 2019;98:e16816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Nersesjan V, Zervides KA, Sørensen AL, Kjaer L, Skov V, Hasselbalch HC. The red blood cell count and the erythrocyte sedimentation rate in the diagnosis of polycythaemia vera. Eur J Haematol. 2020;104:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Orkmez M, Orhan S, Bozdayi MA, Tarakcioglu M. Comparison of the StaRRsed Interliner device with Westergren method in erythrocyte sedimentation rate measurement. Int J Lab Hematol. 2021;43:616-622. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Haraoui B, Khraishi M, Choquette D, Lisnevskaia L, Teo M, Kinch C, Galos C, Roy P, Gruben D, Woolcott JC, Vaillancourt J, Sampalis JS, Keystone EC; Canadian Tofacitinib for Rheumatoid Arthritis Observational Investigators. Effectiveness and Safety of Tofacitinib in Canadian Patients With Rheumatoid Arthritis: Primary Results From a Prospective Observational Study. Arthritis Care Res (Hoboken). 2023;75:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Lapić I, Piva E, Spolaore F, Musso G, Tosato F, Pelloso M, Plebani M. Ves-Matic CUBE 200: is modified Westergren method for erythrocyte sedimentation rate a valid alternative to the gold standard? J Clin Pathol. 2019;72:716-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Li M, Yang C, Wang Y, Song W, Jia L, Peng X, Zhao R. The Expression of P2X7 Receptor on Th1, Th17, and Regulatory T Cells in Patients with Systemic Lupus Erythematosus or Rheumatoid Arthritis and Its Correlations with Active Disease. J Immunol. 2020;205:1752-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Liu SY, Xu YL. [Analysis of the Characteristics of Hypercoagulable State and Prethrombotic State in Patients with Hypertension in Pregnancy]. Xueshuan Yu Zhixue Xue. 2021;27:830-832. |
| 33. | Ye J, Wang Y, Wang Z, Liu L, Yang Z, Wang M, Xu Y, Ye D, Zhang J, Zhou Q, Lin Y, Ji Q, Wan J. The Expression of IL-12 Family Members in Patients with Hypertension and Its Association with the Occurrence of Carotid Atherosclerosis. Mediators Inflamm. 2020;2020:2369279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Zhang LL, Song GH, Xia M. [The predictive value of soluble thrombomodulin detection for cardiovascular complications in patients with chronic kidney disease]. Linchuang Jianyan Zazhi. 2022;40:609-611. |
| 35. | Li SP, Ren H, An ZF, Yang YK, Bai ZH. [The Development of Chemiluminescence Immunoassay Detection Method for Thrombomodulin]. Zhongguo Shengwu Gongcheng Zazhi. 2021;41: 30-36. [DOI] [Full Text] |
| 36. | Wang HH, Wang YF. [The predictive value of thrombomodulin for venous thromboembolism in patients with lung cancer]. Zhongguo Yishi Jinxiu Zazhi. 2022;45:247-251. [DOI] [Full Text] |
| 37. | Niu XY, Xie XX. [The Role of Thrombomodulin in Diseases]. Zhongguo Shengwuhuaxue Yu Fenzishengwu Xuebao. 2021;37:998-1004. [DOI] [Full Text] |
| 38. | Li CB, Xu LN, Bu XX, Liu MX. [The value of soluble thrombomodulin in evaluating endothelial injury in patients with kidney disease]. Zhonghua Yi Xue Za Zhi. 2021;101:1812-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Yan YM, Wang XZ, Zhang ZF, Chang SJ, Chen RH, Jia HJ, Song QF. [Association between Mean Platelet Volume/Platelet Count, MicroRNA-26b Relative Expression Level, MicroRNA-195 Relative Expression Level and Hypertensive Left Ventricular Hypertrophy and Their Predictive Value on Patients' Prognosis]. Shiyong Xinnaofei Xueguanbing Zazhi. 2021;29:39-45. [DOI] [Full Text] |
| 40. | Yue MM, Li XY, Zhao YC, Zhang Y. [Research Advances in Relationship of P-selectin and P-selectin Glycoprotein Ligand-1 Gene Polymorphisms with Ischemic Cerebrovascular Diseases]. Yixue Zongshu. 2019;25:4919-4923. [DOI] [Full Text] |
| 41. | Wang Q, Zhu JB, Wu CG, Zou J, Li S, Hu PP, Yue AP. [P-selectin on activated platelets promotes hematogenous metastasis of primary lung cancer via P-selectin glycoprotein ligand-1]. Zhongguo Bingli Shengli Zazhi. 2019;35:1988-1993. [DOI] [Full Text] |
| 42. | Zhao J, Xu B, Chen G, Zhang Y, Wang Q, Zhao L, Zhou H. Cryopreserved platelets augment the inflammatory response: role of phosphatidylserine- and P-selectin-mediated platelet phagocytosis in macrophages. Transfusion. 2019;59:1799-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Wang WT, Chen YZ, Liu EX, Xu JM, Gu SL, Chen YT, Hu XB, Zhang MQ, Yin W, Li Q. [High expression of P-selectin (CD62P) after platelet activation in vitro inhibits bacterial proliferation and induces apoptosis-like changes]. Xibao Yu Fenzi Mianyixue Zazhi. 2022;38: 781-788. |
| 44. | Fox SC, May JA, Dovlatova N, Glenn JR, Johnson A, White AE, Radhakrishnan A, Heptinstall S. How does measurement of platelet P-selectin compare with other methods of measuring platelet function as a means of determining the effectiveness of antiplatelet therapy? Platelets. 2019;30:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Nurden AT, Caen JP. Specific roles for platelet surface glycoproteins in platelet function. Nature. 1975;255:720-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 276] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 46. | Guan J, Hu XH. [Diagnostic Value of Four Indexes of Coagulation and Platelet in Patients with Gestational Hypertension]. Xueshuan Yu Zhixue Xue. 2022;28:1019-1021. [DOI] [Full Text] |
| 47. | Chen Y, Li J, Gong B. [Correlation between Platelet, Coagulation Function and Hypertensive Disorders Complicating Pregnancy]. Xueshuan Yu Zhixue Xue. 2020;26:553-554. [DOI] [Full Text] |
| 48. | Yao L, Li XY. [Changes and Clinical Significance of Coagulation Factor and D-dimer in Patients with Gestational Diabetes Mellitus and Hypertension]. Xueshuan Yu Zhixue Xue. 2020;26:594-595. [DOI] [Full Text] |